A metal-free “black dye” for panchromatic dye-sensitized solar cells†
Haining
Tian
a,
Xichuan
Yang
*a,
Ruikui
Chen
a,
Anders
Hagfeldt
ab and
Licheng
Sun
*ac
aState Key Laboratory of Fine Chemicals, DUT-KTH Joint Education and Research Center on Molecular Devices, Dalian University of Technology (DUT), 158 Zhongshan Rd, 116012, Dalian, China. E-mail: yangxc@dlut.edu.cn; Fax: +86 411 8370 2185; Tel: +86 411 8899 3886
bSchool of Chemical Science and Engineering, Center of Molecular Devices, Physical Chemistry, Royal Institute of Technology (KTH), Teknikringen 30, 10044, Stockholm, Sweden. E-mail: hagfeldt@kth.se; Fax: +46 8 790 8207; Tel: +46 8 790 8177
cSchool of Chemical Science and Engineering, Department of Chemistry, Organic Chemistry, Royal Institute of Technology (KTH), Teknikringen 30, 10044, Stockholm, Sweden. E-mail: lichengs@kth.se; Fax: +46 8 791 2333; Tel: +46 8 790 8127
First published on 24th March 2009
Abstract
A novel metal-free “black dye” was designed and synthesized for panchromatic dye-sensitized solar cells. Based on this dye, the broader incident photon-to-current conversion efficiency spectrum was obtained over the whole visible range extending into the near-IR region up to 920 nm.
Broader contextDue to high molar extinction coefficient, low cost and prominent solar energy-to-electricity conversion efficiency, metal-free dyes for dye sensitized solar cells (DSCs) have developed rapidly in recent years. However, compared with Ru-complexes dyes, a major drawback of metal-free dyes is the narrower incident photon-to-current conversion efficiency (IPCE). To obtain a panchromatic metal-free dye, Tian et al. employ phenoxazine (electron donor, D), thiophene (π-bridge) and co-rodanmine (electron acceptor, A) to construct a novel D–π–A “black dye”. When the dye was fabricated to DSCs, a broader IPCE spectrum over the whole visible range of solar spectrum extending into the near-IR region up to 920 nm was obtained. The result further proves that metal-free dyes can act as panchromatic sensitizers for DSCs like Ru dyes. |
Dye-sensitized solar cells (DSCs) as promising solar energy-to-electricity conversion devices have been studied extensively since Grätzel and co-workers reported that in 1991.1 The wide-bandgap nanocrystalline TiO2 sensitized by Ru complexes2 and metal-free dyes3 as photoelectrodes can achieve prominent solar energy-to-electricity conversion efficiency (η), over 11% and 9%, respectively, under AM 1.5G simulated solar light of 100 mW cm−2. Organic dyes4–14 have attracted much attention due to their many advantages over Ru dyes, such as diversity of molecule structures, high molar extinction coefficient, simple synthesis as well as low cost and environmental impact. To improve the solar energy-to-electricity conversion efficiency of DSCs based on metal-free dyes, a broader incident photon-to-current conversion efficiency (IPCE) spectrum for metal-free dyes is desirable. However, compared to Ru sensitizers, metal-free dyes showed a narrower light action spectra over the whole spectral distribution of sunlight. So far, only Ru sensitizers can achieve a broader IPCE spectrum from 300 nm to 920 nm.15 To the best of our knowledge due to the difficulties of achieving perfect energy match between the dyes and nano-oxide or electrolyte, few metal-free panchromatic dyes have been reported for Grätzel cells. In this paper, we report a novel metal-free “black dye” (TH304, see Fig. 1) which shows a broader IPCE spectrum, 300 nm to ∼920 nm.
