Catalytic gasification of algae in supercritical water for biofuel production and carbon capture†
Samuel
Stucki
a,
Frédéric
Vogel
*a,
Christian
Ludwig
ab,
Anca G.
Haiduc
b and
Martin
Brandenberger
a
aPaul Scherrer Institut, Laboratory for Energy and Materials Cycles, 5232, Villigen PSI, Switzerland. E-mail: frederic.vogel@psi.ch; Fax: +41 56 310 21 99; Tel: +41 56 310 21 35
bEcole Polytechnique Fédérale de Lausanne, ENAC-ISTE, 1015, Lausanne, Switzerland. E-mail: christian.ludwig@epfl.ch; Fax: +41 21 693 80 35; Tel: +41 21 693 76 78
First published on 20th March 2009
Abstract
There has been growing concern about the way cultivating biomass for the production of agro-biofuels competes with food production. To avoid this competition biomass production for biofuels will, in the long term, have to be completely decoupled from food production. This is where microalgae have enormous potential. Here we propose a novel process based on microalgae cultivation using dilute fossil CO2 emissions and the conversion of the algal biomass through a catalytic hydrothermal process. The resulting products are methane as a clean fuel and concentrated CO2 for sequestration. The proposed gasification process mineralizes nutrient-bearing organics completely. Here we show that complete gasification of microalgae (Spirulina platensis) to a methane-rich gas is now possible in supercritical water using ruthenium catalysts. 60–70% of the heating value contained in the algal biomass would be recovered as methane. Such an efficient algae-to-methane process opens up an elegant way to tackle both climate change and dependence on fossil natural gas without competing with food production.
Broader contextThe expansion of agricultural biofuel production has raised the public awareness of the environmental benefits of this resource and of its impact on global food supply security. However, biofuels can provide a means to stabilize CO2 levels in the atmosphere if not produced on arable land and if the emission of essential nutrients into the environment can be avoided. Exploiting photosynthesis for capturing atmospheric CO2 and producing renewable fuels has been the motivation to develop biofuels. To date, no viable proposal has been made that resolves the dilemma of land competition for producing either food or biofuels. We propose a hybrid route combining the advantages of aquatic photosynthetic organisms with the ones of a new catalytic thermochemical conversion step. This new approach of biofuel production in a truly closed cycle of nutrients and water, is expected to trigger new research in areas as diverse as plant physiology, chemical and bio-chemical engineering, catalysis and materials science. The new algae-based process evolving from our work on catalysis in supercritical water can pave the way to truly sustainable, carbon negative biofuel production. This paper shows a possible way out of the biofuel vs. food dilemma and is therefore of general public interest. |
Introduction
Biofuels derived from crops grown on agricultural land have been shown recently to have a poor overall GHG emission performance as, considered on a global scale, they imply extending the land area for agricultural production. This in turn results in further carbon emissions as it involves indirect land use change, e.g. ploughing up virgin forest or grassland.1,2 Agricultural land is an open system with respect to emissions into delicate environmental compartments, such as the hydrosphere and the atmosphere. In such an open system the application of fertilizers and pesticides inevitably leads to emissions of pollutants into the air (ammonia, nitrous oxide,3 volatile organic compounds, etc.) and to groundwater and runoff (phosphates, nitrates). Although good farming practice can reduce some of these environmentally sensitive losses, the fact remains that intensification of agricultural production to produce conventional biofuels will aggravate the situation.4,5 Sustainable production of biofuels is therefore limited to waste utilization and biomass from extensively cultivated land. Waste biomass is a sensible resource for bioenergy, as any emissions involved in producing the biomass are allocated to the production of the primary product (food, pulp, timber, etc.), albeit its potential is limited.6 Biomass grown extensively on land unsuitable for high-yield agriculture suffers from low area-specific yields and hence expensive logistics.7 The future of bio-energy, if its potential is to be exploited beyond the actual limited sustainable potentials, must rely on biomass which can be produced without emissions of greenhouse gases due to land use change and other pollutants.Productivity of algal biomass
