A completely anoxic microbial fuel cell using a photo-biocathode for cathodic carbon dioxide reduction†
Xiaoxin
Cao
a,
Xia
Huang
*a,
Peng
Liang
a,
Nico
Boon
b,
Mingzhi
Fan
a,
Lin
Zhang
a and
Xiaoyuan
Zhang
a
aState Key Joint Laboratory of Environment Simulation and Pollution Control, Department of Environmental Science and Engineering, Tsinghua University, Beijing, 100084, P.R.China. E-mail: xhuang@tsinghua.edu.cn; Fax: +86 10 62771472; Tel: +86 10 62772324
bLaboratory of Microbial Ecology and Technology, Gent University, Coupure Links 653, B-9000, Gent, Belgium
First published on 23rd February 2009
Abstract
Typical microbial fuel cells (MFCs) rely on precious metals for reduction of oxygen at the cathode, but recently MFCs have been developed that use biocathodes for power generation with alternate electron acceptors. It is shown here that with illumination it is possible to develop a biocathode that uses dissolved carbon dioxide (bicarbonate) as the acceptor. During acclimation, the cathode was set at a potential of 0.242 V (vs.SHE) using a potentiostat. After approximately one month of acclimation, a current of 1 mA was sustained. Bicarbonate was reduced in stoichiometric agreement with current generation, with 0.28 ± 0.02 moles of bicarbonate reduced per mole of electrons. When this biocathode was used in a two-bottle MFC, a power density of 750 mW m−2 was produced. These results demonstrate that MFCs can be used to fix carbon dioxide as well as produce electricity.
Broader contextMicrobial fuel cell (MFC) is an emerging process that can generate electricity with simultaneous organic matter removal from domestic and industrial wastewaters. The main bottleneck perceived at the moment is the cathodic electron transfer. Typical MFC relies on precious metals for reduction of oxygen at the cathode, but recently MFCs have been developed that use biocathodes for power generation with alternate electron acceptors. This paper shows that with illumination it is possible to develop a biocathode that uses dissolved carbon dioxide (bicarbonate) as the acceptor. When this biocathode was used in a prototype MFC, the power density produced was comparable to that produced by common chemical cathode MFCs. These findings demonstrate for the first time the possibility of direct electron transfer between a cathode and microorganisms for fixation of carbon dioxide in biomass. Moreover, the excellent performance of the bicarbonate biocathode makes such a MFC system simpler and more economically viable. |
1 Introduction
The microbial fuel cell (MFC) is an emerging process that can generate electricity with simultaneous organic matter removal from domestic and industrial wastewaters.1,2 Previously, most systems have relied on precious metals for reduction of oxygen at the cathode. However recently, MFCs have been developed that use biocathodes for oxygen, nitrate or perchlorate reduction.3–6Carbon dioxide can also be used by some microorganisms as a terminal electron acceptor, but so far there have been no studies examining the use of biocathodes for reduction of CO2 in MFCs.From a thermodynamic point of view, CO2reduction has a very low redox potential and thus its use in a MFC could result in very low voltage production. The potential for CO2reduction (HCO3− + 4e− + 5H+ → (CH2O) + 2H2O, where (CH2O) represents the approximate formula of cell mass) at pH = 7.0 is −0.420 V (vs.SHE).3,7 In order to generate electricity, the cathode potential must be higher than anode potential. Acetate oxidation at the anode, for example, can produce a minimum potential of −0.280 V (vs.SHE), which is insufficient for current generation. Therefore, energy must be put into the system in order to use CO2 as a final electron acceptor. Recently, CO2 was reduced to methane with hydrogen gas as the intermediate, but an external energy source (electricity) was supplied.8,9
Here, we explore the idea of using sunlight to drive CO2reduction at the cathode in a MFC. We demonstrate that if a cathode is inoculated with bacteria and illuminated, a biocathode capable of CO2reduction can be developed. This advancement may allow the development of more sustainable and cost efficient MFCs capable of electricity production and carbon sequestration.
