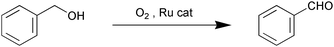Development of concerto metal catalysts using apatite compounds for green organic syntheses
Kiyotomi
Kaneda
*ab and
Tomoo
Mizugaki
b
aResearch Center for Solar Energy Chemistry, Osaka University, 1–3 Machikaneyama, Toyonaka, Osaka 560-8531, Japan. E-mail: kaneda@cheng.es.osaka-u.ac.jp; Fax: +81 6-6850-6260; Tel: +81 6-6850-6260
bDepartment of Materials Engineering Science, Graduate School of Engineering Science, Osaka University, 1–3 Machikaneyama, Toyonaka, Osaka 560-8531, Japan
First published on 21st April 2009
Abstract
Catalytic chemistry and catalyst design play key roles in achieving green sustainable chemistry, which strives for reductions in waste, energy, materials, risks and hazards, and costs. Our approach to green sustainable chemistry described here is the development of highly functionalized heterogeneous metal catalysts based on the unique characteristics of natural inorganic crystallites, such as hydroxyapatite, as macro-ligands for active metal species. These catalysts show the concerto effect between metal active species and surface properties, and so are considered concerto catalysts. This review emphasizes the creation of well-defined active metal sites on apatite compounds that exhibit novel catalytic performance in selective oxidations using molecular oxygen as a clean oxidant, highly efficient carbon–carbon bond formations including asymmetric reactions, and chemical fixation of carbon dioxide.
Broader contextAtom-efficient catalytic reactions are one of the most promising solutions for the environmentally-benign organic synthesis routes, which replace conventional methods employing toxic and/or hazardous stoichiometric reagents. Heterogeneous catalysts have the advantages of being operationally simple and enabling unprecedented reactions by specific ensembles of surface sites based on the crystal structures. Design and synthesis of precise architectures of active metal species on solid surfaces are the most important issues in creating high performance heterogeneous catalysts. We have developed Concerto metal catalysts, novel nanostructured heterogeneous catalysts capable of cooperative activation by several active species, using inorganic crystallite of apatites as unique supports. The concertocatalysts can be easily prepared and exhibit outstanding performances in alcohol oxidations, C–C bond forming reactions, and fixation of carbon dioxide, which open a new avenue for sustainable green organic syntheses. In this review, the prominent catalytic behavior for the above transformations is focused in relation to the concerted functions between the surface properties of the inorganic crystallites and the metal active species. |
1. Introduction
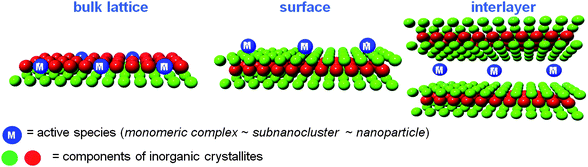
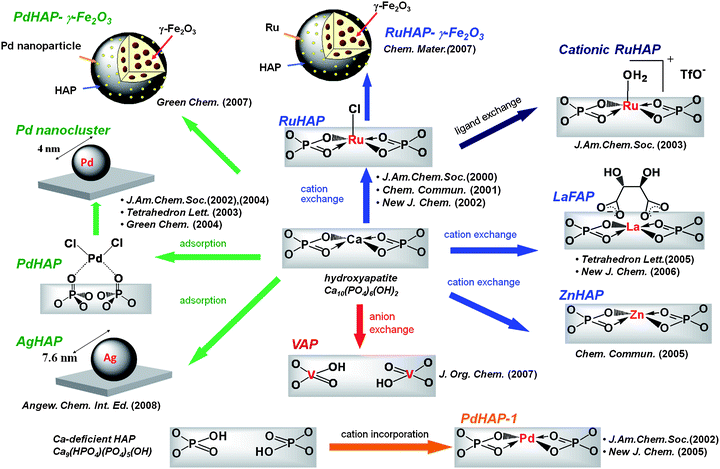
The rapid increase in the Earth's population along with rapid economic growth is causing serious global problems, such as the exhaustion of natural resources, environmental pollution, and anxiety concerning the risks of chemical substances. All of these problems have a strong chemistry component. Therefore, those who work in the fields of chemistry and chemical technology should seek solutions for the above problems to the society. The development of the Green and Sustainable Chemistry (GSC) movement has appeared in response to the challenges. In 2003, the first international symposium on GSC was held in Tokyo, which resulted in the creation of the “Tokyo statement.” A portion of the statement read: “Green chemistry can make major contributions to a sustainable society and a more sustainable future through the innovation of chemistry”.1 The key elements of GSC are reductions in wastes, energy, materials, risks and hazards, and costs. Catalytic chemistry and catalyst design play important roles in achieving this goal. In addition, a sound relationship must be established between the chemical community and society.Our approach to GSC involves the development of highly functionalized heterogeneous metal catalysts based on the unique characteristics of natural inorganic crystallites such as hydroxyapatite, montmorillonite, and hydrotalcite as macro ligands for active metal species.2 These inorganic materials are considered advanced nano-structured catalyst supports that permit control of the location of catalytically active metal species: i.e. in the crystal lattice, on the surface, in the interlayer space (Fig. 1). The creation of well-defined active metal sites on a solid surface not only opens up an avenue to materials that boost catalytic performance, but also aids in understanding of the molecular basis of heterogeneous catalysis. Another advantage of these catalysts is the presence of the concerto effect between active metal species and surface properties, where cooperative catalysis by several immobilized active species on the solid surface can occur. These multifunctional catalysts are considered concertocatalysts.
Hydroxyapatites (HAP), Ca10(PO4)6(OH)2, possess Ca2+ sites surrounded by PO43− tetrahedra parallel to the hexagonal axis, which have potential as biomaterials, adsorbents, and ion exchangers (Fig. 2). However, few applications as catalysts or catalyst supports have been reported.3 A new strategy for the design of high-performance heterogeneous catalysts using apatite compounds has been developed (Fig. 3).4 The choice of hydroxyapatites as a catalyst support is motivated by the following characteristics: (i) well-defined monomeric active species can be immobilized on their surface, based on high ion exchange ability and adsorption capacity; (ii) the nonporous structure can help overcome problems that limit mass transfer; and (iii) weak acid–base properties prevent side reactions induced by the support itself. Our design of the nanostructured concertocatalysts using apatite compounds are based on the properties of (a) cation exchange, (b) anion exchange, and (c) adsorption capacity. Target reactions for the apatite catalysts include selective oxidations using molecular oxygen as a clean oxidant, highly efficient carbon–carbon bond formations including those with asymmetric products, and the chemical fixation of carbon dioxide.
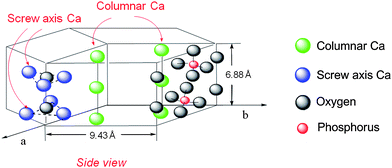 | ||
| Fig. 2 Structure of HAP. | ||
2. Oxidation reactions
2.1. Alcohol oxidations by ruthenium hydroxyapatite (RuHAP) catalyst
The selective oxidation of alcohols to the corresponding carbonyl compounds is one of the most fundamental transformations in laboratory and industrial synthetic chemistry because formyl, keto, and carboxyl groups are versatile functional groups for the synthesis of fine chemicals.5,6 Many oxidizing reagents, such as permanganate and dichromate, have been used to accomplish this transformation.7 These inorganic oxidants, however, have serious disadvantages including high cost and/or toxicity and the production of a large amount of waste. A concern about environmental issues has led to the development of catalytic protocols that are more environmentally friendly in place of classical oxidation methods.8 The use of molecular oxygen as a primary oxidant is advantageous because it is readily available and produces water as the sole byproduct. A significant number of homogeneous and heterogeneous metal-catalyzed aerobic oxidation processes, based on Ru,9Pd,10Pt,11Au,12 and Cu,13 have been developed recently eqn (1). However, there still remain some disadvantages such as the requirement of base additives, high oxygen pressures, and organic solvents.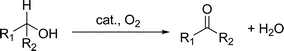 | (1) |
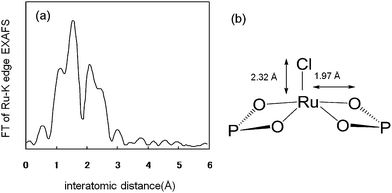
Studies on efficient heterogeneous metal catalysts for aerobic alcohol oxidation have shown that hydroxyapatite acts as a macroligand for the active ruthenium species; monomeric Ru species could be fixed onto the surface of calcium hydroxyapatite by cation exchange.4a The RuHAP was prepared by treatment of HAP with aqueous solution of RuCl3 to yield a dark brown powder. The XRD peak positions of RuHAP were similar to those of the parent HAP. Elemental analysis of RuHAP revealed that the (Ru + Ca) : P ratio of the RuHAP was 1.67 (Ru: 1.69 mmol g−1), which indicates an equimolar substitution of Ru3+ for Ca2+ at the column of Ca and O atoms parallel to the hexagonal axis.4a The XPS and EDX analyses showed that the atomic ratio of Ru to Cl was 1 : 1. The Ru K-edge XANES resembled that of RuCl3 and the Fourier transform (FT) of k3-weighted Ru K-edge EXAFS of the RuHAP indicated no Ru–Ru bond on the RuHAP as shown in Fig. 4a. An inverse Fourier transform of the main peaks was a good fit for three Ru–O and one Ru–Cl shells, as summarized in Table 1. Based on these data, a proposed surface structure of the RuHAP is represented in Fig. 4b; the Ru species exists as monomeric Ru3+ surrounded by oxygen and chlorine. The simple preparation method using the cation-exchange ability of HAP allows creation of a monomeric metal species on the solid surface as macroligands, affording highly efficient heterogeneous catalysts. The RuHAP is an effective heterogeneous catalyst for the oxidation of alcohols. The process is dominated by the formation of monomeric Ru species as a phosphate complex on the RuHAP surface.
| Shell | CNb | Interatomic distance/Å | Δσ/Åc |
|---|---|---|---|
| a The region of 0.8–2.8 Å was inversely Fourier transformed. b Coordination number. c Δσ is the difference between the Debye–Waller factor of RuHAP and that of the reference sample. | |||
| Ru–O (1) | 4.1 | 1.97 | 0.0067 |
| Ru–O (2) | 2.1 | 2.28 | 0.0008 |
| Ru–O (3) | 1.7 | 2.62 | −0.0054 |
| Ru–Cl | 1.2 | 2.32 | 0.0010 |
Table 2 summarizes the oxidation of various alcohols using the RuHAP catalyst at 80 °C under atmospheric O2 pressure. Various alcohols were oxidized efficiently to give the corresponding carbonyl compounds. Benzylic and allylic alcohols showed particularly high reactivity for this oxidation (entries 1–11). Notably, the primary aliphatic alcohol 1-octanol was smoothly converted into 1-octanal without formation of the corresponding carboxylic acid or ester (entry 12). The catalyst also was applicable to the oxidation of heterocyclic alcohols containing nitrogen and sulfur. The corresponding aldehydes of 2-pyridinemethanol and 2-thiophenemethanol were obtained in high yields (entries 14 and 15). In general, previously reported homogeneous transition metal complexes are unable to catalyze the oxidation of alcohols containing heteroatoms because of their strong coordination to the metal center. Even when air was used instead of pure O2, the oxidation of 1-phenylethanol proceeded smoothly to yield a quantitative amount of benzaldehyde (entry 2). The RuHAP catalyst could be facilely reused without any loss of its high catalytic activity and selectivity; the yield of benzaldehyde in the oxidation of benzyl alcohol remained greater than 93% during three recycling experiments. No Ru leaching was observed in the filtrate during the recycling using the ICP method (Ru < 1.6 ppb).
