Alkaline rechargeable Ni/Co batteries: Cobalt hydroxides as negative electrode materials†
Xue-Ping
Gao
*,
Su-Mei
Yao
,
Tian-Ying
Yan
and
Zhen
Zhou
Institute of New Energy Material Chemistry, Nankai University, Tianjin, 300071, China. E-mail: xpgao@nankai.edu.cn; Fax: +86 22-23500876; Tel: +86 22-23500876
First published on 23rd March 2009
Abstract
It is demonstrated that β-Co(OH)2 has a high discharge capacity and good high-rate discharge ability as a negative electrode material. A new rechargeable battery system with higher energy density, consisting of α-phase nickel hydroxides as the positive electrode material and β-cobalt hydroxides as the negative electrode material, is proposed on the basis of multi-electron reactions.
Broader contextAlkaline rechargeable batteries are electrochemical energy storage and conversion devices with high energy densities. Alkaline rechargeable batteries are considered to be one of the most promising power sources for the application in portable electronic devices, electric tools, electric vehicles (EV), and hybrid electric vehicles (HEV). Among alkaline rechargeable Ni-based batteries, nickel/metal hydride (Ni/MH) batteries are widely used owing to their outstanding features of high energy density, high power, environmental issue, and safety. However, further increasing energy density of Ni/MH batteries is limited, because faradaic single electron reactions are dominant in both negative and positive materials. Therefore, multi-electron reactions on electrode materials would be a more effective way to provide high energy density for alkaline rechargeable batteries. Herein, we describe a Ni/Co battery, consisting of α-Ni(OH)2 as the positive material and Co(OH)2 as the negative material on the basis of multi-electron reactions. The fabricated Ni/Co prototype cell shows outstanding features of long durability and high energy. In addition, compared to the toxic feature of a Ni/Cd battery, the Ni/Co battery can be denoted as a green battery. The strategy adapted here could be helpful to explore and develop new and environmentally-friendly power sources with high energy density based on multi-electron reactions. |
Alkaline rechargeable batteries are considered to be one of the most promising power sources for the application in portable electronic devices, electric tools, electric vehicles (EV), and hybrid electric vehicles (HEV). Many electrochemical couples of positive and negative electrode materials have been developed, including nickel/cadmium (Ni/Cd), nickel/iron (Ni/Fe), nickel/zinc (Ni/Zn), and nickel/metal hydride (Ni/MH) alkaline rechargeable Ni-based batteries.1 Among these systems, Ni/MH batteries are widely used owing to their outstanding features of high energy density, high power, environmental issue, and safety. Recently, numerous investigations have been conducted to propose new battery systems without flammable organic electrolytes on the basis of safety concerns, such as aqueous rechargeable lithium batteries,2polymer-based batteries,3 and all-solid-state rechargeable batteries.4 Moreover, high-rate discharge capability and high discharge capacity are also crucial for electrode materials in such rechargeable batteries.
Rechargeable batteries are mostly based on faradaic reactions, and multi-electron reactions on electrode materials are an effective way to provide a high energy density. α-Phase nickel hydroxide as the positive electrode material with 1.6–2.0 exchanged electrons per Ni atom has a higher discharge capacity, in contrast to the capacity limitation of commercial β-phase nickel hydroxide with only one electron reaction.5 On the other hand, negative electrode materials with multi-electron reactions have been explored recently. For example, more than one electron is involved in the electrochemical reaction of metallic cobalt or cobalt hydroxide in alkaline electrolyte.6,7 Moreover, cobalt hydroxide has capacitive characteristics with potential applications as an electrochemical supercapacitor with a high energy density.8
Cobalt hydroxide has a hexagonal layered structure and crystallizes into two polymorphic forms, α and β.9 The α-form is isostructural with hydrotalcite-like compounds that consist of hydroxyl-deficient Co(OH)2−x layers and charge balancing anions (NO3−, CO3−, Cl−, etc.) in the interlayer gallery. On the other hand, the β-form is burcite-like [Mg(OH)2] and consists of a hexagonal packing of hydroxyl ions with Co(II) occupying alternate rows of octahedral sites.9–11 The α-cobalt hydroxide is susceptible to losing interlayer water and anions. In contrast, the β-phase Co(OH)2 is stable and thus is anticipated to be a more promising electrode material.