Algal biomass has been suggested as one promising option to produce biomass for use as fuels and/or chemical feedstocks with high area-specific yields.8 The photosynthetic efficiency of microalgal cultures can theoretically approach 10% of the incoming solar energy. However, practical limitations in real systems exposed to sunlight decrease the efficiency to values of 2–3% of incoming sunlight.9 Practical yields obtained in open ponds have been comparable to conventional tropical agriculture with 25–30 t ha−1 yr−1. Yields in closed photo-bioreactors can approach 150 t ha−1 yr−1.10 An extensive research program on developing algal biomass for energy, the Aquatic Species Program, was started in 1978 in the US. It focused on the development of lipid-producing algae that could be transformed into biodiesel fuel. The program was discontinued in 1996 because the economy of the processes studied did not promise an immediate market introduction of the technologies.8 US bioenergy research has since emphasized the development of alcohol fuels from agricultural biomass. The recent rocketing oil prices together with the aggravated competition for arable land for food production have revived interest in algal biomass both in academia and in industry.11–13 The challenge for algal biomass systems is to increase the efficiency of both the production of algae, and the conversion of the biomass into a useful energy carrier. Methane can be produced from biomass with efficiencies reaching or even exceeding 70% using various pathways14 and has therefore become an interesting option for clean and efficient use of biomass, including algae.15 Biogenic SNG (synthetic natural gas) can be upgraded to natural gas quality through CO2 separation and then distributed via the natural gas pipeline system and used in the same versatile way as natural gas today.To maximize the yield of biomass per unit area of an algal culture, it is important to maintain optimum growth conditions for a given type of microorganism, i.e. with the right intensity of light, partial pressure of CO2, nutrient concentration in the water, pH, temperature and agitation of the culture. Ideally such a system can be closed, with CO2 (e.g. from a stack gas) and water as the sole inputs and a hydrocarbon fuel (methane) as a product. Such conditions can be controlled and contained in a system consisting of essentially two closely coupled processes (see Fig. 1): (i) biomass production under optimum growth conditions in a suitable photo-bioreactor (PBR) system, and (ii) conversion of the biomass product into a useful energy carrier, performed in such a way that all of the organic fraction, including secondary metabolites and nutrient-bearing organics, are completely mineralized and nutrients recycled to the bio-reactor. Anaerobic digestion provides only partial mineralization of the algal biomass,15,16 and is therefore not considered a viable technology for the proposed closed-cycle process. In the following, we provide new experimental evidence indicating that catalytic hydrothermal gasification of microalgae biomass has the potential to become a viable option for sustainable fuel production from CO2 and sunlight according to a coupled process such as the one depicted in Fig. 1.
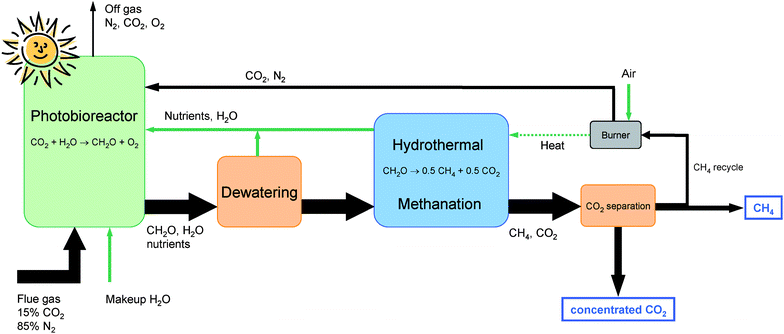 | ||
| Fig. 1 Simplified sketch of the process. The sum formula CH2O is a simplified representation of the algal biomass. The thickness of the black arrows is proportional to the molar flow of carbon. The utilization efficiency of the CO2 injected in the photobioreactor was taken as 90%.8 The green (light) arrows represent non-carbon flows. Their thickness is arbitrary. | ||
Hydrothermal conversion of microalgae
Our research has focused on demonstrating the potential of the catalytic hydrothermal gasification at pressures and temperatures near the critical point of water for complete conversion of organic matter to methane and CO2, and for the efficient recovery of dissolved nutrient salts.33 In such a process, the carbon in the algal biomass (e.g. S. platensis, approximated by C1.0H1.71O0.48N0.19S0.005), is converted to methane and carbon dioxide. Nitrogen and sulfur are expected to form ammonia and hydrogen sulfide, respectively. The overall reaction may be represented by the following simplified stoichiometry| C1.0H1.71O0.48N0.19S0.005 + 0.48 H2O → 0.52 CH4 + 0.48 CO2 + 0.19 NH3 + 0.005 H2S | (1) |
Eqn (1) assumes that the organic sulfur will be released as hydrogen sulfide§ which is a strong catalyst poison. Our approach to avoid catalyst poisoning and to recover all nutrient salts is to precipitate both NH3 and H2S as ammonium and as sulfide salts, respectively, from the supercritical fluid in their appropriate pH range. Note that NH3 and H2S will be separated from the liquefied biomass before entering the catalytic reactor. One issue to be addressed is the documented toxicity of sulfide towards microalgae.17 Current research in our group is aimed at converting sulfide to a harmless species, e.g. sulfate, prior to being recycled to the PBR.