| Entry | Substrate | Product | Time/h | Conv. (%)b | Yield (%)b |
|---|---|---|---|---|---|
| a Reaction conditions: alcohol (2 mmol), RuHAP (Ru: 16.9 mol%), toluene (5 mL), 80 °C, O2 (1 atm). b Determined by GC using an internal standard technique. c Under 1 atm of air instead of pure O2. d N2 atmosphere. e 60 °C. f Alcohol (1 mmol), RuHAP (Ru: 16.9 mol%), toluene (5 mL), 80 °C, O2 (1 atm). | |||||
| 1 |
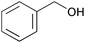
|
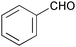
|
3 | 100 | >99 |
| 2c | 3 | 100 | 95 | ||
| 3d | 48 | 20 | 17 | ||
| 4 |
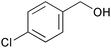
|
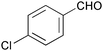
|
3 | 100 | >99 |
| 5 |

|
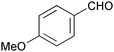
|
3 | 100 | 92 |
| 6 |
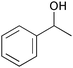
|
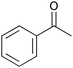
|
2 | 100 | 98 |
| 7 |
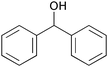
|
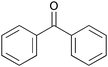
|
2 | 100 | >99 |
| 8 |
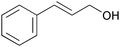
|
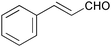
|
1 | 100 | 99 |
| 9 |
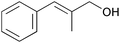
|
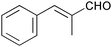
|
2 | 100 | 95 |
| 10 |
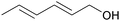
|
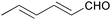
|
4 | 99 | 99 |
| 11 |
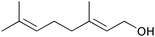
|
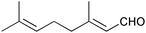
|
3 | 90 | 85 |
| 12 |
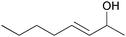
|
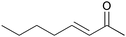
|
5 | 93 | 91 |
| 13 |
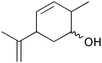
|
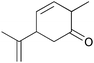
|
6 | 83 | 80 |
| 14e |

|

|
16 | 95 | 94 |
| 15 |
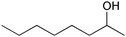
|
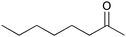
|
6 | 96 | 96 |
| 16f |
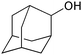
|

|
4 | 100 | 95 |
| 17 |
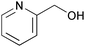
|
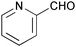
|
10 | 100 | >99 |
| 18 |
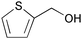
|
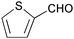
|
2 | 100 | 94 |
Competitive oxidation of an equimolar mixture of p-substituted benzyl alcohols gave a Hammett σ value of 0.429, which is much smaller than the σ = 1.9 for the oxoruthenium compound, e.g., cis-[(N4)RuVIO2]2+,14 but is close to the σ = 0.431 for a stoichiometric reagent of a monomeric [RuCl2(PPh3)3] complex. The present RuHAP catalyst does not contain oxoruthenium species as the active oxidant. In the intramolecular competitive oxidation of 1,7-octanediol, the RuHAP catalyst gave 7-hydroxyoctanal chemoselectively in 81% yield. This high chemoselectivity for a primary hydroxy function is different from that of bulk Ru catalysts. However, it is similar to that of a monomeric [RuCl2(PPh3)3] complex,15 suggesting that the oxidation involves an alcoholate ruthenium intermediate, which is dominated by the formation of a monomeric Ru species on the RuHAP surface.
A possible catalytic cycle for the monomeric Ru complex was proposed as illustrated in Scheme 1. Initially, a Ru-alcoholate intermediate is formed by ligand exchange between an alcohol and a surface Cl moiety of RuHAP. The Ru-alcoholate intermediate then undergoes β-hydride elimination to produce the corresponding carbonyl compounds and a Ru–H species. Finally, an O2 molecule reacts with the hydride species to afford a Ru–OOH species, followed by ligand exchange with the alcohol to regenerate the Ru-alcoholate intermediate along with formation of O2 and H2O.
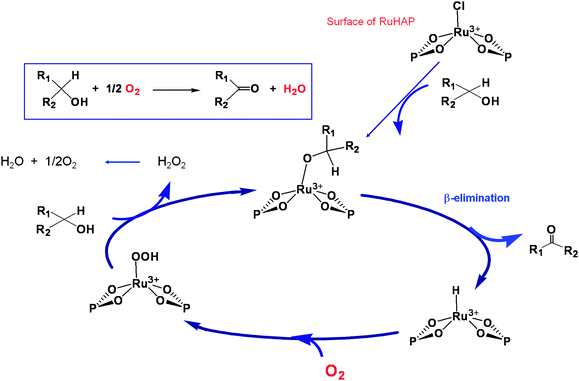 | ||
| Scheme 1 A possible reaction pathway using RuHAP. | ||
It is notable that this RuHAP catalyst does not require any additives for catalytic aerobic oxidation. Furthermore, the mechanism can be shown by the following experiments using benzyl alcohol: (i) addition of a radical trap, 2,6-di-tert-butyl-p-cresol, to the reaction medium hardly influenced the oxidation; (ii) one mole of H2O was produced for every mole of benzaldehyde formed; (iii) the ratio of O2 consumed to benzaldehyde formed was ca. 1 : 2; and (iv) under a N2 atmosphere, benzaldehyde was obtained only in a stoichiometric amount relative to Ru in the RuHAP catalyst. The results of the kinetic isotope effect showed that β-hydride elimination is the rate-determining step of the overall oxidation process.
2.2. Magnetic functionalization of RuHAP catalyst with γ-Fe2O3nanoparticles
Significant improvement in catalytic activity and handling of RuHAP was achieved by functionalizing RuHAP with magnetic γ-Fe2O3nanoparticles.4k Among magnetic nanoparticles, γ-Fe2O3 (maghemite) is technologically important because it plays a key role in catalysis and the formation of magnetic storage media.16The coprecipitation approach to synthesizing γ-Fe2O3 nanocrystallites was employed because basic aqueous conditions allow for subsequent crystallization of the HAP phase and further coating of the γ-Fe2O3 nanocrystallites with HAP. The ruthenium-hydroxyapatite-encapsulated magnetic γ-Fe2O3 (RuHAP-γ-Fe2O3) was prepared as follows: (i) synthesis of hydroxyapatite-encapsulated magnetic γ-Fe2O3 (HAP-γ-Fe2O3) as a reddish-brown powder; (ii) treatment of the HAP-γ-Fe2O3 with an aqueous RuCl3 solution yielded RuHAP-γ-Fe2O3 as a black powder (Ru: 0.1 mmol g−1) (Scheme 2).
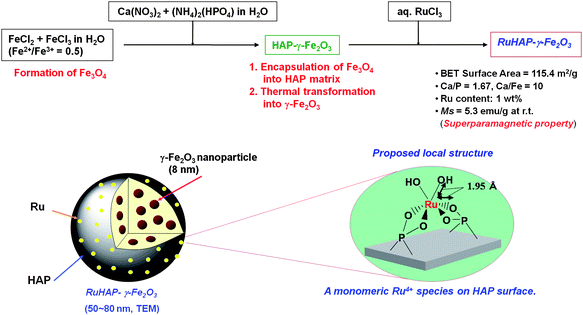 | ||
| Scheme 2 Functionalization of RuHAP catalyst with iron oxide. | ||
The local environment of the Ru species in RuHAP-γ-Fe2O3 was characterized using XPS and Ru K-edge XAFS. The XPS analysis revealed the occurrence of electron transfer from ruthenium to the HAP-γ-Fe2O3 support, and generation of a higher oxidation state of the Ru4+ species in RuHAP-γ-Fe2O3. Electron transfer from metals to γ-Fe2O3 has been reported in Pt/γ-Fe2O317 and Ru/γ-Fe2O318 nanocomposites, as shown by XPS analysis.19 In Fourier transforms (FT) of k3-weighted EXAFS data (Fig. 5), no peaks were produced by RuHAP-γ-Fe2O3, suggestive of contiguous Ru–O–Ru bonds (A), as detected for RuO2 (E) near 3.5 Å. Inverse FT of the main peaks was a good fit using only a Ru–O bond with an interatomic distance (R) of 1.95 Å and coordination number (CN) of 6. These were assigned to Ru–OH and Ru–O–P moieties. A Ru![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O species with a shorter bond distance (ca. 1.85 Å) may not be involved.19 These results suggest that the Ru species on the RuHAP-γ-Fe2O3 surface is monomeric [RuIV(OH)2]2+ surrounded by four phosphate ligands. This Ru species differs from that of previously reported RuHAP.4a Presumably, lowering the Ru content and incorporating γ-Fe2O3 nanocrystallites affect the local environment of the active Ru species. The use of the HAP matrix as a host can provide an effective way for tailoring a uniform particle size and homogeneous dispersion of magnetic γ-Fe2O3 nanocrystallites, while retaining the multiple functionality of HAP.
O species with a shorter bond distance (ca. 1.85 Å) may not be involved.19 These results suggest that the Ru species on the RuHAP-γ-Fe2O3 surface is monomeric [RuIV(OH)2]2+ surrounded by four phosphate ligands. This Ru species differs from that of previously reported RuHAP.4a Presumably, lowering the Ru content and incorporating γ-Fe2O3 nanocrystallites affect the local environment of the active Ru species. The use of the HAP matrix as a host can provide an effective way for tailoring a uniform particle size and homogeneous dispersion of magnetic γ-Fe2O3 nanocrystallites, while retaining the multiple functionality of HAP.
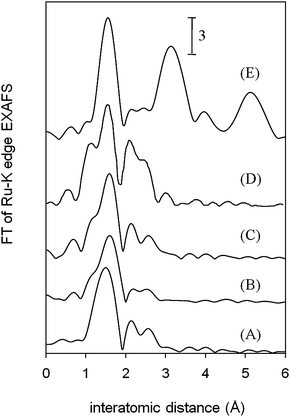 | ||
| Fig. 5 FT of k3-weighted EXAFS for (A) RuHAP-γ-Fe2O3, (B) RuHAP-γ-Fe2O3 reacted under an Ar atmosphere with benzyl alcohol, (C) after the reaction of (B) with O2, (D) RuHAP, and (E) RuO2. | ||
The RuHAP-γ-Fe2O3 exhibited excellent catalytic activity in the oxidation of alcohols using molecular oxygen. As shown in Table 3, various benzylic, allylic, and aliphatic alcohols were selectively converted into the corresponding carbonyl compounds with excellent yields under 1 atm of O2 pressure. A 10 mmol-scale oxidation of benzyl alcohol also was conducted, giving benzaldehyde in 93% yield; the turnover number (TON: mol of product per mol of surface Ru content) based on Ru approached 930 after 24 h. For cyclopropylphenyl carbinol, oxidation of the hydroxyl group occurred without cleavage of the cyclopropyl ring (entry 9). Olefinic function in cinnamyl alcohol remained intact (entry 11). A primary aliphatic alcohol, 1-dodecanol, was successfully oxidized, affording 1-dodecanoic acid without any formation of the corresponding aldehyde or ester (entry 13). The RuHAP-γ-Fe2O3catalyst was effective for the oxidation of heterocyclic alcohols such as 2-pyridinemethanol and 2-thiophenemethanol (entries 19 and 21). The oxidation of sterically bulky alcohols proceeded smoothly with RuHAP-γ-Fe2O3catalyst, which resulted in the successful conversion of 3,5-dibenzyloxybenzyl alcohol and cholestanol into the corresponding carbonyl compounds with excellent yields (eqn 2 and 3, respectively). In particular, the oxidation of 7-hexadecyn-1-ol proceeded quantitatively without any effect on the alkynyl group (eqn 4). These results confirm the absence of micropores, as shown by the N2 adsorption–desorption isotherm and location of the active Ru species mainly on the catalyst surface.