In the present study, we synthesized single-crystal hexagonal platelets of β-Co(OH)2 by homogeneous precipitation,10 and investigated the discharge capability of β-Co(OH)2 at different discharge rates. It is found that β-Co(OH)2 has an excellent high-rate discharge ability and high discharge capacity. In addition, α-Ni(OH)2 microspheres with 1.8 exchanged electrons per Ni atom have a large discharge capacity, excellent high-rate ability and long cycle life as reported in our previous work.12 We describe herein a battery system, consisting of α-Ni(OH)2 as the positive electrode material and β-Co(OH)2 as the negative electrode material on the basis of multi-electron reactions (Scheme l).
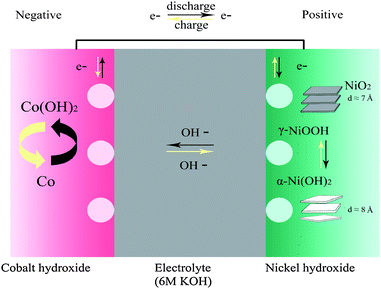 | ||
| Scheme 1 Schematic representation of the electrochemical reaction of Ni/Co batteries. | ||
The XRD pattern (see ESI, Fig. S1†) of the as-prepared β-Co(OH)2 particles matches with those reported earlier.10 All diffraction peaks can be indexed as the hexagonal structure of brucite-like β-Co(OH)2 (JCPDS 74-1057), and the sharp reflections indicate the highly crystalline nature of the pink β-Co(OH)2 product. A thin platelet-like morphology is observed in the β-Co(OH)2 sample from the SEM image (see ESI, Fig. S2†), which is beneficial to the electrochemical reaction on the active surface.
Fig.1 shows the dependence of electrochemical discharge capacity of the β-Co(OH)2 electrode on the cycle number after activation at different discharge rates. Obviously, there is an activation process to reach the maximum discharge capacity of the β-Co(OH)2 electrode. The maximum discharge capacities of 455, 453, 406 and 338 mA h g−1 are achieved at 1, 2, 5 and 10 C rate, respectively, indicating that β-Co(OH)2 exhibits excellent durability at high discharge rate as compared to α-Co(OH)2 (see ESI, Fig. S3†). Moreover, the discharge capacities of 373 and 258 mA h g−1 are retained at 1 C and 10 C rates after 50 cycles for β-Co(OH)2, higher that that of 313, and 240 mA h g−1 for α-Co(OH)2, respectively. The typical discharge curves for the as-prepared β-Co(OH)2 at different discharge rates after activation are shown in Fig. 2. The electrode presents a flat potential plateau around −0.78 V (vs. Hg/HgO) in the discharge curve at a 1 C rate, which is in good agreement with the discharge potential plateau of a metallic cobalt-based composite electrode,7 and the discharge plateau remains unchanged with increasing cycle numbers for both the α-Co(OH)2 and β-Co(OH)2 electrodes (see ESI, Fig. S4†). Although both α-Co(OH)2 and β-Co(OH)2 can be used as a negative electrode, β-Co(OH)2 is more favorable due to the structure transformation from unstable α-Co(OH)2 to stable β-Co(OH)2 during cycling (see ESI†).
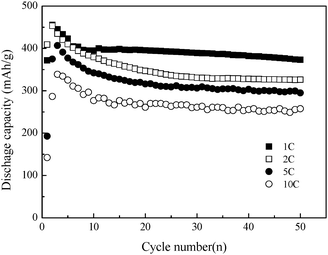 | ||
| Fig. 1 Cycle performance of the β-Co(OH)2 electrode at different discharge rates after charging at a 1, 2, 5 and 10 C rate for 1.5, 0.75, 0.3 and 0.15 h, respectively. | ||
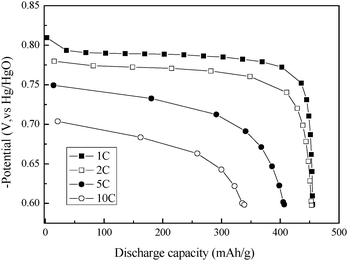 | ||
| Fig. 2 Discharge curves of the β-Co(OH)2 electrode at different discharge rates after activation (charging at a 1, 2, 5 and 10 C rate for 1.5, 0.75, 0.3 and 0.15 h, respectively). | ||
To further investigate the structural change after charging and discharging, XRD patterns of the β-Co(OH)2 electrode at the charge/discharge state in the second cycle are shown in Fig. 3. The pattern of the as-prepared β-Co(OH)2 is also included for comparison. It can be found that the diffraction peaks of metallic Co are detected at the fully charged state in the second cycle. Meanwhile, most of the diffraction peaks can be indexed as β-Co(OH)2 and the diffraction peaks of the metallic Co disappear almost after fully discharging in the second cycle. This means that β-Co(OH)2 can be reduced to metallic Co during the charge process, and the discharge capacity of the electrode is mainly attributed to the electrochemical oxidation of metallic Co. When α-Co(OH)2 is used as a negative electrode, the structure transformation from α-Co(OH)2 to β-Co(OH)2 is found at the fully charged state, showing higher structure stability of β-Co(OH)2 during electrochemical cycling (see ESI, Fig. S5†).