Minowa and Sawayama18 performed a limited number of batch experiments with Chlorella vulgaris in a stirred autoclave at 350 °C and 18 MPa. They used 50% nickel on silica-alumina as a catalyst. All nitrogen in the algae was converted to ammonia. However, a strong deactivation of the catalyst was observed, and a significant amount of the nickel was leached from the support.19 With the highest catalyst loading of 4 g per g of algae dry matter, a maximum of 70% of the carbon contained in the algae could be converted into gaseous products. Recent systematic studies of the effect of sulfur on the activity of noble metal catalysts in supercritical water may explain why microalgae can cause catalyst deactivation.20,21
A certain amount of the active metal will be poisoned by the sulfur released from the biomass and will not contribute to gasifying the biomass. For the algal biomass to be completely converted in batch experiments, for which the separation of the salts from the organic fraction is not feasible, a surplus of catalyst must be employed. This surplus is determined by the total sulfur content of the algae and the number of active surface sites on the catalyst. This aspect was probably overlooked in earlier work on catalytic hydrothermal gasification and explains in part the limited conversion of the algae to gaseous products obtained in the earlier study.18
In the last decade, significant advances in the development of hydrothermal gasification for biomass and organic wastes with a high water content have been made. This has been demonstrated for a range of different feedstocks, including model compounds, organic wastes, and biomass up to a scale of 100 kg h−1.22 We have shown that spruce, a biomass with a low salt content (ca. 0.3 wt%), can be gasified completely to a methane-rich gas in supercritical water with a skeletal nickel catalyst up to high feed concentrations.23 However, the long-term stability of this catalyst was not satisfactory as it sintered rapidly.21 Ruthenium supported on coconut carbon was found to be resistant to sintering and an excellent catalyst for gasifying spruce as well as typical breakdown products of biomass such as ethanol, formic acid, acetic acid, phenol, and anisole in supercritical water. This catalyst was successfully tested in a continuous operation at high space velocities for more than 200 h on stream.24 An important finding was that sulfate, added as sodium sulfate to the feed solution, is a strong poison for the ruthenium catalyst.21 We have, therefore, integrated a salt separation step before the catalytic reactor in our continuous process (see Fig. 2).
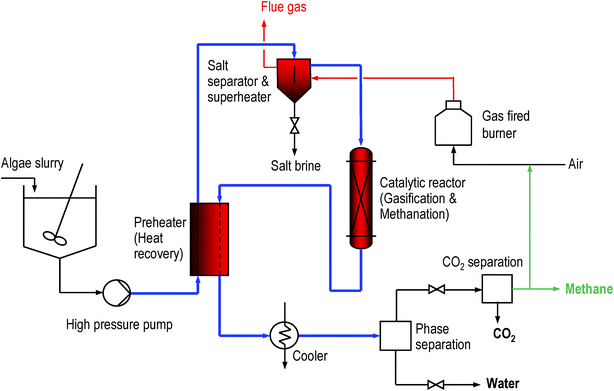 | ||
| Fig. 2 Sketch of PSI's catalytic hydrothermal gasification and methanation process. | ||
An efficient algae-to-methane process
The main flows of the proposed process including the molar carbon flows are shown schematically in Fig. 1. The dilute fossil CO2 (approx. 15 vol%) is captured and assimilated by micro-algae, e.g. Spirulina platensis, in a photo-bioreactor (PBR) exposed to sunlight. The surplus biomass is mechanically dewatered to a dry matter content of 15–20 wt%. This can be achieved with reasonable energy consumption by sedimentation followed by a continuous decanter centrifuge or by a discontinuous filter press.25 If necessary, dissolved secondary organic metabolites can be removed from the filtrate by ultrafiltration or reverse osmosis and fed to the hydrothermal methanation. The separated water and parts of the nutrients are recycled to the PBR. The dewatered biomass slurry is then further processed in the hydrothermal gasification and methanation unit, whose schematic layout is shown in Fig. 2. The algae slurry is pumped to high pressure (ca. 30 MPa) and preheated to a temperature of 300–350 °C using heat from the hot reactor effluent. Heating under high pressure (above the critical pressure of water at 22.1 MPa) avoids evaporation. Further superheating is achieved in the salt separator. At this point, the water is in its supercritical state, exhibiting a very low solubility for salts. The salts are precipitated and separated continuously from the supercritical fluid stream in the salt separator, which is essentially a reverse-flow gravity separator.26 This nutrient concentrate is cooled and conditioned so that it can be recycled to the PBR. The remaining stream, containing the organic fraction and the water, is gasified and converted to methane