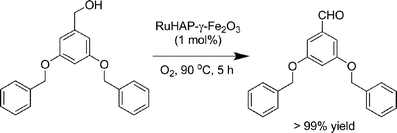 | (2) |
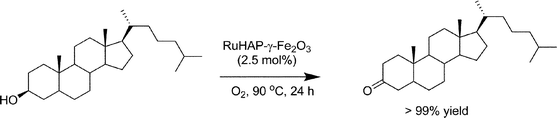 | (3) |
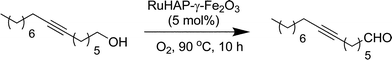 | (4) |
| Entry | Substrate | O2 Pressure/atm | Time/h | Conv. (%)b | Yield (%)b | TOF/h−1 |
|---|---|---|---|---|---|---|
| a Reaction conditions: alcohol (1 mmol), RuHAP-γ-Fe2O3 (Ru: 0.5 mol %), toluene (5 mL), 90 °C, O2 flow. b Determined by GC using an internal standard technique. c RuHAP-γ -FeO3 (Ru: 1 mol %). d RuHAP-γ -Fe2O3 (Ru: 1 mol %), α,α,α,-trifluorotoluene (5 mL). e 1-Octanoic acid was formed. | ||||||
| 1 |
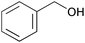
|
1 | 1 | >99 | 98 | 196 |
| 2 | 5 | 0.5 | >99 | 94 | 376 | |
| 3 |
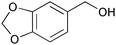
|
1 | 2 | >99 | 99 | 99 |
| 4 | 5 | 1 | >99 | 99 | 198 | |
| 5 |
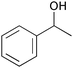
|
1 | 2 | 95 | 91 | 91 |
| 6 | 5 | 1 | 95 | 95 | 160 | |
| 7 |
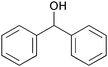
|
1 | 1 | >99 | 98 | 192 |
| 8 | 5 | 0.5 | >99 | 98 | 392 | |
| 9 |
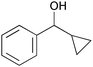
|
1 | 6 | >99 | >99 | 33 |
| 10 | 5 | 3 | >99 | 96 | 64 | |
| 11c |
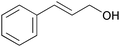
|
1 | 2.5 | 91 | 86 | 34 |
| 12c | 5 | 1.25 | >99 | 96 | 77 | |
| 13d |
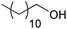
|
1 | 9 | >99 | 98e | 11 |
| 14d | 5 | 9 | >99 | >99e | 11 | |
| 15d |
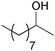
|
1 | 12 | 87 | 83 | 7 |
| 16d | 5 | 10 | 92 | 91 | 9 | |
| 17 |
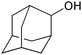
|
1 | 2 | >99 | >99 | 100 |
| 18 | 5 | 1 | 89 | 81 | 162 | |
| 19c |
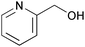
|
1 | 15 | 89 | 88 | 6 |
| 20c | 5 | 15 | 95 | 95 | 6 | |
| 21c |

|
1 | 5 | >99 | 96 | 19 |
| 22c | 5 | 2.5 | >99 | 98 | 39 |
The characteristic feature of the RuHAP-γ-Fe2O3catalyst is a high initial turnover frequency (TOF: mol of product per mol of surface Ru content per hour). For example, the oxidation of benzyl alcohol using 0.5 mol % RuHAP-γ-Fe2O3catalyst afforded benzaldehyde in 98% yield after 1 h; the corresponding TOF based on Ru was 196 h−1 (Table 3). The TOF was significantly greater than those reported for Ru catalysts (Table 4): RuHAP (2 h−1),4aRuCo–HAP (78 h−1),20RuCl2(PPh3)3/TEMPO (80 h−1),22Ru/Al2O3 (40 h−1),23Ru/CeO2/CoO(OH) (10 h−1),24 HB–Ru(10 h−1),25Ru-Co-Al-hydrotalcite (9 h−1),26 RuO2/FAU zeolite (8.7 h−1),27 and (NPr)+(RuO4)−–SiO2 (1.6 h−1).28 The improved catalytic activity of RuHAP-γ-Fe2O3 is attributed to generation of monomeric Ru species with a higher oxidation state. Interestingly, the activity of the RuHAP-γ-Fe2O3catalyst was accelerated significantly at elevated O2 pressures, as shown in Table 3. The reaction rate for oxidation of benzyl alcohol under 5 atm of O2 pressure was nearly two-fold faster than that under atmospheric O2 pressure, in which the TOF value approached 376 h−1. Similarly, other benzylic and allylic alcohols could be converted into the corresponding carbonyl compounds with enhanced oxidation rates at 5 atm of O2 pressure. The acceleration effect of the RuHAP-γ-Fe2O3catalyst is distinct from that of the RuHAP4a and Ru/Al2O323catalysts, where initial oxidation rates were independent of O2 pressure. Another important advantage over other catalyst systems is the smooth oxidation of alcohols at room temperature under atmospheric pressure, as summarized in Table 3. Benzyl alcohol, 1-phenylethanol, benzhydrol, and 2-adamantanol also acted as good substrates for producing the corresponding carbonyl compounds (entries 1–4). Several studies of alcohol oxidation proceeding at room temperature have been reported, but the reactions have disadvantages of long reaction times, low yields, and requirement for additional base and/or co-catalyst. The RuHAP-γ-Fe2O3catalyst described here did not require any additives or co-catalysts to facilitate an efficient catalytic cycle even under mild reaction conditions. We anticipate that this protocol will be of broad interest and applicable to thermally unstable alcohols.
The RuHAP-γ-Fe2O3 can be separated easily from the reaction system by applying an external permanent magnet followed by decantation, as illustrated in Fig. 6. The isolated catalyst could be recycled during the oxidation of benzyl alcohol without significant loss of activity, giving benzaldehyde in 92% yield. Ru K-edge XAFS and TEM analyses support that the local structure of the Ru center and the size distribution of the γ-Fe2O3 nanocrystallites within the HAP matrix remained virtually unchanged even after oxidation. The ICP analysis of the filtrate confirmed that the metal content was negligible (Ru < 1.6 ppb, Fe < 8 ppb). Thus, the present reaction undoubtedly proceeds on a solid surface.
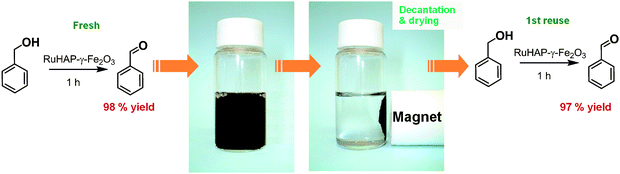 | ||
| Fig. 6 Reuse experiment of RuHAP-γ-Fe2O3. | ||
2.3. Oxidation of silanes to silanols catalyzed by silver nanoparticles supported on HAP (AgHAP)
Silanols are valuable building blocks for silicon-based polymeric materials29 and nucleophilic coupling partners in organic synthesis.30 Toxic reagents, which generate environmentally damaging waste, usually are needed for silanol synthesis.31 To overcome this problem, alternative catalytic transformations of silanes into silanols using H2O as a green oxidant, have been reported in the presence of organic solvents.32–34 However, using the reported synthetic methods without organic solvents is prevented because of the production of disiloxanes.4lAdsorption capacity of HAP can be applied to the preparation of metal nanoparticles on the surface of HAP. For example, HAP-supported Pd nanoparticles showed excellent catalysis for alcohol oxidations,4g hydrodehalogenation,4h hydrogenolysis,4e and dehydrogenation reactions.4f We developed Ag nanoparticles supported on hydroxyapatite (AgHAP), which exhibit high catalytic activity for the selective oxidation of silanes into silanols using water as an oxidant and no need for organic solvents. This is the first catalytic system that uses water as a solvent for the selective oxidation of silanes into the corresponding silanols.4l Of all metal nanoparticles, silver nanoparticle catalysts are generally considered to have low activity for many liquid-phase organic reactions, and their catalytic properties have been not been thoroughly investigated.
The AgHAP was synthesized by treatment of HAP with an aqueous solution of AgNO3, followed by reduction with KBH4 (Fig. 7) (Ag: 0.3 mmol g−1). A plasmon peak at 414 nm indicated that the diameter of the Ag nanoparticles was 6–8 nm.35 The TEM images showed that Ag nanoparticles with a mean diameter of 7.6 nm formed on the surface of the HAP substrate. When a triphasic mixture of dimethylphenylsilane (1), water, and AgHAP was heated at 80 °C under an argon atmosphere for 15 min, oxidation of 1 occurred quantitatively to afford dimethylphenylsilanol (2) with evolution of an equimolar amount of H2. The use of Ag salts and other supported Ag catalysts gave poor to moderate yields of 2 (Table 5). The combination of Ag with HAP was most efficient for achieving high catalytic activity for the selective oxidation of 1 into 2. The AgHAP catalyst exhibited extremely high activity in the oxidation of 1, even at −40 °C (entry 7). Interestingly, AgHAP also catalyzed the quantitative formation of 2 and the concerted formation of an equimolar amount of H2 in an air atmosphere (entry 8). The ICP analysis of the liquid phase confirmed that no Ag leached into solution (detection limit: 7 ppb). These results demonstrate that the present oxidation occurred exclusively on the surface of AgHAP. Solid AgHAP could be recovered easily from the reaction mixture by simple filtration and was reusable without any loss of activity and selectivity during four recycles of the catalyst (entries 1–5).
|
|
|||||
|---|---|---|---|---|---|
| Entry | Catalyst | Time/min | Conv. (%)b | Select. (2:3) (%)b | V m/mL m−2c |
| a Reaction conditions: dimethylphenylsilane (1 mmol), catalyst (Ag: 3 mol%), H2O (2 mL), 80 °C, Ar flow. b Determined by GC using internal standard technique. c H2O monolayer adsorption capacity. d Room Temperature. e Ethyl acetate (5.0 mL) was added into reaction mixture, −40 °C. f Air atmosphere. | |||||
| 1 | AgHAP | 15 | 99 | >99 : 1 | 0.311 |
| 2 | reuse 1 | 15 | 99 | >99 : 1 | — |
| 3 | reuse 2 | 15 | 99 | >99 : 1 | — |
| 4 | reuse 3 | 15 | 99 | >99 : 1 | — |
| 5 | reuse 4 | 15 | 99 | >99 : 1 | — |
| 6d | AgHAP | 90 | 97 | >99 : 1 | — |
| 7e | AgHAP | 600 | 98 | >99 : 1 | — |
| 8f | AgHAP | 15 | 99 | >99 : 1 | — |
| 9 | Ag–Al2O3 | 15 | 79 | >99 : 1 | 0.271 |
| 10 | Ag–TiO2 | 15 | 71 | >99 : 1 | 0.246 |
| 11 | Ag–SiO2 | 15 | 29 | >99 : 1 | 0.157 |
| 12 | Ag–TC | 15 | 25 | >99 : 1 | — |
| 13 | Ag–NO3 | 15 | 24 | >99 : 1 | — |
| 14 | Ag2O | 15 | 27 | >99 : 1 | — |
| 15 | AgPF6 | 15 | 23 | 82 : 18 | — |
| 16 | AgOTf | 15 | 30 | 88 : 12 | — |
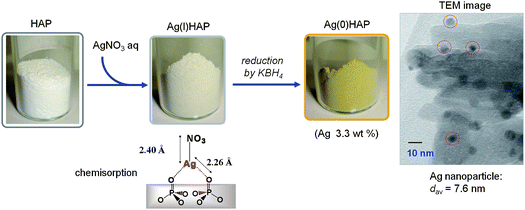 | ||
| Fig. 7 Preparation of AgHAP. | ||
A range of phenylsilanes was oxidized in high yield with greater than 99% selectivity for the silanols (Table 6). An olefinic group was tolerant to oxidation of methylphenylvinylsilane. AgHAP could smoothly catalyze oxidations of less reactive bulky silanes, such as triphenylsilane, in high yields. Aliphatic silanes were barely oxidized under the reaction conditions.
| Entry | Silane | Silanol | Time/min | Conv. (%)b | Select. to silanol (%)b |
|---|---|---|---|---|---|
| a Reaction conditions: silane (1 mmol), AgHAP (Ag: 3 mol%), H2O (2 mL), 80 °C, Ar flow. b Determined by GC using internal standard technique. c EtOAc (5 mL), H2O (5 mmol). | |||||
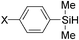
|
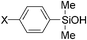
|
||||
| 1 |
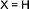
|
15 | 99 | >99 | |
| 2 |
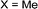
|
180 | 99 | >99 | |
| 3 |

|
30 | 97 | >99 | |
| 4 |
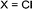
|
30 | 97 | >99 | |
| 5 |
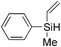
|
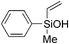
|
60 | 97 | >99 |
| 6 |

|
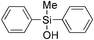
|
60 | 97 | >99 |
| 7c |
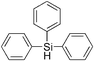
|

|
5 | 99 | >99 |
| 8 |
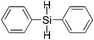
|
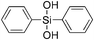
|
15 | 99 | >99 |
| 9 |
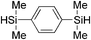
|
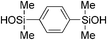
|
240 | 96 | >99 |
| 10 |
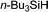
|
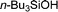
|
24 h | Trace | — |
| 11 |
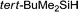
|
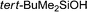
|
24 h | Trace | — |
The highly specific activity of AgHAP for oxidation of aromatic silanes can be explained by the strong adsorption of aromatic silanes via an aromatic group interaction with the surface of AgHAP as revealed by IR studies: the aliphatic silanes did not adsorb on AgHAP. According to the water adsorption capacity, the high hydrophilicity of the HAP surface also affects the high activity of the AgHAP catalyst. In addition, microwave dielectric studies on the dynamics of water demonstrated that the water molecules interact strongly with the surface of Ag nanoparticles to generate a nucleophilic OH− species.36
The cooperative action between Ag nanoparticles and HAP plays an important role in the specific activity for phenylsilanes in this aqueous catalytic system. A possible mechanism is shown in Scheme 3. The first step is absorption of phenylsilane dispersed in water onto the surface of Ag nanoparticles by a strong aryl-ring–Ag interaction. Then, a nucleophilic OH− species from water activated on the Ag surface attacks the adsorbed phenylsilane to form phenylsilanol through a pentacoordinate silicon intermediate.37 This hydrophilic environment of the AgHAP surface increases the concentration of nucleophiles (OH− or H2O) and promotes formation of the silanol by suppressing condensation to the disiloxane.