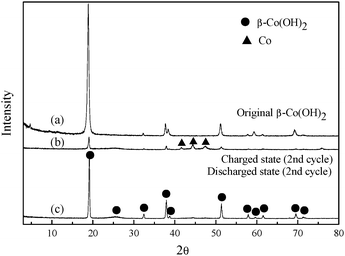 | ||
| Fig. 3 XRD patterns of the β-Co(OH)2 electrode at the charge and discharge state in the second cycle. | ||
To elucidate the electrochemical reaction process occurring on the β-Co(OH)2 electrode, cyclic voltammograms (CVs) of β-Co(OH)2 electrode from −0.4 V to −1.2 V (vs. Hg/HgO) at different scan rates are presented (see ESI, Fig. S6†). A pair of redox peaks is detected, indicating that the reversible capacity is mainly based on the faradaic redox mechanism. The cathodic peak is due to the reduction of Co(OH)2 to metallic Co and the anodic peak is attributed to the oxidation of metallic Co to Co(OH)2. An excellent electrochemical reaction activity and a good high rate capability are verified by the results of CV at higher scan rate. For the β-Co(OH)2 electrode material, it is well accepted that the surface faradaic reaction with 2 electrons is dominant and can be expressed as6,13
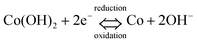 | (1) |
According to the above two-electron reaction process, the theoretical electrochemical capacity of Co(OH)2 is 576 mA h g−1 based on Faraday's law. The discharge capacity of Co(OH)2 at 1 C discharge rate is 455 mA h g−1 and the utilization of Co(OH)2 is 79%. From XRD patterns, it can be observed that β-Co(OH)2 is still detected as the coexisting phase with metallic Co at the end of the charging state. It implies that the partial irreversible conversion between β-Co(OH)2 and metallic Co is involved in the faradic reaction, resulting in the incomplete utilization of Co(OH)2. Therefore, on average, 1.58 electrons are involved in the practical electrochemical process of the β-Co(OH)2 electrode. It is reported that Co(OH)2 undergoes dissolution in alkaline electrolytes and the amount of cobalt formed from the dissolved Co(OH)2 is 8 mg L−1 in 5 M KOH.14 In addition, Co(OH)2 can be transformed to HCoO2− to some extent13 and the equilibrium concentration of HCoO2− is >10−4 M in 6 M KOH solution at 25 °C.15 Thus, the partial dissolution of Co(OH)2 also accounts for the decay of the discharge capacity during cycling.7
The promising values with respect to both discharge capacity and high rate capability of the β-Co(OH)2 in the half-cell clearly demonstrate a suitability for fabricating alkaline rechargeable batteries. A novel alkaline rechargeable battery system is designed with β-Co(OH)2 as the negative electrode. The electrode was prepared by mixing β-Co(OH)2 with carbonyl nickel powder (NICO 255) in a weight ratio of 1 : 3 and then pressed into a small pellet. As α-Ni(OH)2 microspheres with 1.8 exchanged electrons per Ni atom have a large discharge capacity, excellent high-rate ability and long cycle life,12 they were used here as the positive electrode material (see ESI, Experimental and Fig. S7†). α-Ni(OH)2, cobalt oxide powders, and Ni powders were mixed with a weight ratio of 65.6 : 7.7 :26.7, and pasted into a nickel foam. Then, the resultant positive electrode was coupled with a β-Co(OH)2 negative electrode in 6 M KOH. The fabricated Ni/Co prototype cell exhibits a flat discharge voltage plateau, high power density and long-term endurance at a 1 C rate as presented in Fig. 4. The output energy density reaches 165 W h kg−1 (both α-Ni(OH)2 and β-Co(OH)2 as the active materials) and stabilizes at 158.8 W h kg−1 after 50 cycles, revealing an energy retention of 96.2%. In the Ni/Co prototype cell, the overall battery reaction is expressed as
 | (2) |
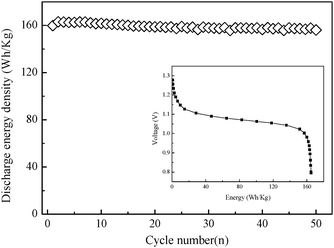 | ||
| Fig. 4 Cycling performance of the fabricated Ni/Co prototype cell at a 1 C rate after charging at a 1 C rate for 1.5 h. Inset is the discharge curve of the Ni/Co prototype cell in the second cycle at a 1 C rate. | ||
The operating principle is similar to that of Ni/Cd batteries,16 and the Ni/Co cell can be expected as a new member in alkaline rechargeable battery systems. In addition, if the weight percentage of 75–80% is considered in a practical battery, the energy density of 120–130 W h kg−1 would be obtained for the Ni/Co cell, which is higher than that of commercial Ni/Cd and Ni/MH batteries. (see ESI, Table S1†).1 Moreover, cobalt is an element that occurs naturally in many different chemical forms throughout the environment in air, water, soil, rocks, plants and animals with trace amounts. Small amounts of cobalt are accessible and essential for health because cobalt is a part of vitamin B12.17 Therefore, compared to the toxic features of a Ni/Cd battery, the Ni/Co battery can be denoted as a green battery.