in a catalytic reactor. After cooling and condensing out the water, the remaining CO2 is separated from the methane in a concentrated form and may be fed to an underground store. Several commercial technologies are available for this task: pressure swing adsorption (PSA), organic solvent or water-based absorption (scrubbing), and membrane separation. Optionally, if no such storage is available, the CO2 can be completely recycled to the PBR. Depending on the separation technology chosen and the intended use of the CO2, the depressurization of the product gas may be carried out before CO2 separation (as shown in Fig. 2), or after CO2 separation (e.g. for high pressure scrubbing), providing the separated CO2 either at low or at high pressure.Some of the methane produced has to be burned to supply enough heat to drive the hydrothermal gasification. This is achieved by heating the salt separator with the flue gases exiting the burner. The flue gases are then fed back to the photo-bioreactor. The net overall process efficiency is expected to reach 60–70%, defined as the heating value of the net methane produced to the heating value of the algae dry matter fed to the hydrothermal methanation.27
A prerequisite for such coupled processes is the full mineralization of nutrient-bearing organic molecules in the biomass to achieve a closed nutrient loop. Furthermore, recycling of partially degraded biomass must be avoided because it may adversely interfere with the algal metabolism.28
Results and discussion
We performed a series of batch experiments aimed at reaching full conversion of the algal biomass to gaseous products. Due to the poisoning effect of molecules with heteroatoms present in the biomass, in particular S, a surplus of catalyst is needed in order to obtain meaningful data from batch experiments. In the continuous, full-scale process (see Fig. 2) we expect that the heteroatoms are split off during preheating under hydrothermal conditions,29–32 forming inorganic ions, i.e. ammonium from N, sulfide from S, and phosphate from P, which are separated as salts continuously from the supercritical fluid before the catalytic reactor. In the batch experiments, a fraction of the catalyst particles is sacrificed as adsorbent for these poisons. The free sites of the active metal are then able to gasify and convert the organic intermediates to methane, CO2, and hydrogen. To test this hypothesis, we varied the mass ratio of catalyst to algae dry matter from 0.1 to 8.1.| Exp. no. | Catalyst | Feed conc. (wt%) | m cat/mDM g/g | RT/min | C gas/Cfeed % (g/g) | CH4 vol% | C2H6 vol% | C3H8 vol% | CO2 vol% | CO vol% | H2 vol% | Y C1–C3 gC1–C3/gDM |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a Blank run with only catalyst and water (the carbon of the support is considered as the feed here). | ||||||||||||
| C01 | Ru/C | 10.0 | 0.2 | 62 | 24 | 7.5 | 5.0 | 5.5 | 63.7 | 0.5 | 17.8 | 0.05 |
| Z01 | Ru/ZrO2 | 20.0 | 0.1 | 61 | 18 | 4.2 | 4.0 | 5.0 | 76.5 | 0.4 | 9.9 | 0.03 |
| Z02 | Ru/ZrO2 | 10.0 | 1.5 | 63 | 25 | 20.1 | 3.6 | 3.0 | 51.1 | 0.3 | 22.0 | 0.05 |
| C02 | Ru/C | 9.8 | 1.5 | 60 | 32 | 14.9 | 5.2 | 3.7 | 64.1 | 0.2 | 12.0 | 0.06 |
| C03 | Ru/C | 5.1 | 1.9 | 60 | 45 | 20.5 | 4.6 | 2.8 | 57.6 | 0.1 | 14.4 | 0.09 |
| C04 | Ru/C | 2.5 | 8.1 | 361 | 109 | 41.7 | 0.7 | <0.01 | 49.6 | 0.1 | 8.0 | 0.26 |
| N01 | — | 2.5 | 0 | 65 | 10 | 5.1 | 1.7 | 5.5 | 57.6 | 0.7 | 29.3 | 0.02 |
| Z03 | Ru/ZrO2 | 2.5 | 8.0 | 62 | 67 | 41.3 | 1.6 | 1.1 | 38.1 | 0.1 | 17.8 | 0.21 |
| C05 | Ru/C | 2.5 | 8.0 | 63 | 93 | 42.7 | 1.7 | 0.6 | 49.0 | 0.1 | 5.8 | 0.26 |
| Z04 | Ru/ZrO2 | 2.5 | 7.9 | 360 | 93 | 52.4 | 1.2 | 0.2 | 38.0 | <0.01 | 8.2 | 0.32 |
| C06 | Ru/C | 10.1 | 0.9 | 61 | 37 | 21.2 | 8.9 | 7.8 | 52.3 | 0.3 | 9.6 | 0.12 |
| Z05 | Ru/ZrO2 | 10.0 | 8.0 | 360 | 66 | 43.1 | 1.9 | 1.5 | 44.5 | 0.1 | 8.8 | 0.21 |
| C07a | Ru/C | 13.8 | — | 361 | 1 | 1.5 | 0.4 | 0.02 | 98.1 | <0.01 | <1 | <0.001 |
| EQ | — | 2.5 | — | — | 100 | 43.4 | 5.7 × 10−4 | 4.6 × 10−8 | 45.5 | 0.03 | 11.1 | 0.31 |
Table 1 shows experimental results for S. platensis using two different ruthenium catalysts. The ruthenium on activated coconut carbon (Ru/C) is a commercial catalyst and has been used successfully at hydrothermal conditions.21,24 The ruthenium on zirconia (Ru/ZrO2) was prepared in our laboratory and exhibited a performance similar to the Ru/C in screening experiments but with a potentially better long-term stability than the carbon support.