2 Methods
2.1 MFC construction and operation
Two-botttle “H” type MFCs were constructed as previously described10 with the bottles separated by a cation membrane (CMI7000, Membranes International Inc., USA). Both chambers were initially flushed with nitrogen gas, sealed with rubber stoppers, and stirred slowly using magnetic stir bars at 60 rpm. Plain graphite felt (Sanye, China) was used for both electrodes (projected surface area of 8 cm2), with the electrodes connected to the circuit using graphite rods. A saturated calomel electrode (SCE, 0.242 V vs.SHE, Leici, China) was fitted through the rubber stopper and used as a reference electrode. The cathodes were illuminated at 900 lx (JD-3 light meter, China) using 25 W incandescent lamps. When operated in dark, the cathode chamber was wrapped in aluminium foil. All experiments were conducted in duplicate.The cathode was filled with medium and inoculated using a mixture of aerobic and anaerobic sludge from a phototrophic anode chamber.10 The medium (pH = 7) contained (per liter): 4.4 g KH2PO4, 3.4 g K2HPO4·3H2O, 1.0 g NaHCO3, 1.5 g NH4Cl, 0.1 g MgCl2·6H2O, 0.1g CaCl2·2H2O, 0.1 g KCl, and 10 mL of trace mineral metals solution.4 The counter chamber was filled with the same medium except NH4Cl was replaced by 1.6 g NaCl. The reactor was then operated with the cathode potential set to 0.242 V (vs.SHE) to omit the generation of hydrogen using a potentiostat (MSTAT T8000, Arbin, USA) until a steady current was obtained.
Following the startup period, the potentiostat was removed and the biocathodes were used in MFCs. The anode chamber was filled with the same medium used for the cathode except that the NaHCO3 was replaced with sodium acetate (1.6 g). The anodes used in MFC experiments were taken from other MFCs operating with the same acetate medium. All MFCs were operated in batch mode at 30 ± 0.5 °C.
2.2 Analytical techniques
Current produced during poised potential experiments was obtained from a potentiostat. During MFC studies, the voltage across an external resistor (500 Ω) was monitored using a multimeter (DAQ2213), with the current and power calculated as previously described.10Cyclic voltammetry (CV) was carried out using a potentiostat with a three-electrode arrangement. The working electrode was connected to the cathode and the scan rate was 5 mV s−1.
Bicarbonate concentrations were measured using a total organic carbon analyzer (TOC-V CPH, Shimadzu, Japan). Biofilm biomass was determined by phospholipid analysis.11 NO2− and NO3− were measured using standard methods. Dissolved oxygen was measured by an HACH LDO probe.
3. Results and discussion
3.1 Current generation coupled to bicarbonate reduction
After approximately one month of acclimation with a cathode potential set at 0.242 V, the current reached a plateau of about 1 mA (3.2 A m−2). The reactor was then operated for four cycles each approximately 190 h long, with the reduction in bicarbonate concentration measured at the beginning and end of each cycle (Fig. 1A). Based on the measured current and the decrease in bicarbonate, the ratio was 0.28 ± 0.02 mole of bicarbonate consumed per mole of electrons (Fig. 1B). In contrast, for control reactors inoculated in dark, inoculated with a heat-killed sludge, or not inoculated, there was negligible current generation or bicarbonate consumption. No nitrite or nitrate was detected in solution, eliminating the possibility that these chemicals were acting as the electron acceptor.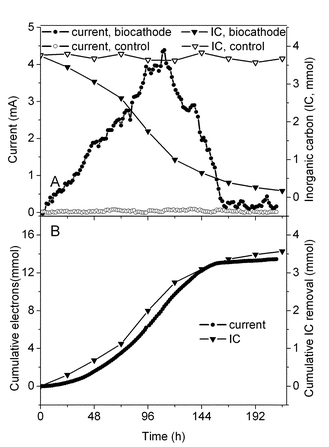 | ||
| Fig. 1 Electricity generation coupled to bicarbonate reduction in poised potential experiments at 0.242 V. (A) Current and bicarbonate concentration in the biocathode and abiotic control chamber. (B) Cumulative electrons and bicarbonate consumption in the biocathode chamber. | ||
During operation, no or low concentrations (maximum 0.2 mg L−1) of dissolved oxygen were detected in the chambers. Even if oxygen was generated by the cathodic biofilm, its low concentration in solution would not have substantially contributed to current generation. For Pt-catalyzed cathodes and oxygen biocathodes, dissolved oxygen concentrations below 2 mg L−1 substantially reduce current generation.12 The lack of dissolved oxygen makes it unlikely that dissolved oxygen served as a substantial final electron acceptor. Therefore, it is believed that current generation was due to bicarbonate reduction at cathode.