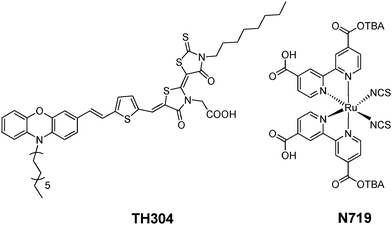 | ||
| Fig. 1 The structure of the TH304 and N719 dyes. | ||
In TH304, the phenoxazine (POZ) subunit is employed as an electron donor and dodecyl is adopted to increase the solubility and suppress aggregation of the dye, co-rhodanine is used as the electron acceptor for electron injection to the conduction band (CB) of TiO2, and thiophene acts as π-bridge for extending absorption spectrum. The synthesis of this dye including the related precursors and the experimental details are provided in ESI†
The UV–vis absorption spectrum of the TH304 dye shows a broader light harvesting range from 400 nm to 700 nm in CH2Cl2 solution with a maximum wavelength 568 nm (see Fig. 2.). The molar extinction coefficient, ε (λ), was determined to be 41000 M−1 cm−1 at the absorption maximum. When the dye was adsorbed on TiO2 film, slight blue-shift of absorption maximum and broadening of absorption spectrum were found probably due to the coaction of dyeH-aggregation and J-aggregation on the TiO2electrode surface. When excited at absorption maximum, the dye exhibits a fluorescence spectrum consisting of a single band with a maximum at 772 nm. Based on the intersection of absorption and fluorescence spectra, the zeroth–zeroth transition energy (E0–0), 1.90 V, was obtained.
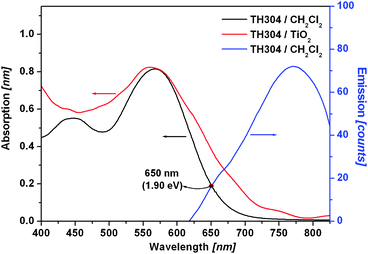 | ||
| Fig. 2 The absorption spectra of the TH304 dye in solution (black line) and on TiO2 film (red line), and the emission spectrum in solution (blue line). | ||
To estimate the HOMO level of the dye, cyclic voltammetry was employed using a three-electrode cell and an electrochemistry workstation (BAS100B, USA). The working electrode was a glass carbon disc, the auxiliary electrode was a Pt wire and Ag/Ag+ was used as reference electrode. Tetrabutylammonium hexaflourophosphate (TBAPF6) 0.1 M was used as the supporting electrolyte in CH2Cl2. Ferrocene was added to the solution at the end of the experiment and the ferrocenium/ferrocene (Fc/Fc+) redox couple was used as an internal potential reference. The potentials vsNHE were calculated by addition of 630 mV to the potentials vsFc/Fc+.9c The first oxidation potential (Eox) corresponds to the HOMO level and is determined to be 0.85 V vsNHE. It is shown that the HOMO level of the dye is sufficiently more positive than the iodine/iodide redox potential value, 0.4 V vsNHE, indicating that the oxidized dye formed after electron injection into the conduction band of TiO2 can accept an electron from iodide ions thermodynamically. LUMO level of the dye, −1.05 V vsNHE can be obtained by Eox − E0–0, and is sufficiently more negative than Ecb (conduction-band-edge energy level) of the TiO2electrode, which is −0.5 V vsNHE16 implying that the electron from the excited dye can be injected to CB of TiO2.
For the measurement of DSCs performance and IPCE spectra, 16 µm thickness TiO2 films (12 µm transparent layer + 4 µm scattering layer) were prepared by the screen-print method. The concentrations of dye-baths for N719 dye and TH304 dye are 0.3 mM and 0.2 mM in EtOH and CH2Cl2 respectively, and saturated chenodeoxycholic acid (CDCA) was added as co-adsorbent into the TH304 dye-bath to improve DSCs performance. The electrolyte, consisting of 0.6 M 1,2-dimethyl-3-propylimidazolium iodide (DMPII), 0.06 M LiI, 0.04 M I2 and 0.4 M 4-tert-butylpyridine (TBP) in dry acetonitrile was employed for N719 dye. However, the optimized electrolyte for TH304 dye was 0.2 M tetrabutylammonium iodide (TBAI), 0.7 M LiI, 0.07 M I2 in dry acetonitrile. The TiO2 films were sensitized for optimized time in dye-baths (N719 for 24 h, TH304 for 2 h), and then the DSCs were fabricated by assembling the dye-loaded film as the working electrode and Pt-coated conducting glass as the counter electrode coated with a hot-melt Surlyn1702 film (25 µm), from Dupont. The electrolyte was introduced into the cellvia vacuum backfilling through the hole in the back of the counter electrode. Finally, the hole was sealed using Surlyn 1702 film. The photovoltaic performance was measured at 100 mW cm−2 under AM 1.5G conditions with a mask (0.159 cm2). For the best cell, the photocurrent density–photovoltage (J–V) curve is shown in Fig. 3, and the overall solar energy-to-electricity conversion efficiency (η) of TH304 dye based DSCs is 3.0% with short-circuit photocurrent density JSC = 14.4 mA cm−2, open-circuit photovoltage VOC = 0.39 V and fill factor ff = 0.54.