The maximum yield of C1–C3 hydrocarbons, calculated at chemical equilibrium, is 0.31 gC1–C3/gDM for 2.5 wt% S. platensis at 400 °C and 30 MPa. The corresponding gas composition (dry basis; free of NH3 and H2S) was also calculated (Table 1, entry EQ)¶. No solids (except the catalyst) were present after the experiments. The liquid phase, depending on the degree of organic conversion, ranged from a clear transparent water-like liquid, over a slightly yellow homogeneous oil-like solution to a deeply yellow/brownish liquid with two liquid phases. Without a catalyst the conversion to gases was only 10% and the C1–C3 yield was very low (exp. N01 in Table 1). Feed carbon gasification greater than 50%, and methane concentrations greater than 40 vol% could only be achieved with high catalyst loadings. Complete gasification was achieved with both Ru/C and Ru/ZrO2 at a catalyst to biomass ratio of 8, and C1–C3 yields close to the thermodynamic equilibrium were reached (C04, C05, Z04). Higher hydrocarbons, such as ethane and propane, which are not thermodynamically stable products, were mainly formed at low feed conversions where they can represent up to 16 vol% of the dry product gas (C06). CO did not exceed 0.7 vol% in the dry product gas. CO is known to be a primary product of biomass gasification that can undergo further conversion to CO2 by the watergas shift reaction, promoted by the high partial pressure of water. The highest concentrations of CO were found for the experiments with high feed concentrations (10 and 20 wt%), and for the experiment without catalyst (N01). Interestingly, experiment Z04 exhibited a methane concentration of 52 vol%, which is significantly higher than the 43% calculated for chemical equilibrium (compared to entry EQ in Table 1). It is possible that, in experiment Z04, more CO2 dissolved in the remaining alkaline aqueous phase than in the other experiments, thus reducing the concentration of CO2 in the product gas and increasing the concentration of all other components including methane. This is supported by the fact that the methane yield of experiment Z04 did not exceed the calculated equilibrium yield by the same proportion as the methane concentration did (approx. 3% vs. 21%, respectively).
Additional information on the pH and the nonpurgeable organic carbon content (NPOC) of the aqueous phase recovered from the reactor is given in Table 2. Note that NPOC corresponds to the total organic carbon content, determined by the differential method, if no volatile organic carbon is present in the sample. The ratio Argas/Arfeed is the amount of argon recovered after the experiment to the amount of argon added for pressurizing the reactor. Significant deviations from 100% indicate a leakage, losses during gas sampling, or errors in the gas analysis. As the argon balance of experiment Z03 deviates by 14%, the data from this experiment should be used with caution. “Self-gasification” of the Ru/C catalyst was found to be insignificant (C07), because the value of 1% for the carbon gasification is well within the experimental uncertainty. Longer term experiments with this catalyst in the absence of poisons have previously demonstrated its hydrothermal stability for more than 200 h.24 Based on the data obtained from these experiments, both 2%Ru/C and 2%Ru/ZrO2 are considered suitable catalysts for the complete conversion of algal biomass into a methane-rich gas. However, further research needs to include long-term catalyst stability testing.
| Exp. no. | Argas/Arfeed (%) (mol/mol) | pH | NPOC (mg L−1) | T end (°C) | p end (MPa) | ΔYC1–C3/YC1–C3 (%) |
|---|---|---|---|---|---|---|
| a Blank run with only catalyst and water (the carbon of the support is considered as the feed here). NPOC: nonpurgeable organic carbon. | ||||||
| C01 | 104.0 | 9.0–9.5 | 14051 | 409 | 33.7 | 4.3 |
| Z01 | 96.6 | 9.0 | 32626 | 400 | 34.1 | 3.9 |
| Z02 | 106.1 | 8.0 | 9212 | 402 | 31.9 | 3.8 |
| C02 | 99.4 | 9.0 | 4023 | 401 | 32.7 | 2.8 |
| C03 | 102.3 | 8.5–9.0 | 3772 | 400 | 30.8 | 3.0 |
| C04 | 97.2 | 7.5–8.0 | 333 | 402 | 31.5 | 2.7 |
| N01 | 100.7 | 8.0 | 5316 | 401 | 31.8 | 3.6 |
| Z03 | 85.9 | 8.0 | 2455 | 400 | 30.9 | 2.4 |
| C05 | 96.6 | 8.0 | 328 | 399 | 32.4 | 2.1 |
| Z04 | 102.5 | 8.5 | 277 | 403 | 33.6 | 3.2 |
| C06 | 99.3 | 8.5 | 6125 | 402 | 34.5 | 5.1 |
| Z05 | 100.1 | 8.5–9.0 | 3738 | 403 | 31.1 | 2.5 |
| C07a | 99.0 | 6.0 | 229 | 401 | 32.1 | 4.0 |
| EQ | 100.0 | N/A | 0 | 400 | 30.0 | 0.0 |
One important question to be answered for the proposed cyclic process is the suitability of the recycled streams for microalgae growth. In particular, the recovered nutrient solution may contain trace elements from the reactor materials and from the catalyst, e.g. nickel, chromium, or ruthenium. Nickel will inhibit algae growth if present at greater than ca. 10 ppm.34 Thus corrosion-resistant materials of construction are essential in this process. However, the corrosion rates acceptable for safe operation of the pressurized vessels are so low that reactor corrosion is not expected to lead to problematic concentrations of heavy metals in the recycled process effluent if they are not allowed to accumulate.