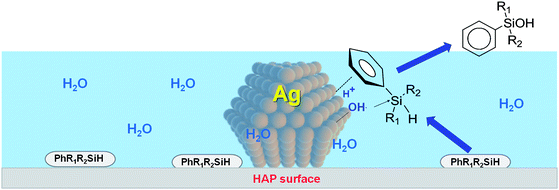 | ||
| Scheme 3 Proposed reaction pathway of the oxidation of silane by AgHAP. | ||
3. C–C bond formation
3.1. Michael additions by vanadate apatite (VAP) catalyst
Conducting reactions under aqueous conditions may provide advantages such as reduced pollution, low cost, and simplicity in process and handling. In addition, unique reactivity and selectivity that cannot be achieved under dry conditions are often observed in aqueous organic reactions due to the hydrophilic and hydrogen-bonding properties of water, even when solid catalysts are employed.38The Michael addition of 1,3-dicarbonyl compounds with activated enones provides access to 1,5-dioxo synthons, which can be easily transformed into cyclohexanone derivatives for use as intermediates in steroid and terpenoid syntheses.39 Traditionally, this reaction is promoted by strong bases such as alkali metal alkoxides or hydroxides; however, it suffers from low selectivity due to side reactions and subsequent reactions, e.g., ester solvolysis, aldol cyclizations, and retro-Claisen-type decompositions.40 Therefore, a number of catalytic systems employing neutral conditions have been developed to avoid these problems.41
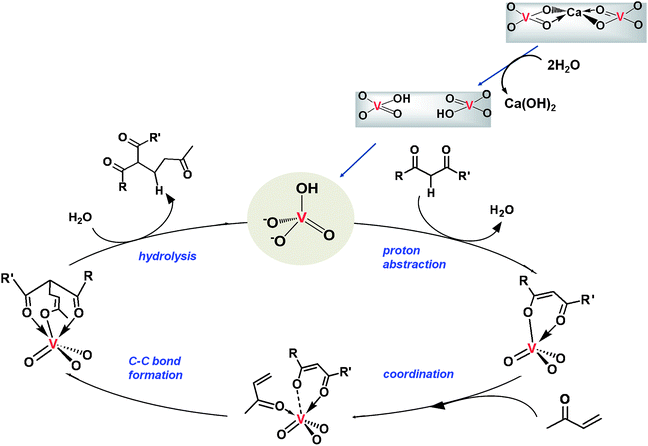
Using the anion exchange ability of apatite compounds (Fig. 1), a calcium vanadate apatite (VAP), Ca10(VO4)6(OH)2, was synthesized by substituting VO43− for PO43− in the apatite matrix. VAP acts as a Brønsted base and exhibits exceptionally high catalytic activity for carbon–carbon bond-forming reactions in water as the reaction medium.
VAP was synthesized from CaSO4·2H2O and Na3VO4 using excess NaOH, followed by calcination in air at 800 °C, yielding the VAP as a white powder.42 The Ca/V ratio of the VAP was 1.67, which agrees with the stoichiometric value of the apatite components. XRD patterns of the VAP calcined at various temperatures revealed that the CaO phase was generated by calcination at temperatures greater than 600 °C, concomitant with the VAP phase. TEM images of the VAP calcined at 800 °C show the VAP covered with amorphous CaO. Thus, the VAP consists of three phases: CaO, a nonstoichiometric VAP shell [e.g., Ca9(HVO4)(VO4)5(OH)] on the surface, and a stoichiometric VAP core. The present methodology of catalyst preparation is a promising protocol for creation of a stable nonstoichiometric apatite phase containing isolated tetrahedral V5+ species on an apatite surface.
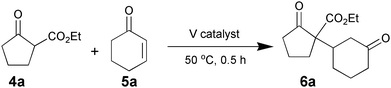
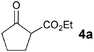
To investigate the catalytic activity of VAP, the Michael reaction of 2-oxo-cyclopentane carboxylic acid ethyl ester (4a) with 2-cyclohexen-1-one (5a) was conducted at 50 °C in various solvents (Table 7). VAP showed extremely high catalytic activity for the Michael reaction under aqueous conditions, giving only 2-oxo-1-(3-oxocyclohexyl)-cyclopentane carboxylic acid ethyl ester (6a) in a quantitative yield within 30 min (entry 1). The reaction did not proceed without VAP (entry 26), and no product was formed when a polar or nonpolar organic solvent was used instead of water (entries 6–15). The calcination process of VAP is indispensable for the high activity; as the temperature of calcination increased to 800 °C, the catalytic activity of the resulting VAPs increased (entries 1–4). The findings of the XRD analysis, in which the peak intensities of the resulting VAP lattice increased with calcination temperature, supports these results. Orthovanadate and pyrovanadate derivatives containing isolated VO4 tetrahedra, such as Na3VO4, K3VO4, and Mg2V2O7, also gave 6a in moderate yields of 39%, 32%, and 17%, respectively (entries 16–18), followed by deactivation of these catalysts caused by a structural change from monomeric to polymerized species during the reaction. The use of other vanadium reagents did not afford any products (entries 19–24), and the reaction did not proceed in the presence of the HAP by itself (entry 25). These results support the contention that the presence of isolated VO4 tetrahedral species on a solid surface plays a key role in the success of aqueous Michael reactions.
|
|
||||
|---|---|---|---|---|
| Entry | Catalyst b | Solvent | Conv. (%)c | Yield (%)c |
| a Reaction conditions: 4a (1 mmol), 5a (1.5 mmol), catalyst (V: 0.012 mmol), solvent (3 mL). b Values in parentheses are calcination temperature. c Determined by GC using an internal standard technique. d Vanadium leaching was observed. | ||||
| 1 | VAP (800 °C) | Water | >99 | >99 |
| 2 | VAP (600 °C) | Water | 20 | 28 |
| 3 | VAP (400 °C) | Water | 11 | 10 |
| 4 | VAP (200 °C) | Water | 2 | 2 |
| 5d | VAP (uncalcined) | Water | 38 | 36 |
| 6 | VAP (800 °C) | — | 0 | 0 |
| 7 | VAP (800 °C) | Acetone | 0 | 0 |
| 8 | VAP (800 °C) | CH3CN | 0 | 0 |
| 9 | VAP (800 °C) | EtOAc | 0 | 0 |
| 10 | VAP (800 °C) | DMF | 0 | 0 |
| 11 | VAP (800 °C) | DMSO | 0 | 0 |
| 12 | VAP (800 °C) | 1,4-dioxane | 0 | 0 |
| 13 | VAP (800 °C) | CH3NO2 | 0 | 0 |
| 14 | VAP (800 °C) | THF | 0 | 0 |
| 15 | VAP (800 °C) | Toluene | 0 | 0 |
| 16 | Na3VO4 | Water | 41 | 39 |
| 17 | K3VO4 | Water | 33 | 32 |
| 18 | Mg2V2O7 | Water | 19 | 17 |
| 19 | NaVO3 | Water | 0 | 0 |
| 20 | VO(acac)2 | Water | 0 | 0 |
| 21 | V(acac)3 | Water | 0 | 0 |
| 22 | V2O5 | Water | 0 | 0 |
| 23 | VOSO4·nH2O | Water | 0 | 0 |
| 24 | VCl3 | Water | 0 | 0 |
| 25 | HAP | Water | 0 | 0 |
| 26 | — | Water | 0 | 0 |
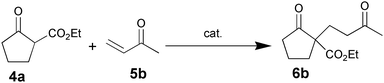
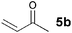
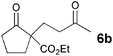
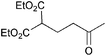
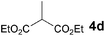
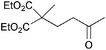
The VAP-catalyzed Michael reaction in water was applicable to various sets of donors and acceptors as summarized in Table 8. The aqueous Michael reaction of 4a with 3-buten-2-one (5b) proceeded efficiently in the presence of the VAP catalyst to give 2-oxo-1-(3-oxobutyl)-cyclopentanecarboxylic acid ethyl ester (6b) quantitatively in 10 min (entry 1). The Michael reactions of diethylmalonate derivatives (4c and 4d) with 5b, which did not easily proceed when using traditional Lewis acid catalysts, gave the corresponding products in excellent yields (entries 13 and 14). In all cases, the 1,4-addition products were obtained exclusively without formation of 1,2-adducts. Unfortunately, the use of 2-acetylpentanone, acetylacetone, and ethyl benzoyl acetate as donors, which have low pKa values of 7.8, 9.0, and 9.4, respectively,43 were not effective in the present Michael reaction, and resulted in the dissolution of the vanadium species into the reaction mixture. This leaching of vanadium may be the result of strong coordination by the donors to the surface vanadium species, thus preventing smooth reaction.44 In the reaction of 4a with acrolein (5e), sequential intramolecular 1,2-addition reaction and acetylation proceeded to give 4-acetoxy-8-oxobicyclo-[3.2.1]-octan-1-carboxylic acid ethyl ester (6f) (Scheme 4).45
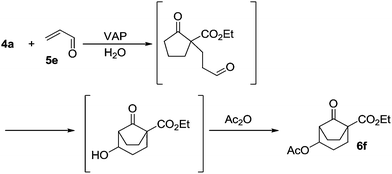 | ||
| Scheme 4 Sequencial Michael and 1,2-addition reaction. | ||
| Entry | Donorb | Acceptor | Time/h | Product | Yield (%)c |
|---|---|---|---|---|---|
| a Reaction conditions: donor (5 mmol), acceptor (5.5 mmol), VAP (50 mg), H2O (5 mL), 30 °C. b Determined by GC using internal standard technique. Values in parentheses are isolated yield. c VAP (25 mg). d 50 °C. e 5b (20 mmol), without solvent, 80 °C. f 5b (10 mmol), without solvent, 80 °C. | |||||
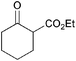
| 1 |
|
10 min |
|
>99(96) | |
| 2 |
|
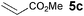
|
3 |
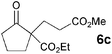
|
92 |
| 3 |
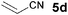
|
3 |
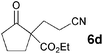
|
93 | |
| 4 |
|
5b | 1 |
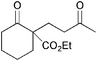
|
98 |
| 5 |
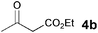
|
5b | 1.5 |
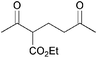
|
93 |
| 6d |
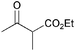
|
5b | 2 |
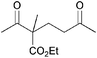
|
>99 |
| 7e |
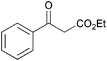
|
5b | 2 |
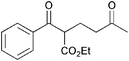
|
98(90) |
| 8f |
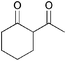
|
5b | 1 |
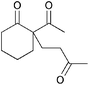
|
>99 |
| 9 |
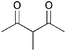
|
5b | 1 |
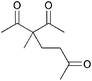
|
>99 |
| 10 | 5b | 2 |
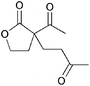
|
94 | |
| 11 |
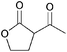
|
5c | 2 |
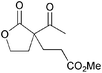
|
98 |
| 12 | 5d | 2 |
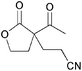
|
96 | |
| 13 |
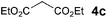
|
5b | 1 |
|
94(92) |
| 14 |
|
5b | 3 |
|
98 |
The workup procedure is straightforward due to the heterogeneous nature of the catalyst and the three-phase reaction mixture, as shown in Fig. 8. The product could be separated easily by extraction after filtering the solid catalyst. No spectral change was observed by UV-Vis or XAFS spectra of the recovered VAP, which indicates that the vanadium species remained in a monomeric state throughout the reaction. The catalyst was capable of being reused at least four times without any reduction in activity during the reaction of 4a and 5b. To demonstrate the effectiveness of the VAP, the catalyst was removed by filtration from the reaction of 4b with 5b after 55% conversion. Continuation of the reaction for 1 h under identical reaction conditions afforded no additional product. In the aqueous phase, no vanadium was detected by ICP analysis, indicating that this reaction proceeds on the VAP surface and does not involve dissolved vanadium. More significantly, the use of 8 mg of the VAP catalyst under aqueous conditions allowed 32.2 g (200 mmol) of 4a to react with 15.4 g (210 mmol) of 5b in a highly effective manner, giving a 92% isolated yield (41.6 g) of 6b at 40 °C in 1.5 h (Scheme 5). Based on the surface vanadium content,46 the VAP catalyst showed a high TON of 260![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 and TOF of 48 s−1. The TON and TOF values of the VAP catalyst are the highest ever reported for transition-metal-catalyzed Michael reactions (Table 9).47–56
400 and TOF of 48 s−1. The TON and TOF values of the VAP catalyst are the highest ever reported for transition-metal-catalyzed Michael reactions (Table 9).47–56
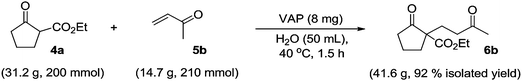 | ||
| Scheme 5 200 mmol scale reaction using VAP catalyst. | ||
|
|
|||
|---|---|---|---|
| Catalyst | TONa | TOF/h−1a | Ref. |
| a TONs and TOFs were calculated according to the results in the references. | |||
| VAP | 236![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
172![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
4j |
| LaHAP | 4500 | 375 | 4n |
| Sc3+-mont | 1000 | 29 | 46 |
| Cu2+-mont | 400 | 29 | 47 |
| Ni(acac)2 | 100 | 100 | 48 |
| FeCl3·6H2O | 100 | 100 | 49 |
| Fe-mica | 100 | 5 | 50 |
| Yb(OTf)3 | 100 | 1.4 | 51 |
| RuH2(PPh3)4 | 33.3 | 67 | 52 |
| Yb(OTf)3/SiO2 | 17 | 0.7 | 53 |
| InCl3 | 10 | 1.7 | 54 |
| CeCl3·7H2O/NaI | 5 | 0.8 | 55 |
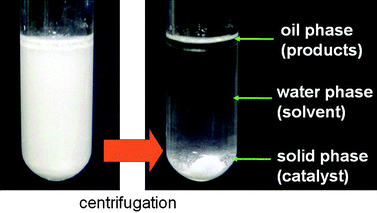 | ||
| Fig. 8 Triphase separation of VAP catalyst. | ||
After aqueous Michael reaction of 4a with 5b, 7% of Ca leached from the VAP calcined at 800 °C and the XRD spectrum of the catalyst showed the disappearance of the CaO phase. The Ca/V ratio of the recovered VAP catalyst was 1.50.