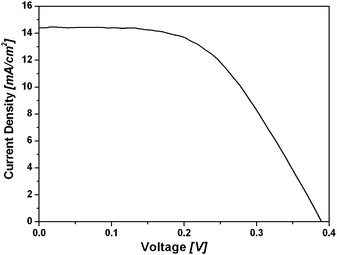 | ||
| Fig. 3 The J–V curve of the DSCs based on TH304 dye. | ||
Fig. 4 shows the incident photon-to-current conversion efficiency (IPCE) spectra of DSCs based on N719 dye (purple line) and TH304 (black line) dye, respectively. Notably, it shows a broader IPCE spectrum from 300 nm to 920 nm with a maximum value 67% at 580 nm for TH304-based DSCs than that of the N719-based DSCs where the IPCE spectrum is from 300 nm to 800 nm with maximum value 85% at 520 nm. The IPCE values from 660 nm to 920 nm of TH304 dye-based DSCs are apparently superior to N719-based DSCs. The result shows that in addition to Ru complexes15 the metal-free, pure organic dyes can also achieve a broad spectrum response up to the near-IR region. Although the TH304 dye cannot obtain a higher η value, the discovery of this dye exhibiting enhanced light harvesting in the near-IR region opens up the way to improve significantly the overall efficiency of nanocrystalline photovoltaic devices based on metal-free dyes in the future.
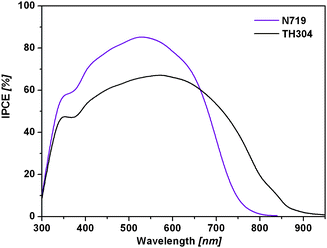 | ||
| Fig. 4 The IPCE spectra of DSCs based on N719 dye (purple line) and TH304 dye (black line). | ||
Density functional theory (DFT) calculations were made on a B3LYP/6-31G level with Gaussian 0317,18 to get a further insight into electron distribution of TH304 at different energy levels. The electronic redistribution shows a pronounced intramolecular charge separation between the HOMO and LUMO levels (depicted in Fig. 5). However, the LUMO electron density geometry distribution of the dye is mainly concentrated on the thienyl and co-rhodanine framework, and resulting in the position of LUMO isolated from the carboxyl anchoring group due to the presence of the methylene group.11 This result implies that a strong orbital overlap between the LUMO level of the excited dye and Ecb of TiO2 will be required to achieve more effective electron injection, where the addition of 4-tert-butlypyridine (TBP) into the electrolyte can not help for faster electron injection.19
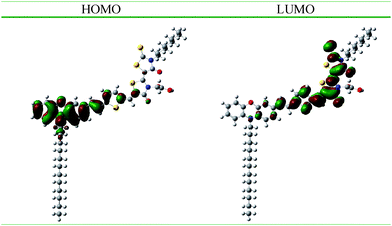 | ||
| Fig. 5 Graphic representation of the frontier molecular orbitals of TH304 dye (O red, S yellow, N blue, C gray). | ||
In summary, a novel phenoxazine metal-free dye (TH304) was designed and synthesized for panchromatic dye-sensitized solar cells. Based on this dye, a broader incident photon-to-current conversion efficiency spectrum has been obtained over the whole visible range extending into the near-IR region up to 920 nm. Although the TH304 dye did not obtain higher η value than 3.0% under our experimental conditions, the enhanced light harvesting in the near-IR region provides possibilities for further improvement of overall efficiency of nanocrystalline photovoltaic devices based on metal-free dyes in the future. Further structural modification of the TH304 dye to get even better DSCs performance is therefore in progress.
Acknowledgements
We gratefully acknowledge the financial support of this work from the following sources: China Natural Science Foundation (Grant 20633020), the Ministry of Science and Technology (MOST) (Grant 2001CCA02500), the Ministry of Education (MOE), the Program for Changjiang Scholars and Innovation Research Team in University (IRT0711), the NKBRSF (2009CB220009), the Swedish Energy Agency, the Swedish Research Council and the K & A Wallenberg Foundation.Notes and References
- B. O'Regan and M. Grätzel, Nature, 1991, 353, 737–740 CrossRef CAS.