An energy balance performed for a range of other biomass feedstocks (liquid manure, wood, sewage sludge) indicates that 60–70% of the heating value of the biomass can be recovered as methane.27 Due to the similar heating value and elemental composition of microalgae, we expect the efficiency of the hydrothermal gasification of microalgae to fall within the same range as for the other types of biomass. Note that the hydrothermal process is designed to be thermally self-sufficient, as all heat demands are met either by heat recovery or by combusting some of the methane produced. Large-scale plants would be designed to also meet their own electricity demand, e.g. by incorporating a Rankine steam power cycle. Preliminary calculations of the energy balance for the whole algae-to-methane process indicate that dewatering of the PBR effluent to 15–20 wt% may be a critical issue.
Experimental
The catalytic hydrothermal gasification of S. platensis was conducted in a small unstirred batch reactor system developed in our laboratory with a total internal volume of approximately 30 mL. Details can be found in the ESI†. S. platensis was obtained as a fine powder from a commercial food supplier (Josefs NaturBiokraft, Hasle, Switzerland). The food grade microalgae (BAG-N-Nr. 50511, PharmaCode 2219895, Art.I-Nr. Spp00125) contained less than 3 wt% of water. Before each experiment the microalgae were dried for 1 h at 105 °C in a drying oven. Table 3 lists the elemental composition and the ash content of S. platensis on a dry basis.| C | H | N | O | S | Ash |
|---|---|---|---|---|---|
| 47.8 | 6.8 | 10.6 | 30.6 | 0.6 | 7.3 |
Water and biomass were mixed in a beaker such as to yield the desired feed concentration (2.5, 5, 10, and 20 wt%, respectively). Then the amount of catalyst (2% Ru on carbon or 2% Ru on ZrO2) corresponding to the desired ratio of catalyst to biomass was added (0.15 g–5.6 g), and the mixture was transferred into the reactor. The reactor tube was closed tightly with the help of a torque wrench and finally connected to a capillary tube. Air was removed by evacuating the apparatus with a vacuum pump and by flushing twice with argon before each experiment. Before starting the gasification experiment, the reactor was pressurized with 2.0–4.2 MPa of argon to avoid water evaporation and dry out of the biomass slurry during heat-up. This procedure also served as a leak test (see argon mass balance closure in Table 2).
The reaction was initiated by the immersion of the reactor assembly into a preheated fluidized sand bath (Techne SBL-2D). The desired reaction temperature Tend was reached in less than 10 min. after immersion. Before each experiment, the expected pressure pend was calculated, including preliminary trials to verify the calculations before the actual experiment. The actual temperature Tend and pressure pend reached in each experiment are given in Table 2.