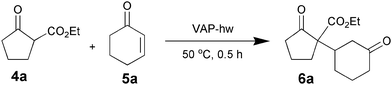
Hot water pretreatment of the VAP calcined at 800 °C (VAP-hw), also reduced the Ca/V ratio to 1.50 together with disappearance of the CaO peaks. The peak positions of the CO2-TPD profile indicated that the surface basicity of VAP-hw was stronger than that of the VAP calcined at 800 °C. Furthermore, the amount of base in the VAP-hw was approximately 0.32 mmol g−1, two orders of magnitude greater than that of the VAP calcined at 800 °C (6.6 × 10−3 mmol g−1). Furthermore, in the reaction of 4a with 5b, an addition of 1.5 equiv of benzoic acid based on the amount of surface vanadium species resulted in a significant decrease in product yield from 99% to 37%. These results show that water triggers a nonstoichiometric apatite phase by removal of the CaO phase from the surface, and the distorted vanadate species (such as V–OH) acts as a Brønsted base site (Scheme 6). The VAP-hw catalyst promoted Michael reaction of 4a with 5a even in organic solvents (Table 10) such as 1,2-dichloroethane, toluene, and ethanol (entries 2–4). In recycling experiments under aqueous conditions, no dissolution of Ca species was detected in the filtrate.
3.2. Asymmetric Michael addition: Tartaric acid modified fluoroapatite (TA-LaFAP) catalyst
Developing an asymmetric version of the Michael reaction is challenging for synthetic organic chemists.57 Various chiral catalysts have been examined including cinchona alkaloids,58alkoxide complexes,59crown ether-metal alkoxide complexes,60 transition metal complexes,61 and bimetallic lanthanide complexes.62 Although remarkable progress has been achieved using these homogeneous catalysts, only a few heterogeneous catalysts are available, such as polymer-supported metal complexes,63polymer-supported cinchona alkaloids,64 layered double hydroxides containing chiral organic guests,65 and MCM-41-grafted chiral amines.66 We developed a lanthanum-exchanged apatite, LaHAP, as a heterogeneous Lewis acid catalyst for Michael reactions by applying the cation exchange property of HAP.4mPreparation of the heterogeneous asymmetric catalysts is straightforward. A fluoroapatite (FAP), Ca10(PO4)6F2, was chosen as a macroligand and cation exchange of the surface calcium sites with lanthanum, followed by treatment with (R,R)-tartaric acid (TA), afforded TA-LaFAP (La content: 0.36 mmol g−1, La : TA = 1 : 1) as a white powder.4m,n Since the immobilized La species still have vacant coordination sites owing to their large ionic radii, further binding of the chiral ligands allows formation of an enantioselective sphere on the solid surface. The La K-edge XAFS measurements showed that the La species exist in a highly dispersed form with a +3 oxidation state (Fig. 9).
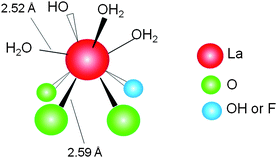 | ||
| Fig. 9 A proposed surface structure of apatite-bound La complexes. | ||
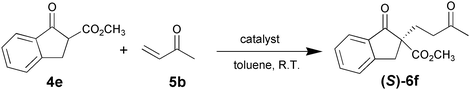
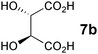
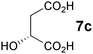
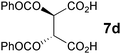
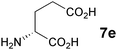
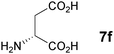
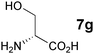
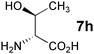
In the reaction of methyl 1-oxoindan-2-carboxylate (4e) with 5a in toluene, the TA-LaFAP (La: 1.2 mol%) effectively served as a chiral heterogeneous catalyst to afford (S)-methyl 1-oxo-2-(3′-oxobutyl)-2-indanecarboxylate ((S)-6f) in 97% yield with enantioselectivity of 60% ee (Table 11, entry 1). Note that no enantioselectivity occurred when using the combination of either parent FAP or La(OTf)3 with 7a (entries 2 and 3), showing that immobilization of La species on the FAP surface is essential for attaining asymmetric catalysis. This is apparently the first demonstration of an asymmetric reaction utilizing an apatite catalyst.67 The enantioselectivity achieved by TA-LaFAP for the asymmetric Michael reaction of 4e with 5a was greater than those reported for other catalyst systems, such as the N,N′-dioxide-Sc(OTf)3 complex (39% ee),61fdiaminodiol-CoII complex (38% ee),61c chiral amidine (8% ee),68 and poly(cinchona alkaloid-co-acrylonitrile) (42% ee).64a This value also is comparable with those using polymer-supported quinine (65% ee),64b1,10-bi-2-naphthol-Na complex (57% ee),59 and quinine (56% ee).58a Interestingly, the use of the HAP instead of FAP as a support gave a 30% ee with the opposite configuration (entry 6). For (S,S)-tartaric acid (7b), (R)-6f was obtained with the same chemical yield and enantioselectivity as those of 7a, revealing that the absolute configuration of the Michael adducts is determined by the ligand used. Other dicarboxylic acid ligands, such as (S)-malic acid (7c) and (R,R)-O,O′-dibenzoyltartaric acid (7d), favored excess formation of the (R)-isomer with moderate enantioselectivity (entries 8 and 9). The reaction involving (S)-serine (7g) and (S)-threonine (7h) containing amino and monocarboxyl groups was less efficient (entries 12 and 13). As a consequence, the presence of a dicarboxylic acid aided enantioselectivity. Furthermore, solvent polarity strongly influenced catalytic performance. Non-polar solvents with low dielectric constants, such as toluene, n-hexane, and diethyl ether, were good solvents, whereas polar solvents with higher dielectric constant values, such as methanol and DMF, gave poor results with respect to single enantiomer products. The use of water afforded a racemic product despite providing a good chemical yield, which suggests that specific interactions (such as hydrogen bonding) between the solvent and catalyst surface significantly influence the conformation of the transition state in the enantioselectivity determining step. Another important parameter that affects the activity and enantioselectivity of the reaction is the choice of metal precursors. Fig. 10 shows the effect of rare earth metal triflates (RE(OTf)3) having various ionic radii.43 Note that catalytic performance varied significantly with ionic radius.44 The largest La3+ cation afforded the Michael adduct with the highest yield and enantioselectivity, while the use of smaller cations, such as Y, Yb, and Sc, gave unsatisfactory results in terms of both chemical and optical yields. The higher activity and enantioselectivity obtained with larger cations might be attributed to increased coordination numbers, which would allow the further binding of chiral organic modifiers and substrates to metals to promote a reasonable transition state even on the solid surface.
|
|
|||||
|---|---|---|---|---|---|
| Entry | Catalyst | Ligand | Yield (%)b | Ee (%)c | Configurationd |
| a Reaction conditions: 4a (0.5 mmol), 5b (0.75 mmol), catalyst (La: 1.2 mol%), toluene (2 mL), 6 h, Ar atmosphere. b Determined by GC using internal standard technique. c Determined by HPLC (Daisel Chiralpak ADH). d Assigned by optical rotation. e 7a (1.2 mol%). f 10 °C. g 40 °C. | |||||
| 1 | LaFAP |
|
97 | 60 | S |
| 2 | FAP | 7a | 18 | — | — |
| 3e | La(OTf)3 | 7a | 29 | <1 | S |
| 4f | LaFAP | 7a | 5 | 44 | S |
| 5g | LaFAP | 7a | >99 | 50 | S |
| 6 | LaFAP | 7a | >99 | 30 | R |
| 7 | LaFAP |
|
98 | 59 | R |
| 8 | LaFAP |
|
>99 | 50 | R |
| 9 | LaFAP |
|
>99 | 46 | R |
| 10 | LaFAP |
|
>99 | 32 | R |
| 11 | LaFAP |
|
55 | 29 | S |
| 12 | LaFAP |
|
>99 | 19 | R |
| 13 | LaFAP |
|
>99 | 7 | S |
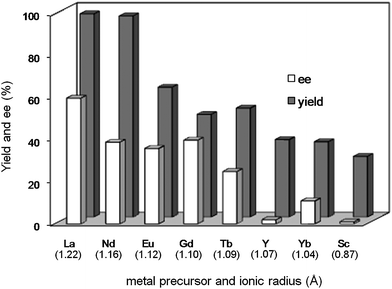 | ||
| Fig. 10 Effect of rare Earth metal precursors (RE(OTf)3) for asymmetric Michael reaction of 4e with 5b. Reaction was conducted with catalyst (1.2 mol % based on 4e), 4e (0.5 mmol), 5b (0.75 mmol), and toluene (2 mL) at room temperature for 6 h under Ar atmosphere. Values in parentheses are ionic radii of 8-coordination for Sc3+ and 9-coordination for other rare Earth metal cations (RE3+). | ||
The enantioselectivity appeared to be independent of conversion level and remained unchanged at approximately 60% ee during the reaction, suggesting that racemization of the Michael adduct did not occur (Fig. 11). The TA-LaFAP catalyst was reused with retention of activity and enantioselectivity in the absence of an externally added chiral organic ligand; yields greater than 97% and 59% ee were maintained for at least three recycling experiments in reaction of 4e and 5a. Thus, the present asymmetric Michael reaction proceeds on the chirally modified heterogeneous apatite surface.
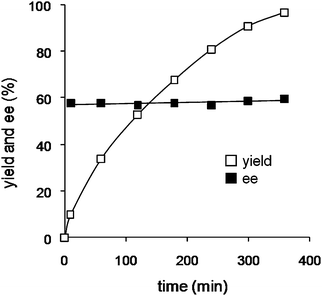 | ||
| Fig. 11 Time profile for the asymmetric Michael reaction of 4c with 5b catalyzed by TA-LaFAP. Reaction conditions: TA-LaFAP (0.016 g, 1.2 mol % based on 4c), 4c (0.5 mmol), 5b (0.75 mmol), toluene (2 mL), R.T., 6 h, Ar atmosphere. | ||
The preliminary screening of the chiral organic modifiers revealed that (R,R)-tartaric acid (7a) is the most suitable ligand to achieve high enantioselectivity, suggesting that dicarboxylic acids play an important role in the conformation of the enantio-differentiating complex on the solid surface. Organic modifiers might bind with La species via coordination of two carboxylate groups in a bidentate manner. Although the precise transition state is not known, obtaining the desirable geometry may be directed by hydrogen-bonding between OH groups of 7a and the substrate, which is inhibited by the use of polar solvents with high dielectric constants, resulting in poor enantiomeric results.