- (a) M. K. Nazeeruddin, A. Kay, I. Rodicio, R. Humphry-Baker, E. Müller, P. Liska, N. Vlachopoulos and M. Grätzel, J. Am. Chem. Soc., 1993, 115, 6382–6390 CrossRef CAS; (b) M. K. Nazeeruddin, S. M. Zakeeruddin, R. Humphry-Baker, M. Jirousek, P. Liska, N. Vlachopoulos, V. Ahklover, C. H. Fischer and M. Grätzel, Inorg. Chem., 1999, 38, 6298–6305 CrossRef CAS; (c) M. Grätzel, J. Photochem. Photobiol., A, 2004, 164, 3–14 CrossRef CAS.
- S. Ito, S. M. Zakeeruddin, R. Humphry-Baker, P. Liska, R. Charvet, P. Comte, M. K. Nazeeruddin, P. Péchy, M. Takata, H. Miura, S. Uchida and M. Grätzel, Adv. Mater., 2006, 18, 1202–1205 CrossRef CAS.
- (a) K. Hara, K. Sayama, Y. Ohga, A. Shinpo, S. Suga and H. Arakawa, Chem. Commun., 2001, 569–570 RSC; (b) K. Hara, Z. S. Wang, T. Sato, A. Furube, R. Katoh, H. Sugihara, Y. Dan-oh, C. Kasada, A. Shinpo and S. Suga, J. Phys. Chem. B, 2005, 109, 15476–15482 CrossRef CAS.
- K. Sayama, K. Hara, N. Mori, M. Satsuki, S. Suga, S. Tsukagoshi, Y. Abe, H. Sugihara and H. Arakawa, Chem. Commun., 2000, 1173–1174 RSC.
- (a) T. Horiuchi, H. Miura and S. Uchida, Chem. Commun., 2003, 3036–3037 RSC; (b) T. Horiuchi, H. Miura, K. Sumioka and S. Uchida, Chem. Commun., 2004, 126, 12218–12219 Search PubMed; (c) S. Ito, H. Miura, S. Uchida, M. Takata, K. Sumioka, P. Liska, P. Comte, P. Péchy and M. Grätzel, Chem. Commun., 2008, 5194–5196 RSC; (d) B. Liu, W. Zhu, Q. Zhang, W. Wu, M. Xu, Z. Ning, Y. Xie and H. Tian, Chem. Commun., 2009, 1766–1768 RSC.
- K. Hara, T. Sato, R. Katoh, A. Furube, T. Yoshihara, M. Murai, M. Kurashige, S. Ito, A. Shinpo, S. Suga and H. Arakawa, Adv. Funct. Mater., 2005, 15, 246–252 CrossRef CAS.
- (a) Z.-S. Wang, F.-Y. Li and C.-H. Huang, Chem. Commun., 2000, 2063–2064 RSC; (b) Q.-H. Yao, F.-S. Meng, F.-Y. Li, H. Tian and C.-H. Huang, J. Mater. Chem., 2003, 13, 1048–1053 RSC.
- (a) M. Velusamy, K. R. J. Thomas, J. T. Lin, Y. C. Hsu and K. C. Ho, Org. Lett., 2005, 7, 1899–1902 CrossRef CAS; (b) T. Kitamura, M. Ikeda, K. Shigaki, T. Inoue, N. Anderson, X. Ai, T. Lian and S. Yanagida, Chem. Mater., 2004, 16, 1806–1812 CrossRef CAS; (c) D. P. Hagberg, T. Edvinsson, T. Marinado, G. Boschloo, A. Hagfeldt and L. Sun, Chem. Commun., 2006, 2245–2247 RSC; (d) D. P. Hagberg, T. Marinado, M. Karlsson, K. Nonomura, P. Qin, G. Boschloo, T. Brinck, A. Hagfeldt and L. Sun, J. Org. Chem., 2007, 72, 9550–9556 CrossRef CAS; (e) D. P. Hagberg, J. Yum, H. Lee, F. D. Angelis, T. Marinado, K. M. Karlsson, R. Humphry-Bake, L. Sun, A. Hagfeld, M. Grätzel and M. K. Nazeeruddin, J. Am. Chem. Soc., 2008, 130, 6259–6266 CrossRef CAS; (f) H. Tian, X. Yang, R. Chen, R. Zhang, A. Hagfeld and L. Sun, J. Phys. Chem. C, 2008, 112, 11023–11033 CrossRef CAS; (g) Z. Ning, Q. Zhang, W. Wu, H. Pei, B. Liu and H. Tian, J. Org. Chem., 2008, 73, 3791–3797 CrossRef CAS.