In summary, β-Co(OH)2 presents good electrochemical performances at high discharge rates based on the faradic redox reaction with 1.58 exchanged electrons on average. A new alkaline rechargeable battery system, consisting of α-Ni(OH)2 as the positive electrode material and β-Co(OH)2 as the negative electrode material is proposed on the basis of multi-electron reactions. The fabricated Ni/Co prototype cell shows outstanding features of long durability and high power. The strategy adapted in the present study could be helpful to explore and develop new power sources with high energy density based on multi-electron reactions.
Financial support from the 973 Program (2009CB220100) and NSFC (50671049) of China is greatly appreciated.
References
- U. Köhler, C. Antonius and P. Bäuerlein, J. Power Sources, 2004, 127, 45 CrossRef CAS.
- G. J. Wang, L. J. Fu, N. H. Zhao, L. C. Yang, Y. P. Wu and H. Q. Wu, Angew. Chem., Int. Ed., 2007, 46, 295 CrossRef CAS.
- H. K. Song and G. T. R. Palmore, Adv. Mater., 2006, 18, 1764 CrossRef CAS.
- P. H. L. Notten, F. Roozeboom, R. A. H. Niessen and L. Baggetto, Adv. Mater., 2007, 19, 4564 CrossRef CAS.
- W. K. Hu and D. Noréus, Chem. Mater., 2003, 15, 974 CrossRef CAS.
- P. Elumalai, H. N. Vasan and N. Munichandraiah, J. Power Sources, 2001, 93, 201 CrossRef CAS.
- Z. W. Lu, S. M. Yao, G. R. Li, T. Y. Yan and X. P. Gao, Electrochim. Acta, 2008, 53, 2369 CrossRef CAS.
- L. Cao, F. Xu, Y. Y. Liang and H. L. Li, Adv. Mater., 2004, 16, 1853 CrossRef CAS.
- Z. P. Xu and H. C. Zeng, Chem. Mater., 1999, 11, 67 CrossRef CAS.
- Z. P. Liu, R. Z. Ma, M. Osada, K. Takada and T. Sasaki, J. Am. Chem. Soc., 2005, 127, 13869 CrossRef CAS.
- P. V. Kamath, G. H. A. Therese and J. Gopalakrishnan, J. Solid State Chem., 1997, 128, 38 CrossRef.
- Q. D. Wu, X. P. Gao, G. R. Li, G. L. Pan, T. Y. Yan and H. Y. Zhu, J. Phys. Chem. C, 2007, 111, 17082 CrossRef CAS.
- B. S. Haran, B. N. Popov and R. E. White, J. Electrochem. Soc., 1998, 145, 3000 CrossRef CAS.
- V. Pralong, A. Delahaye-Vidal, B. Beaudoin, B. Gerand and J. M. Tarascon, J. Mater. Chem., 1999, 9, 955 RSC.
- H. Senoh, M. Ueda, N. Furukawa, H. Inoue and C. Iwakura, J. Alloys Compd., 1998, 280, 114 CrossRef CAS.
- A. K. Shukla, S. Venugopalan and B. Hariprakash, J. Power Sources, 2001, 100, 125 CrossRef CAS.
- T. Brock and W. Stopford, J. Environ. Monit., 2003, 5, 71N RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Complete experimental details of syntheses, XRD patterns, SEM images and cyclic voltammograms. See DOI: 10.1039/b901934k |
| This journal is © The Royal Society of Chemistry 2009 |