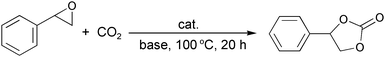
3.3. Cycloaddition of CO2 to epoxides catalyzed by zinc hydroxyapatite (ZnHAP)
Chemical fixation of CO2 into synthetically beneficial compounds is of great interest for GSC because CO2 is an inexpensive, nontoxic, and abundant C1 feed stock.69 One of the most powerful approaches is the coupling of CO2 and epoxides to provide five-membered cyclic carbonates with 100% atom efficiency, which are prominently utilized for the production of engineering plastics as well as for the synthesis of pharmaceuticals and fine chemicals.70By applying the cation exchange ability of HAP, a hydroxyapatite-bound zinc complex (ZnHAP) possessing robust stability was developed for chemical fixation of CO2 onto epoxides in the presence of a Lewis base as a co-catalyst. The ZnHAP was obtained as a white powder (Zn: 0.3 mmol g−1) by treatment of aqueous Zn(NO3)2 with HAP.40 The monomeric Zn species of ZnHAP exist in tetrahedral geometry with a +2 oxidation state.71 The proposed local structure of Zn on HAP is shown in Fig. 12 as a monomeric Zn2+ phosphate complex surrounded by four oxygen atoms in tetrahedral coordination.
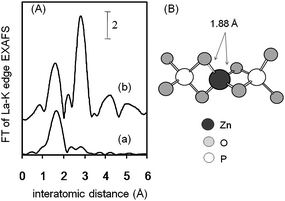 | ||
| Fig. 12 (A) FT of k3-weighted Zn K-edge EXAFS for (a) ZnHAP and (b) ZnO. (B) Proposed surface structure around Zn2+ in the ZnHAP. The nearest oxygen and phosphorus atoms are shown. | ||
The addition of CO2 to styrene oxide using several cation-exchanged HAPs was conducted in the presence of Lewis base co-catalyst of triethylamine (TEA) under 5 atm of CO2 pressure at 100 °C, as summarized in Table 12. ZnHAP was the best catalyst among all of those examined, affording the corresponding carbonate in 98% yield with >99% selectivity (entry 1). Note that both ZnHAP and the Lewis base were indispensable for attaining high carbonate yields (entry 1 vs. entries 12 and 13). The choice of Lewis base has a crucial influence on efficiency of the coupling reaction; 4-(dimethylamino)pyridine (DMAP) gave the best result (entry 6). Other bases, such as TEA, 1,8-diazabicyclo[5.4.0]-7-undecene (DBU), and pyridine, were slightly less effective (entries 7–9). Interestingly, ZnHAP exhibited superior activity under solvent-free conditions, whereas the use of organic solvents led to a significant decrease in catalytic activity (entries 10 and 11). The spent catalyst could be reused without any loss in catalytic performance; yields of greater than 95% and selectivity of nearly 100% were obtained for three recycling experiments of styrene oxide with CO2. ICP analysis of the filtrate confirmed that the Zn content was below the detection limit. More significantly, the recovered ZnHAP catalyst maintained its original monomeric Zn2+ structure as confirmed by XAFS analysis, suggesting that the Zn center is not only catalytically active but also extremely durable under the present reaction conditions.
|
|
||||
|---|---|---|---|---|
| Entry | Catalyst (metal mol %) | Solvent | Base | Yield (%)b |
| a Reaction conditions: styrene oxide (10 mmol), base (4 equiv. of catalyst metal), CO2 (5 atm). b Determined by GC using an internal standard technique. | ||||
| 1 | ZnHAP (0.3) | — | TEA | 98 |
| 2 | CuHAP (0.3) | — | TEA | 60 |
| 3 | AlHAP (0.3) | — | TEA | 50 |
| 4 | CoHAP (0.3) | — | TEA | 21 |
| 5 | CrHAP (0.3) | — | TEA | 19 |
| 6 | ZnHAP (0.15) | — | 4-DMAP | 79 |
| 7 | ZnHAP (0.15) | — | TEA | 62 |
| 8 | ZnHAP (0.15) | — | DBU | 60 |
| 9 | ZnHAP (0.15) | — | Pyridine | 57 |
| 10 | ZnHAP (0.15) | DMF (5 mL) | TEA | 29 |
| 11 | ZnHAP (0.15) | CH2Cl2 (5 mL) | TEA | 5 |
| 12 | — | — | 4-DMAP | 12 |
| 13 | ZnHAP (0.15) | — | — | Trace |
As shown in Table 13, terminal epoxides possessing aromatic, aliphatic, and both electron-donating and -withdrawing substituents were successfully converted into the corresponding cyclic carbonates in excellent yield. In all cases, the selectivity was almost 100% without the formation of the corresponding diols and polycarbonates. The present catalytic system also is suitable for large-scale operations. For example, a 100 mmol scale reaction of epichlorohydrin provided 4-(chloromethyl)-1,3-dioxolan-2-one in 90% yield within 24 h (entry 1). Note that the coupling reaction proceeded even under atmospheric CO2 pressure, giving a remarkably high TON of up to 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 with a TOF of approximately 1100 h−1 (entry 2). These values for epichlorohydrin are considerably higher than those of analogous binary catalyst systems comprising a metal complex and Lewis base under high CO2 pressure, such as salen-CrIII (50 psig, TON and TOF, 98 and 65 h−1),72binaphthyldiamino salen-ZnII (500 psi, 100, 6 h−1),73 and CoIII-porphyrin (300 psig, 250, 250 h−1).74 Furthermore, the ZnHAP-catalyzed coupling reactions proceeded exclusively with retention of the epoxide configuration; the reactions of enantiomerically pure (R)- and (S)-benzyl glycidyl ethers with CO2 yielded (R)- and (S)-4-(benzyloxymethyl)-1,3-dioxolan-2-one in 82% and 84% yields, respectively, with over 99% ee.
600 with a TOF of approximately 1100 h−1 (entry 2). These values for epichlorohydrin are considerably higher than those of analogous binary catalyst systems comprising a metal complex and Lewis base under high CO2 pressure, such as salen-CrIII (50 psig, TON and TOF, 98 and 65 h−1),72binaphthyldiamino salen-ZnII (500 psi, 100, 6 h−1),73 and CoIII-porphyrin (300 psig, 250, 250 h−1).74 Furthermore, the ZnHAP-catalyzed coupling reactions proceeded exclusively with retention of the epoxide configuration; the reactions of enantiomerically pure (R)- and (S)-benzyl glycidyl ethers with CO2 yielded (R)- and (S)-4-(benzyloxymethyl)-1,3-dioxolan-2-one in 82% and 84% yields, respectively, with over 99% ee.
| Entry | Epoxide/mmol | Zn (mol%) | Yield (%)b | TON |
|---|---|---|---|---|
| a Reaction conditions: 4-DMAP (0.02 mmol), CO2 (10 atm), 100 °C, 24 h. b Determined by GC using an internal standard technique. c CO2 (1 atm). d DMF (5 mL) was used as solvent. e 130 °C. | ||||
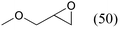
| 1 |
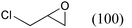
|
0.003 | 90 | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| 2c | 86 | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
||
| 3d |

|
0.003 | 95 | 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
| 4c,d | 90 | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
||
| 5 |
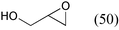
|
0.006 | 96 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| 6 | 84 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
||
| 7 |
|
0.006 | 95 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
| 8e |
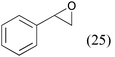
|
0.012 | 89 | 7400 |
| 9 |
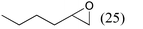
|
0.012 | 86 | 7100 |
| 10 |

|
0.012 | 81 | 6700 |
A possible catalytic cycle of the ZnHAP is proposed in Scheme 7.62 The coupling reaction proceeds through effective cooperation between the ZnHAP and the Lewis base; the former activates the epoxide as a Lewis acid, while the latter attacks the less sterically hindered carbon atom to open the epoxide ring. The generated oxy anion species then reacts with CO2 to give the corresponding cyclic carbonate (Path A in Scheme 7). This mechanism is exemplified by the reaction of trans-1-deuterio-1,2-hexene oxide with CO2, which showed 82% selectivity for the corresponding trans-1-deuterio-1,2-hexene carbonate. Minor formation of the cis-isomer means that the participation of activated CO2 by Lewis base, i.e. zwitterion [R3N+C(O)O−], in the ring opening step of the epoxide cannot be ruled out (Path B in Scheme 7).75
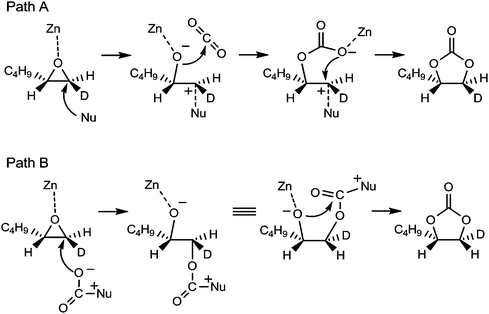 | ||
| Scheme 7 Possible reaction pathways for cycloaddition of CO2 to epoxide by ZnHAP catalyst. | ||
Conclusions
An effective approach to green and sustainable chemistry was developed by designing nanostructured concertocatalysts using apatite compounds as macroligands, and their catalytic performance for a variety of organic syntheses was demonstrated. Catalytic systems incorporating heterogeneous metal catalysts offers significant benefits for achieving simple and clean organic syntheses because they allow: (i) use of nonpolluting oxidants; (ii) high catalytic activity and selectivity; (iii) high substrate tolerance; and (iv) a simple work-up procedure and easy recovery of the catalyst with recyclability. In catalytic chemistry, recyclability is an important feature for helping to solve environmental and energy issues. The design of such high-performance catalysts requires not only modification of conventional catalysts, but also breakthroughs in science and technology that result from a knowledge of biological and materials sciences.References
- The 1st International Symposium on Green Sustainable Chemistry (GSC TOKYO 2003), Green Chem., 2003, 5, G74 RSC.
- K. Kaneda, K. Ebitani, T. Mizugaki and K. Mori, Bull. Chem. Soc. Jpn., 2006, 79, 981 CrossRef CAS.
- Book and review (a) J. C. Elliott, Structure and Chemistry of the Apatites and Other Calcium Orthophosphates, Elsevier, Amsterdam, 1994 Search PubMed; (b) S. Sebti, M. Zahouily, H. B. Lazrek, J. A. Mayoral and D. J. Macquarrie, Curr. Org. Chem., 2008, 12, 203 CrossRef CAS.
- (a) K. Yamaguchi, K. Mori, T. Mizugaki, K. Ebitani and K. Kaneda, J. Am. Chem. Soc., 2000, 122, 7144 CrossRef CAS; (b) K. Mori, K. Yamaguchi, T. Mizugaki, K. Ebitani and K. Kaneda, Chem. Commun., 2001, 461 RSC; (c) K. Mori, M. Tano, T. Mizugaki, K. Ebitani and K. Kaneda, New J. Chem., 2002, 26, 1536 RSC; (d) K. Mori, K. Yamaguchi, T. Hara, T. Mizugaki, K. Ebitani and K. Kaneda, J. Am. Chem. Soc., 2002, 124, 11572 CrossRef CAS; (e) M. Murata, T. Hara, K. Mori, M. Ooe, T. Mizugaki, K. Ebitani and K. Kaneda, Tetrahedron Lett., 2003, 44, 4981 CrossRef CAS; (f) T. Hara, K. Mori, M. Ooe, T. Mizugaki, K. Ebitani and K. Kaneda, Tetrahedron Lett., 2003, 44, 6207 CrossRef CAS; (g) K. Mori, T. Hara, T. Mizugaki, K. Ebitani and K. Kaneda, J. Am. Chem. Soc., 2003, 125, 11460 CrossRef CAS; (h) T. Hara, K. Mori, M. Oshiba, T. Mizugaki, K. Ebitani and K. Kaneda, Green Chem., 2004, 6, 507 RSC; (i) T. Hara, T. Kaneta, K. Mori, T. Mitsudome, T. Mizugaki, K. Ebitani and K. Kaneda, Green Chem., 2007, 9, 1246 RSC; (j) T. Hara, S. Kanai, K. Mori, T. Mizugaki, K. Ebitani, K. Jitsukawa and K. Kaneda, J. Org. Chem., 2007, 71, 7455; (k) K. Mori, S. Kanai, T. Hara, T. Mizugaki, K. Ebitani, K. Jitsukawa and K. Kaneda, Chem. Mater., 2007, 19, 1249 CrossRef CAS; (l) T. Mitsudome, S. Arita, H. Mori, T. Mizugaki, K. Jitsukawa and K. Kaneda, Angew. Chem., Int. Ed., 2008, 47, 7938 CrossRef CAS; (m) K. Mori, M. Oshiba, T. Hara, T. Mizugaki, K. Ebitani and K. Kaneda, Tetrahedron Lett., 2005, 46, 4283 CrossRef CAS; (n) K. Mori, M. Oshiba, T. Hara, T. Mizugaki, K. Ebitani and K. Kaneda, New J. Chem., 2006, 30, 44 RSC; (o) K. Mori, Y. Mitani, T. Hara, T. Mizugaki, K. Ebitani and K. Kaneda, Chem. Commun., 2005, 3331 RSC.