- (a) S. Kim, J. K. Lee, S. O. Kang, J. Ko, J.-H. Yum, S. Fantacci, F. D. Angelis, D. D. Censo, M. K. Nazeeruddin and M. Grätzel, J. Am. Chem. Soc., 2006, 128, 16701–16707 CrossRef CAS; (b) D. Kim, J. K. Lee, S. O. Kang and J. Ko, Tetrahedron, 2007, 63, 1913–1922 CrossRef CAS; (c) H. Choi, J. K. Lee, K. Song, S. O. Kang and J. Ko, Tetrahedron, 2007, 63, 3115–3121 CrossRef CAS; (d) H. Choi, C. Baik, S. O. Kang, J. Ko, M.-S. Kang, M. K. Nazeeruddin and M. Grätzel, Angew. Chem., Int. Ed., 2008, 47, 327–320 CrossRef CAS.
- H. Tian, X. Yang, R. Chen, Y. Pan, L. Li, A. Hagfeldt and L. Sun, Chem. Commun., 2007, 3741–3743 RSC.
- (a) R. Chen, X. Yang, H. Tian, X. Wang, A. Hagfeldt and L. Sun, Chem. Mater., 2007, 19, 4007–4015 CrossRef CAS; (b) R. Chen, X. Yang, H. Tian and L. Sun, J. Photochem. Photobiol., A, 2007, 189, 295–300 CrossRef CAS.
- D. Kuang, S. Uchida, R. Humphry-Baker, S. M. Zakeeruddin and M. Grätzel, Angew. Chem., Int. Ed., 2008, 47, 1923–1927 CrossRef CAS.
- K. R. J. Thomas, Y.-C. Hsu, J. T. Lin, K.-M. Lee, K.-C. Ho, C.-H. Lai, Y.-M. Cheng and P.-T. Chou, Chem. Mater., 2008, 20, 1830–1840 CrossRef CAS.
- (a) M. K. Nazeeruddin, P. Péchy and M. Grätzel, Chem. Commun., 1997, 1705–7106 RSC; (b) M. K. Nazeeruddin, P. Péchy, T. Renouard, S. M. Zakeeruddin, R. Humphry-Baker, P. Comte, P. Liska, L. Cevey, E. Costa, V. Shklover, V. Shklover, L. Spiccia, G. B. Deacon, C. A. Bignozzi and M. Grätzel, J. Am. Chem. Soc., 2001, 123, 1613–1624 CrossRef CAS.
- A. Hagfeldt and M. Grätzel, Chem. Rev., 1995, 95, 49–68 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, J. A. Montgomery, Jr., T. Vreven, K. N. Kudin, J. C. Burant, J. M. Millam, S. S. Iyengar, J. Tomasi, V. Barone, B. Mennucci, M. Cossi, G. Scalmani, N. Rega, G. A. Petersson, H. Nakatsuji, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, M. Klene, X. Li, J. E. Knox, H. P. Hratchian, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, P. Y. Ayala, K. Morokuma, G. A. Voth, P. Salvador, J. J. Dannenberg, V. G. Zakrzewski, S. Dapprich, A. D. Daniels, M. C. Strain, O. Farkas, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. V. Ortiz, Q. Cui, A. G. Baboul, S. Clifford, J. Cioslowski, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. L. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A. Nanayakkara, M. Challacombe, P. M. W. Gill, B. Johnson, W. Chen, M. W. Wong, C. Gonzalez and J. A. Pople, Gaussian 03, revision C.02; Gaussian, Inc.: Wallingford, CT, 2004 Search PubMed.
- P. Qin, X. Yang, R. Chen, L. Sun, T. Marinado, T. Edvinsson, G. Boschloo and A. Hagfeldt, J. Phys. Chem. C, 2007, 111, 1853–1860 CrossRef CAS.
- G. Boschloo, L. Häggman and A. Hagfeldt, J. Phys. Chem. B, 2006, 110, 13144–13150 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details. See DOI: 10.1039/b901238a |
| This journal is © The Royal Society of Chemistry 2009 |