To stop the reaction after a predetermined time, the reactor was lifted out of the fluidized sand bath and quenched in a cold water bath. The pressure inside the reactor and the temperatures were measured and recorded at intervals of 1 s using a LabView™-based data acquisition system. Gas samples were taken with a gas sampling bag (volume 1 L, SKC). The gas samples were analyzed off-line with a gas chromatograph (Agilent 6890). Liquid samples were obtained through filtration of the batch reactor content over a membrane filter (regenerated cellulose, pore size 0.45 µm) with a vacuum filtration apparatus. Nonpurgeable organic carbon (NPOC) was measured on a TOC – Vwp (Total Organic Carbon Analyzer) from Shimadzu connected to an ASI-V autosampler. The NPOC was measured by the persulfate/UV oxidation method at 80 °C. The pH of the aqueous samples was measured with color-fixed pH indicator sticks (pH 7–14, Fisherbrand). Additional details on analytical methods can be found in the ESI.†
Summary and outlook
We have demonstrated that the microalga S. platensis can be gasified completely to a methane-rich gas in supercritical water. The carbon contained in the algae could be transferred completely (within experimental uncertainty) into gaseous products (CH4, CO2, C2H6, C3H8). This was made possible by using supported Ru catalysts and suitable operating conditions. This result is an important prerequisite for the development of new photosynthetic fuel production systems with closed nutrient cycles. The experimental results also show that the catalyst converting the biomass to methane and CO2 needs to be protected from poisoning by heteroatoms present in algal biomass. Future development will hence have to focus on the efficient separation of heteroatoms before the catalytic conversion by exploiting, e.g., salt precipitation above the critical point of water.Our proposed process allows the efficient conversion of algal biomass into methane fuel and CO2 suitable for sequestration using catalytic gasification and methanation in supercritical water. If the separation of the heteroatoms and salts can be controlled, this process does not rely on specific products of algal cultures (e.g. lipids), but allows to exploit algal strains with optimized photosynthetic efficiency. The hydrothermal process mineralizes the organic matter of the aquatic biomass, and allows the nutrients for the algal culture to be kept in a closed loop. Further research is needed to assess the impact of the recycled nutrient solution on microalgae growth.
The proposed system is a process that not only captures carbon, converting flue gas into a pure CO2 product at elevated pressure, but also produces pipeline quality SNG. The economic attractiveness of the proposed process may be further improved by coupling the biomass gasification and mineralization process with the direct biotechnical production of high value speciality chemicals by making use of appropriately chosen algae cultures.
Acknowledgements
The authors thank A. Wokaun, M. Schubert, R. Bernier-Latmani (EPFL), and W. H. Casey (UC Davis) for valuable discussions. We thank L. van Loon, W. Müller, and J. Müller for assistance with the NPOC analyses, M. Schneider (ETHZ) for performing the elemental analyses of the algae, and E. De Boni, M. Elsener and P. Ruch for technical and analytical support. We thank C. Cavenaghi (Engelhard-BASF) for providing catalyst samples, and T.-B. Truong for the Ru/ZrO2 catalyst synthesis. This project was made possible through financial support from the Velux Foundation, Zürich (project Nr. 405).References
- J. Fargione, J. Hill, D. Tilman, S. Polasky and P. Hawthorne, Science, 2008, 319, 1235–1238 CrossRef CAS.
- T. Searchinger, R. Heimlich, R. A. Houghton, F. Dong, A. Elobeid, J. Fabiosa, S. Tokgoz, D. Hayes and T.-H. Yu, Science, 2008, 319, 1238–1240 CrossRef CAS.
- P. J. Crutzen, A. R. Mosier, K. A. Smith and W. Winiwarter, Atmos. Chem. Phys., 2008, 8, 389–395 CAS.
- J. W. Erisman, A. Bleeker, J. Galloway and M. S. Sutton, Environ. Pollut., 2007, 150, 140–149 CrossRef CAS.
- European Environment Agency, Source apportionment of nitrogen and phosphorus inputs into the aquatic environment, EEA report no. 7/2005, ISSN 1725–9177, 2005 Search PubMed.
- E. M. W. Smeets, A. P. C. Faaij, I. M. Lewandowski and W. C. Turkenburg, Prog. Energy Combust. Sci., 2007, 33, 56–106 CrossRef CAS.
- A. Kumar, J. B. Cameron and P. C. Flynn, Biomass Bioenergy, 2003, 24(6), 445–464 CrossRef.
- J. Sheehan, T. Dunahay, J. Benemann and P. Roessler, A Look Back at the U.S. Department of Energy's Aquatic Species Program—Biodiesel from Algae, Close-out report, NREL/TP-580–24190, July, 1998 Search PubMed.
- J. R. Benemann and W. J. Oswald, Systems and economic analysis of microalgae ponds for conversion of CO2 to biomass, U.S. Department of Energy, Pittsburgh, PA, 1996 Search PubMed.
- A. S. Carlsson, J. B. van Beilen, R. Möller and D. Clayton, Micro- and macro-algae: utility for industrial applications. Outputs from the EPOBIO project, CPL Press, Newbury, UK, 2007, http://www.epobio.net/pdfs/0709AquaticReport.pdfAccessed October 3, 2007 Search PubMed.
- Y. Li, M. Horsman, N. Wu, C. Q. Lan and N. Dubois-Calero, Biotechnol. Prog., 2008, 24, 815–820 CAS.
- Y. Chisti, Biotechnol. Adv., 2007, 25, 294–306 CrossRef CAS.
- B. Wang, Y. Li, N. Wu and C. Q. Lan, Appl. Microbiol. Biotechnol., 2008, 79, 707–718 CrossRef CAS.
- R. W. R. Zwart and H. Boerrigter, Energy Fuels, 2005, 19, 591–597 CrossRef CAS.