- (a) T. Matsumoto, M. Ueno, N. Wang and S. Kobayashi, Chem.–Asian J., 2008, 3, 196 CrossRef CAS; (b) M. J. Schultz and M. S. Sigman, Tetrahedron, 2006, 62, 8227 CrossRef CAS.
- (a) R. A. Sheldon and J. K. Kochi, Metal-Catalyzed Oxidations of Organic Compounds; Academic Press: New York, 1981 Search PubMed; (b) C. L. Hill, Advances in Oxygenated Processes, A. L. Baumstark, Ed.; JAI Press: London, 1988; Vol. 1, p 1 Search PubMed; (c) M. Hudlucky, Oxidations in Organic Chemistry; ACS Monograph Series; American Chemical Society: Washington, DC, 1990 Search PubMed; (d) G. Tojo and Gabriel Fernández, Oxidation of Alcohols to Aldehydes and Ketones, Springer, 2005 Search PubMed.
- (a) G. Cainelli and G. Cardillo, Chromium Oxidants in Organic Chemistry; Springer: Berlin, 1984 Search PubMed; (b) D. G. Lee and U. A. Spitzer, J. Org. Chem., 1970, 35, 3589 CrossRef CAS; (c) F. M. Menger and C. Lee, Tetrahedron Lett., 1981, 22, 1655 CrossRef CAS.
- (a) B. M. Trost, Science, 1991, 254, 1471 CrossRef CAS; (b) P. T. Anastas and J. C. Warner, Green Chemistry, Theory and Practice; Oxford University Press: Oxford, U.K., 1998 Search PubMed; (c) J. H. Clark, Green Chem., 1999, 1, 1 RSC; (d) R. A. Sheldon, Green Chem., 2000, 2, G1 RSC.
- (a) R. Tang, S. E. Diamond, N. Neary and F. Mares, J. Chem. Soc., Chem. Commun., 1978, 562 RSC; (b) C. Bilgrien, S. Davis and R. S. Drago, J. Am. Chem. Soc., 1987, 109, 3786 CrossRef CAS; (c) J.-E. Bäckvall, R. L. Chowdhury and U. Karlsson, J. Chem. Soc., Chem. Commun., 1991, 473 RSC; (d) T. Iwahama, S. Sakaguchi, Y. Nishiyama and Y. Ishi, Tetrahedron Lett., 1995, 36, 6923 CrossRef CAS; (e) R. Lenz and S. V. Ley, J. Chem. Soc., Perkin Trans. 1, 1997, 3291 RSC; (f) I. E. Markó, P. R. Giles, M. Tsukazaki, I. Chellé-Regnaut, C. J. Urch and S. M. Brown, J. Am. Chem. Soc., 1997, 119, 12661 CrossRef CAS; (g) A. Hanyu, E. Takezawa, S. Sakaguchi and Y. Ishii, Tetrahedron Lett., 1998, 39, 5557 CrossRef CAS; (h) K. S. Coleman, C. Y. Lorber and J. A. Osborn, Eur. J. Inorg. Chem., 1998, 1673 CrossRef CAS; (i) M. Lee and S. Chang, Tetrahedron Lett., 2000, 41, 7507 CrossRef CAS; (j) P. A. Shapley, N. Zhang, J. L. Allen, D. H. Pool and H.-C. Liang, J. Am. Chem. Soc., 2000, 122, 1079 CrossRef CAS; (k) G. Csjernyik, A. H. Ell, L. Fadini, B. Pugin and J.-E. Bäckvall, J. Org. Chem., 2002, 67, 1657 CrossRef CAS.
- (a) T. F. Blackburn and J. Schwartz, J. Chem. Soc., Chem. Commun., 1977, 157 RSC; (b) E. Gómez-Bengoa, P. Noheda and A. M. Echavarren, Tetrahedron Lett., 1994, 35, 7097 CrossRef CAS; (c) S. Aiet-Mohand, F. Henin and J. Muzart, Tetrahedron Lett., 1995, 36, 2473 CrossRef; (d) K. Kaneda, M. Fujii and K. Morioka, J. Org. Chem., 1996, 61, 4502 CrossRef CAS; (e) K. Kaneda, Y. Fujie and K. Ebitani, Tetrahedron Lett., 1997, 38, 9023 CrossRef CAS; (f) K. P. Peterson and R. C. Larock, J. Org. Chem., 1998, 63, 3185 CrossRef CAS; (g) T. Nishimura, T. Onoue, K. Ohe and S. Uemura, Tetrahedron Lett., 1998, 39, 6011 CrossRef CAS; (h) T. Nishimura, T. Onoue, K. Ohe and S. Uemura, J. Org. Chem., 1999, 64, 6750 CrossRef CAS; (i) G.-J. ten Brink, I. W. C. E. Arends and R. A. Sheldon, Science, 2000, 287, 1636 CrossRef; (j) S. S. Stahl, J. L. Thorman, R. C. Nelson and M. A. Kozee, J. Am. Chem. Soc., 2001, 123, 7188 CrossRef CAS; (k) M. A. Steinhoff, S. R. Fix and S. S. Stahl, J. Am. Chem. Soc., 2002, 124, 766 CrossRef CAS; (l) G.-J. ten Brink, I. W. C. E. Arends and R. A. Sheldon, Adv. Synth. Catal., 2002, 344, 355 CrossRef CAS; (m) B. A. Steinhoff and S. S. Stahl, Org. Lett., 2002, 4, 4179 CrossRef CAS; (n) M. J. Schultz, C. C Park and M. S. Sigman, Chem. Commun., 2002, 3034 RSC; (o) D. R. Jensen, M. J. Schultz, J. A. Mueller and M. S. Sigman, Angew. Chem., Int. Ed., 2003, 42, 3810 CrossRef CAS.
- (a) Y. Uozumi and R. Nakao, Angew. Chem., Int. Ed., 2003, 42, 194 CrossRef CAS; (b) T. Wang, H. Shou, Y. Kou and H. Liu, Green Chem., 2009 10.1039/b818560c.
- (a) A. Abad, P. Concepción, A. Corma and H. Garciá, Angew. Chem., Int. Ed., 2005, 44, 4066 CrossRef CAS; (b) A. S. K. Hashmi and G. J. Hutchings, Angew. Chem., Int. Ed., 2006, 45, 7896 CrossRef; (c) A. S. K. Hashmi, Chem. Rev., 2007, 107, 3180 CrossRef.
- (a) I. E. Markó, P. R. Giles, M. Tsukazaki, S. M. Brown and C. J. Urch, Science, 1996, 274, 2044 CrossRef CAS; (b) I. E. Markó, M. Tsukazaki, P. R. Giles, S. M. Brown and C. J. Urch, Angew. Chem., Int. Ed., 1997, 36, 2208 CrossRef CAS; (c) Y. Wang, J. L. Dubois, B. Hedman, K. O. Hodgson and T. D. P. Stack, Science, 1998, 279, 537 CrossRef CAS; (d) I. E. Markó, A. Gautier, I. Chellé-Regnaut, P. R. Giles, M. Tsukazaki, C. J. Urch and S. M. Brown, J. Org. Chem., 1998, 63, 7576 CrossRef CAS; (e) I. E. Markó, P. R. Giles, M. Tsukazaki, A. Gautier, I. Chellé-Regnaut, S. M. Brown and C. J. Urch, J. Org. Chem., 1999, 64, 2433 CrossRef CAS; (f) P. Gamez, I. W. C. E. Arends, J. Reedijk and R. A. Sheldon, Chem. Commun., 2003, 2414 RSC.
- W.-C. Chen, W.-Y. Yu, C.-K. Li and C.-M. Che, J. Org. Chem., 1995, 60, 6840 CrossRef.
- (a) S. Kanemoto, S. Matsubara, K. Takai, K. Oshima, K. Utimoto and H. Nozaki, Bull. Chem. Soc. Jpn., 1988, 61, 3607 CAS; (b) R. Lenz and S. V. Ley, J. Chem. Soc., Perkin Trans. 1, 1997, 3291 RSC.
- (a) M. Sugimoto, J. Am. Ceram. Soc., 1999, 82, 269 CAS; (b) G. Bate, J. Magn. Magn. Mater., 1999, 100, 413.
- J. Zhang, Y. Wang, H. Ji, Y. Wei, N. Wu, B. Zuo and Q. Wang, J. Catal., 2005, 229, 114 CrossRef CAS.
- O. Helgason, J.-M. Greneche, F. J. Berry and F. Mosselmans, J. Phys.: Condens. Matter, 2003, 15, 2907 CrossRef CAS.
- K. Motokura, D. Nishimura, K. Mori, T. Mizugaki, K. Ebitani and K. Kaneda, J. Am. Chem. Soc., 2004, 126, 5662 CrossRef CAS.
- H.-B. Ji, K. Ebitani, T. Mizugaki and K. Kaneda, Catal. Commun., 2002, 3, 511 CrossRef CAS.
- (a) Z. Opre, J.-D. Grunwaldt, M. Maciejewski, D. Ferri, T. Mallat and A. Baiker, J. Catal., 2005, 230, 415.
- A. Dijksman, A. Marino-Gonzalez, A. M. I. Payeras, I. W. C. E. Arends and R. A. Sheldon, J. Am. Chem. Soc., 2001, 123, 6826 CrossRef CAS.
- K. Yamaguchi and N. Mizuno, Chem.–Eur. J., 2003, 9, 4353 CrossRef CAS.
- K. Ebitani, H.-B. Ji, T. Mizugaki and K. Kaneda, J. Mol. Catal., A, 2004, 212, 161 CrossRef CAS.
- T. Matsumoto, M. Ueno, N. Wang and S. Kobayashi, Chem.–Asian J., 2008, 3, 239 CrossRef CAS.
- T. Matsushita, K. Ebitani and K. Kaneda, Chem. Commun., 1999, 265 RSC.
- B. Z. Zhan, M. A. White, T. K. Sham, J. A. Pincock, R. J. Doucet, K. V. R. Rao, K. N. Robertson and T. S. Cameron, J. Am. Chem. Soc., 2003, 125, 2195 CrossRef CAS.
- R. Ciriminna, S. Campestrini and M. Pagliaro, Adv. Synth. Catal., 2004, 212, 161.
- (a) R. Corriu, C. Guerin and J. Moreau, Top. Stereochem., 1984, 15, 43 Search PubMed; (b) P. D. Lickiss, Adv. Inorg. Chem., 1995, 42, 147 CrossRef CAS; (c) I. Ojima, Z. Li, J. Zhu in The Chemistry of Organic Silicon Compounds (Eds.: S. Rappoport, Y. Apeloig), Wiley, New York, 1998, chap. 29 Search PubMed; (d) E. W. Colvin, Silicon Reagents in Organic Synthesis, Academic Press, London, 1988, p. 7 Search PubMed.