When the reactors were switched from light to dark conditions, the current immediately began to decrease (Fig. 2). When light was restored to the cathode, current generation resumed and increased over time. These results therefore demonstrate that current generation depends on both bicarbonate utilization and light.
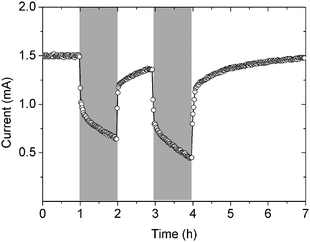 | ||
| Fig. 2 Response of current to light and dark cycling (the grey bars indicate dark conditions). | ||
3.2 Power generation using the biocathode
When the biocathode was used in a MFC, the maximum power generated with acetate as the electron donor was 750 mW m−2 (Fig. 3). In contrast, the maximum power generated by the same reactor, using a plain carbon cathode, was only 50 mW m−2. The power density of the biocathode was therefore 15-fold larger than that achieved with abiotic cathode control. Similar observations of an increase in power due to a reduction in cathode overpotential have been made using biocathodes with oxygen and nitrate.4,13,14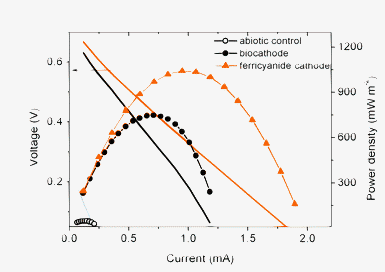 | ||
| Fig. 3 Comparison of MFC performance with abiotic control cathode, biocathode and ferricyanide cathode, respectively. | ||
The biocathode performance was also compared to ferricyanide, a commonly used chemical catholyte (50 mM K3[Fe(CN)6], 100 mM PBS, pH = 7.0). Under these conditions, the maximum power generated was 1050 mW m−2, which was 40% greater than that of the biocathode. These results showed that the biocathode performance was comparable to the chemical cathode.
3.3 Electrochemical characterization of the biocathode
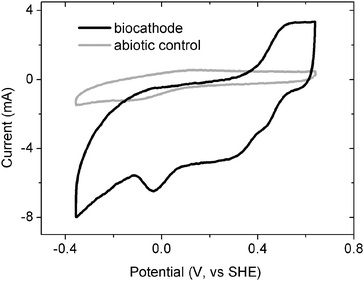
To determine if current was affected by suspended cells or soluble mediators, the solution was replaced with fresh anaerobic medium once the current declined below 10 µA (due to depletion of bicarbonate at the end of the batch cycle). The current rapidly increased indicating mediators were not needed for current flow. The cathodes were also examined using CV. Decreasing the cathode potential increased the current (Fig. 4) and a reduction peak was observed at −0.040 V (vs.SHE). These CV results are similar to those obtained by Clauwaert et al. using a biocathode for nitrate.4 They also observed a current peak in the potential range of +0.050 to −0.030 V (vs.SHE).These results demonstrate that the biofilm is the key factor for extracellular electron transfer, not mediators or cells in solution. Also no hydrogen was generated at this cathode potential (0.242 V), indicating hydrogen was not an intermediate needed for current flow. Taken together, these results indicate that direct electron transfer is occurring from the cathode to the microorganisms.6 This is different from previous results where CO2reduction is sustained by hydrogen production and its conversion into methane using a mediator (neutral red).15 Recently, algae have been used in the cathode chamber to reduce CO2, but this process also requires a mediator (methylene blue).16
3.4 Biomass production
At the end of the experiment (after four months of operation for set potential and MFC experiments) the electrodes were removed and the cathode biomass measured to demonstrate microbial growth on the cathode. The biomass on the cathode increased from 0.19 ± 0.01 mg (measured for the inoculum) to 1.12 ± 0.06 mg lipid phosphorus. Scanning electron micrographs of carbon fibers from the cathode showed a sparse coverage of bacteria (Fig. 5). These results were similar to previous biocathode studies that also indicated low microbial densities on the biocathode for oxygen reduction.6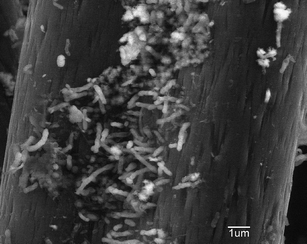 | ||