- C. G. Golueke and W. J. Oswald, Sol. Energy, 1963, 7, 86–92 CrossRef CAS.
- P. H. Chen and W. J. Oswald, Environ. Int., 1998, 24, 889–897 CrossRef CAS.
- Y. Cohen, B. B. Jørgensen, N. P. Revsbech and R. Poplawski, Appl. Environ. Microbiol., 1986, 51, 398–407 CAS.
- T. Minowa and S. Sawayama, Fuel, 1999, 78, 1213–1215 CrossRef CAS.
- K. Tsukahara, T. Kimura, T. Minowa, S. Sawayama, T. Yagishita, S. Inoue, T. Hanaoka, Y. Usui and T. Ogi, J. Biosci. Bioeng., 2001, 91(3), 311–313 CrossRef CAS.
- M. Osada, N. Hiyoshi, O. Sato, K. Arai and M. Shirai, Energy Fuels, 2007, 21(3), 1400–1405 CrossRef CAS; M. Osada, N. Hiyoshi, O. Sato, K. Arai and M. Shirai, Energy Fuels, 2007, 21(4), 1854–1858 CrossRef CAS.
- M. H. Waldner, F. Krumeich and F. Vogel, J. Supercrit. Fluids, 2007, 43(1), 91–105 CrossRef CAS.
- A. A. Peterson, F. Vogel, R. P. Lachance, M. Fröling, M. J. Antal, Jr. and J. W. Tester, Energy Environ. Sci., 2008, 1, 32–65 RSC.
- M. H. Waldner and F. Vogel, Ind. Eng. Chem. Res., 2005, 44(13), 4543–4551 CrossRef CAS.
- F. Vogel, M. H. Waldner, A. A. Rouff and S. Rabe, Green Chem., 2007, 9(6), 616–619 RSC.
- F. H. Mohn, in Micro-Algal Biotechnology, ed. M. A. Borowitzka and L. J. Borowitzka, Cambridge Univ. Press, Cambridge, 1988, ch. 15 Search PubMed.
- A. A. Peterson, P. Vontobel, F. Vogel and J. W. Tester, J. Supercrit. Fluids, 2008, 43(3), 490–499 CrossRef CAS.
- J. S. Luterbacher, M. Fröling, F. Vogel, F. Maréchal and J. W. Tester, Environ. Sci. Technol., 2009, 43(5), 1578–1583 CrossRef CAS.
- L. Rodolfi, G. Chini Zittelli, L. Barsanti, G. Rosati and M. R. Tredici, Biomol. Eng., 2003, 20, 243–248 CrossRef CAS.
- O. M. Ogunsola and N. Berkowitz, Fuel, 1995, 74(10), 1485–1490 CrossRef CAS.
- Y. Dote, S. Inoue, T. Ogi and S. Yokoyama, Biomass Bioenergy, 1996, 11(6), 491–498 CrossRef CAS.
- T. J. Houser, Y. Zhou and X. Liu, J. Supercrit. Fluids, 1996, 9, 106–112 CrossRef CAS.
- M. Akimoto, T. Sato and T. Nagasawa, Ind. Eng. Chem. Res., 2003, 42(10), 2074–2080 CrossRef.
- F. Vogel, in Handbook of Green Chemistry, P. Anastas (Series Editor), Volume 2Heterogeneous Catalysis, R. Crabtree (Volume Editor), Wiley-VCH, Weinheim, 2009, ch. 12, pp. 281–324, ISBN 978-3-527-32497-2 Search PubMed.
- A. G. Haiduc, M. Brandenberger, S. Suquet, F. Vogel, R. Bernier-Latmani and C. Ludwig, J. Appl. Phycol. DOI:10.1007/s10811-009-9403-3.
Footnotes |
| † Electronic supplementary information (ESI) available: Supplementary methods. See DOI: 10.1039/b819874h |
| ‡ The partial pressure of CO2 in the stack gas with 15 vol% CO2 at ambient pressure is 0.015 MPa. The concentration of CO2 in the dry product gas of the hydrothermal gasification after separation of the aqueous and the gas phase is 40–50 vol% at 30 MPa (compare Table 1), corresponding to a partial pressure of 12–15 MPa and to enrichment by a factor of 800–1000. After separating the methane from the product gas mixture, the concentration of CO2 increases further. The total and hence the partial pressure, however, will depend on the technology used for separating the methane. |
| § The distribution of the actual species, H2S, HS− or S2−, is determined by the pH and the dissociation equilibria at reaction conditions. |
| ¶ Equilibrium composition was calculated from elemental composition data using Aspen Plus™ 2006 (Aspen Technology, Cambridge, USA). The Peng–Robinson equation of state was used with the Boston–Mathias alpha function. |
| This journal is © The Royal Society of Chemistry 2009 |