- (a) K. Hirabayashi, Y. Nishihara, A. Mori and T. Hiyama, Tetrahedron Lett., 1998, 39, 7893 CrossRef CAS; (b) K. Hirabayashi, J. Kawashima, Y. Nishihara, A. Mori and T. Hiyama, Org. Lett., 1999, 1, 299 CrossRef CAS; (c) S. E. Denmark and D. Wehrli, Org. Lett., 2000, 2, 565 CrossRef CAS; (d) S. Chang, S. H. Yang and P. H. Lee, Tetrahedron Lett., 2001, 42, 4883 CrossRef; (e) K. Hirabayashi, A. Mori, J. Kawashima, M. Suguro, Y. Nishihara and T. Hiyama, J. Org. Chem., 2000, 65, 5342 CrossRef CAS.
- (a) L. H. Sommer, L. A. Ulland and G. A. Parker, J. Am. Chem. Soc., 1972, 94, 3469 CrossRef CAS; (b) W. Adam, R. Mello and R. Curci, Angew. Chem., Int. Ed. Engl., 1990, 29, 890 CrossRef; (c) E. G. Rochow and W. F. Gilliam, J. Am. Chem. Soc., 1941, 63, 798 CrossRef CAS; (d) R. O. Sauer, J. Am. Chem. Soc., 1944, 66, 1707 CrossRef CAS; (e) S. M. Sieburth and W. Mu, J. Org. Chem., 1993, 58, 7584 CrossRef; (f) K. Hirabayashi, A. Mori and T. Hiyama, Tetrahedron Lett., 1997, 38, 461 CrossRef CAS.
- (a) M. Lee, S. Ko and S. Chang, J. Am. Chem. Soc., 2000, 122, 12011 CrossRef CAS; (b) Y. Lee, D. Seomoon, S. Kim, H. Han, S. Chang and P. H. Lee, J. Org. Chem., 2004, 69, 1741 CrossRef CAS; (c) L. H. Sommer and J. E. Lyons, J. Am. Chem. Soc., 1969, 91, 7061 CrossRef CAS; (d) E. Choi, C. Lee, Y. Na and S. Chang, Org. Lett., 2002, 4, 2369 CrossRef CAS.
- K. Mori, M. Tano, T. Mizugaki, K. Ebitani and K. Kaneda, New J. Chem., 2002, 26, 1536 RSC.
- E. A. Ison, R. A Corbin and M. M. A. Omar, J. Am. Chem. Soc., 2005, 127, 11938 CrossRef CAS.
- J. P. Wilcoxon, J. E. Martin and P. Provencio, J. Chem. Phys., 2001, 115, 998 CrossRef.
- A. Ghosh, M. Smits, J. Bredenbeck and M. Bonn, J. Am. Chem. Soc., 2007, 129, 9608 CrossRef CAS.
- C. Eaborn and L. D. Jenkins, J. Organomet. Chem., 1974, 69, 185 CrossRef CAS.
- (a) C.-J. Li and T.-H. Chan, Eds.; Organic Reactions in Aqueous Media; Wiley: New York, 1997 Search PubMed; (b) S. Kobayashi and K. Manabe, Acc. Chem. Res., 2002, 35, 209 CrossRef CAS; (c) K. Manabe and S. Kobayashi, Chem.–Eur. J., 2002, 8, 4095 CrossRef.
- (a) T.-L. Ho, Tactics of Organic Synthesis; Wiley: New York, 1994 Search PubMed; (b) M. E. Jung, In ComprehensiVe Organic Synthesis; B. M. Trost and I. Fleming, Ed.; Pergamon: Oxford, 1991; Vol. 4; pp 1 Search PubMed; (c) H. O. House, Modern Synthetic Reactions, 2nd edn, Benjamin,Menlo Park, CA, 1972; p 595 Search PubMed.
- (a) P. Perlmutter, Conjugate Addition Reactions in Organic Synthesis; Tetrahedron Organic Chemistry Series, Vol. 9; Pergamon: Oxford, 1992 Search PubMed; (b) D. A. Oare and C. H. Heathcock, Top. Stereochem., 1989, 19, 227 Search PubMed; (c) E. D. Bergmann, D. Ginsburg and R. Pappo, Org. React. (N. Y.), 1959, 10, 179 CAS.
- (a) S. Pelzer, T. Kauf, C. van Wüllen and J. Christoffers, J. Organomet. Chem., 2003, 684, 308 CrossRef CAS; (b) J. Christoffers, Eur. J. Org. Chem., 1998, 1259, 9.
- I. Mayer, S. Wahnon and S. Cohen, Mater. Res. Bull., 1979, 14, 1479 CrossRef CAS.
- Y. Fu, L. Liu, R.-Q. Li, R. Liu and Q.-X. Guo, J. Am. Chem. Soc., 2004, 126, 814 CrossRef CAS.
- J. Selbin, G. Maus and D. L. Johnson, J. Inorg. Nucl. Chem., 1967, 29, 1735 CrossRef CAS.
- (a) G. L. Buchanan and G. W. McLay, Tetrahedron, 1966, 22, 1521 CrossRef; (b) W. Kraus, H. Patzelt, H. Sadlo, G. Sawitzki and G. Schwinger, Liebigs Ann. Chem., 1981, 1826 CrossRef CAS.
- H. Tanaka, A. Yasukawa, K. Kandori and T. Ishikawa, Colloids Surf., A, 1997, 125, 53 CrossRef CAS.
- T. Kawabata, T. Mizugaki, K. Ebitani and K. Kaneda, J. Am. Chem. Soc., 2003, 125, 10486 CrossRef CAS.
- T. Kawabata, M. Kato, T. Mizugaki, K. Ebitani and K. Kaneda, Chem.–Eur. J., 2005, 11, 288 CrossRef.
- T. Saegusa, Y. Ito, S. Tomita and H. Kinoshita, Bull. Chem. Soc. Jpn., 1972, 45, 496 CrossRef CAS.
- J. Christoffers, J. Chem. Soc., Perkin Trans. 1, 1997, 3141 RSC.
- K.-I. Shimizu, M. Miyagi, T. Kan-no, T. Kodama and Y. Kitayama, Tetrahedron Lett., 2003, 44, 7421 CrossRef CAS.
- E. Keller and B. L. Feringa, Tetrahedron Lett., 1996, 37, 1879 CrossRef CAS.
- S.-I. Murahashi, T. Naota, H. Taki, M. Mizuno, H. Takaya, S. Komiya, Y. Mizuho, N. Oyasato, M. Hiraoka, M. Hirano and A. Fukuoka, J. Am. Chem. Soc., 1995, 117, 12436 CrossRef CAS.
- H. Kotsuki and K. Arimura, Tetrahedron Lett., 1997, 38, 7583 CrossRef CAS.
- J. S. Yadav, V. Geetha and B. V. S. Reddy, Synth. Commun., 2002, 32, 3519 CrossRef CAS.
- G. Bartoli, M. Bosco, M. C. Bellucci, E. Marcantoni, L. Sambri and E. Torregiani, Eur. J. Org. Chem., 1999, 617, 7.
- (a) E. N. Jacobsen, in Comprehensive Asymmetric Catalysis, ed. E. N. Jacobsen, A. Pfaltz and H. Yamamoto, Springer, Berlin, 1999 Search PubMed; (b) N. Krause and A. Hoffmann-Röder, Synthesis, 2001, 171 CrossRef CAS; (c) M. P. Sibi and S. Manyem, Tetrahedron, 2000, 56, 8033 CrossRef CAS.
- (a) H. Wynberg and R. Helder, Tetrahedron Lett., 1975, 31, 4057 CrossRef; (b) K. Hermann and H. Wynberg, J. Org. Chem., 1979, 44, 2238 CrossRef CAS.
- Y. Tamai, A. Kamifuku, E. Koshiishi and S. Miyano, Chem. Lett., 1995, 957 CAS.
- D. J. Cram and G. D. J. Sogah, J. Chem. Soc., Chem. Commun., 1981, 625 RSC.
- (a) H. Brunner and H. Benedikt, Angew. Chem., Int. Ed. Engl., 1984, 23, 312 CrossRef; (b) G. Desimoni, P. Quadrelli and P. P. Righetti, Tetrahedron, 1990, 46, 2927 CrossRef CAS; (c) C. Botteghi, S. Paganelli, A. Schionato, C. Boga and A. Fava, J. Mol. Catal., 1991, 66, 7 CAS; (d) M. Sawamura, H. Hamashima and Y. Ito, J. Am. Chem. Soc., 1992, 114, 8295 CrossRef CAS; (e) T. Suzuki and T. Torii, Tetrahedron: Asymmetry, 2001, 12, 1077 CrossRef CAS; (f) Y. Hamashima, D. Hotta and M. Sodeoka, J. Am. Chem. Soc., 2002, 124, 11240 CrossRef CAS; (g) M. Nakajima, S. Yamamoto, Y. Yamaguchi, S. Nakamura and S. Hashimoto, Tetrahedron, 2003, 59, 7307 CrossRef CAS.
- M. Shibasaki, H. Sasai and T. Arai, Angew. Chem., Int. Ed. Engl., 1997, 36, 1236 CrossRef.
- (a) S. Matsunaga, T. Ohshima and M. Shibasaki, Tetrahedron Lett., 2000, 41, 8473 CrossRef CAS; (b) T. Arai, Q.-S. Hu, A.-F. Zheng, L. Pu and H. Sasai, Org. Lett., 2000, 2, 4261 CrossRef CAS; (c) T. Sekiguti, Y. Lizuka, S. Takizawa, D. Jayaprakash, T. Arai and H. Sasai, Org. Lett., 2003, 5, 2647 CrossRef CAS; (d) Y. Hamashima, H. Takano, D. Hotta and M. Sodeoka, Org. Lett., 2003, 5, 3225 CrossRef CAS; (e) S. Takizawa, H. Somei, D. Jayaprakash and H. Sasai, Angew. Chem., Int. Ed., 2003, 42, 5711 CrossRef CAS.
- (a) N. Kobayashi and K. Iwai, J. Am. Chem. Soc., 1978, 100, 7071 CrossRef CAS; (b) M. Inagaki, J. Hiratake, Y. Yamamoto and J. Oda, Bull. Chem. Soc. Jpn., 1987, 60, 4121 CAS; (c) R. Alvarez, M.-A. Hourdin, C. Cavé, J. d′Angelo and P. Chaminade, Tetrahedron Lett., 1999, 40, 7091 CrossRef CAS.
- B. M. Choudary, B. Kavita, N. S. Chowdari, B. Sreedhar and M. L. Kantam, Catal. Lett., 2002, 78, 373 CrossRef CAS.
- A. Corma, S. Iborra, I. Rodríguez, M. Iglesias and F. Sánchez, Catal. Lett., 2002, 82, 237 CrossRef CAS.
- (a) P. McMorn and G. J. Hutchings, Chem. Soc. Rev., 2004, 33, 108 RSC; (b) C. E. Song and S.-G. Lee, Chem. Rev., 2002, 102, 3495 CrossRef CAS.
- H. Kotsuki, A. Sugino, H. Sakai and H. Yasuoka, Heterocycles, 2000, 53, 2561 CrossRef CAS.
- (a) A. Behr, Carbon Dioxide Activation by Metal Complexes, VCH publishers, New York, 1988 Search PubMed; (b) Carbon Dioxide Fixation and Reduction in Biological and Model Systems, ed. C.-I. Brauden and G. Schneider, Oxford University Press, Oxford, UK, 1994 Search PubMed.
- (a) A. A. G. Shaikh and S. Sivaram, Chem. Rev., 1996, 155, 2063 CrossRef CAS; (b) D. J. Darensbourg and M. W. Holtcamp, Coord. Chem. Rev., 1996, 153, 155 CrossRef CAS; (c) M. Yoshida and M. Ihara, Chem.–Eur. J., 2004, 10, 2886 CrossRef CAS.
- H. Yoshida, T. Shimizu, C. Murata and T. Hattori, J. Catal., 2003, 220, 226 CrossRef CAS.
- R. L. Paddock and S. T. Nguyen, J. Am. Chem. Soc., 2001, 121, 11498 CrossRef CAS.
- Y. M. Shen, W. L. Duan and M. Shi, J. Org. Chem., 2003, 68, 1559 CrossRef CAS.
- R. L. Paddock, Y. Hiyama, J. M. McKay and S. T. Nguyen, Tetrahedron Lett., 2004, 45, 2023 CrossRef CAS.
- (a) M. George and R. G. Weiss, J. Am. Chem. Soc., 2001, 123, 10393 CrossRef CAS; (b) E. R. Perez, M. O. Silva, V. C. Costa, U. P. Rodringnes-Filho and D. W. Franco, Tetrahedron Lett., 2002, 43, 4091 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2009 |