| Fig. 5 Scanning electron micrographs of the carbon fibers obtained from the biocathode. | ||
4. Conclusions
In the presence of light, it was shown that a biocathode could be used to directly reduce bicarbonate. The amount of bicarbonate reduced was in stoichiometric agreement with that theoretically expected from the current, with an average of 0.28 ± 0.02 mole biocarbonate/mole electron. When the biocathode was used in an MFC, the maximum power density obtained was 15-fold larger than that produced using a plain carbon cathode. These findings demonstrate for the first time the possibility of direct electron transfer between a cathode and microorganisms for fixation of carbon dioxide in biomass, while at the same time allowing the generation of electricity from biodegradable organic matter.Acknowledgements
This study was supported by the NSFC (20577027), International Program of MOST (2006DFA91120) and 863 Project (2006AA06Z329). We thank Professor Xie in China NCNT for the SEM (FEI QUANTA 200 FEG) observations and Professor B. E. Logan in PSU for valuable discussions and comments on manuscript revision.References
- B. E. Logan and J. M. Regan, Environ. Sci. Technol., 2006, 40, 5172–5180 CAS.
- B. M. Antonio Rinaldi, Virgilio Garavaglia, Silvia Licoccia, Paolo Di Nardo and Enrico Traversa, Energy Environ. Sci., 2008, 1, 417–429 RSC.
- Z. He and L. T. Angenent, Electroanalysis, 2006, 18, 2009–2015 CrossRef CAS.
- P. Clauwaert, K. Rabaey, P. Aelterman, L. De Schamphelaire, T. H. Ham, P. Boeckx, N. Boon and W. Verstraete, Environ. Sci. Technol., 2007, 41, 3354–3360 CrossRef CAS.
- J. C. Thrash, J. I. Van Trump, K. A. Weber, E. Miller, L. A. Achenbach and J. D. Coates, Environ. Sci. Technol., 2007, 41, 1740–1746 CrossRef CAS.
- K. Rabaey, S. T. Read, P. Clauwaert, S. Freguia, P. L. Bond, L. L. Blackall and J. Keller, ISME J., 2008, 2, 519–527 CrossRef CAS.
- F. Widdel, S. Schnell, S. Heising, A. Ehrenreich, B. Assmus and B. Schink, Nature, 1993, 362, 834–836 CrossRef CAS.
- H. Liu, S. Grot and B. E. Logan, Environ. Sci. Technol., 2005, 39, 4317–4320 CrossRef CAS.
- P. Clauwaert, R. Toledo, D. Van der Ha, R. Crab, W. Verstraete, H. Hu, K. M. Udert and K. Rabaey, Water Sci. Technol., 2008, 57, 575–579 Search PubMed.
- X. X. Cao, X. Huang, N. Boon, P. Liang and M. Z. Fan, Electrochem. Commun., 2008, 10, 1392–1395 CrossRef CAS.
- R. H. Findlay, G. M. King and L. Watling, Appl. Environ. Microbiol., 1989, 55, 2888–2893 CAS.
- S. Oh, B. Min and B. E. Logan, Environ. Sci. Technol., 2004, 38, 4900–4904 CrossRef CAS.
- P. Clauwaert, D. Van der Ha, N. Boon, K. Verbeken, M. Verhaege, K. Rabaey and W. Verstraete, Environ. Sci. Technol., 2007, 41, 7564–7569 CrossRef CAS.
- A. Bergel, D. Feron and A. Mollica, Electrochem. Commun., 2005, 7, 900–904 CrossRef CAS.
- D. H. Park, M. Laivenieks, M. V. Guettler, M. K. Jain and J. G. Zeikus, Appl. Environ. Microbiol., 1999, 65, 2912–2917 CAS.
- E. E. Powell, M. L. Mapiour, R. W. Evitts and G. A. Hill, Bioresour. Technol., 2009, 100, 269–274 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental and proposed electron transfer pathway. See DOI: 10.1039/b901069f |
| This journal is © The Royal Society of Chemistry 2009 |