A review of metal separator plate materials suitable for automotive PEM fuel cells
N.
de las Heras
a,
E. P. L.
Roberts
*a,
R.
Langton
b and
D.
R. Hodgson
c
aSchool of Chemical Engineering and Analytical Science, University of Manchester, M60 1QD, UK. E-mail: edward.roberts@manchester.ac.uk
bINEOS Technologies, Runcorn, WA7 4JE, UK
cAIC Europe, Hethel, Norfolk NR14 8FB, UK
First published on 14th November 2008
Abstract
In this paper we review the range of materials which have been studied for use as separator plates in automotive PEM fuel cells, with particular emphasis on metals. For commercial application separator plates must be resistant to corrosion, durable and offer low contact resistance in a fuel cell stack. Graphite and carbon composite materials have been widely used, as they offer durability and give reliable performance. For portable and transport applications, new materials offering reduced cost, weight and volume are needed. Metal plates may offer a compact, low cost alternative to graphite and carbon based composites. However in the aggressive fuel cell environment, corrosion of metal plates can significantly effect fuel cell performance while passivation can also lead to increased ohmic losses. The only metal plate material studied in the literature which meets the performance targets for contact resistance and corrosion is gold coated stainless steel. New corrosion resistant coatings are being developed in order to address these significant issues and this review paper evaluates them in the context of the DOE targets established for 2010.
Nuria de las Heras studies the development and evaluation of metal bipolar plates for polymer electrolyte membrane (PEM) fuel cells. She has developed experimental techniques for characterising the corrosion and contact resistance characteristics of plate materials. |
 E. P. L. Roberts E. P. L. Roberts | Edward Roberts studies the design of electrochemical processes for energy and environmental applications. He has worked with industrial collaborators on the development of new metal plate materials for fuel cells. He has shown how membrane materials can be optimised for direct methanol fuel cells. He is co-inventor of the Arvia Process™ for wastewater treatment. |
Richard Langton led the PEMcoat group at INEOS Technologies Ltd who developed and manufactured coated metal plates for PEM fuel cells. |
David Hodgson has developed electrode materials and electrocatalysts for a wide variety of applications and is the inventor of the PEMcoat materials, developing it into a business. |
Broader contextFuel cells are widely considered to be a sustainable energy conversion system and are a key technology for the development of a hydrogen economy. Low temperature (<100 °C) fuel cells, which use a polymer membrane to separate the electrodes, have been undergoing rapid development for mobile applications and in particular for the transport sector. Currently the cost of fuel cell systems is too high for them to be able to compete with conventional systems such as the internal combustion engine. One of the important challenges is the development of materials for electrode plates which are able to offer long term durability, reduced volume, lightweight and minimum manufacturing costs. These plates make up a very significant part of the weight and cost of a fuel cell system. In this paper the range of available plate materials is reviewed, with particular emphasis on metal plate materials which have the potential to offer significantly reduced weight and volume. Currently no metal plate material has been demonstrated to deliver the required performance at a competitive cost. However stainless steel plates coated with a thin corrosion resistant layer have the potential to achieve these requirements. |
1 Introduction
Polymer electrolyte membrane fuel cells (PEMFC) have been undergoing rapid development in the last few years for portable, mobile applications and in particular for the transport sector.1,2 PEMFCs are relatively compact, have a high power density, rapid start up and operate at low temperatures (60–120 °C). These characteristics make PEMFCs very attractive as a power source for vehicles in comparison with other fuel cell systems. However, there are many technological challenges to overcome before mass production and commercialisation of this technology becomes a reality. One of these challenges is the development of materials for bipolar separator plates able to offer long term durability, reduced volume, lightweight and minimum manufacturing costs. In this paper the range of bipolar plate materials are reviewed, with particular emphasis on metal plate materials which have the potential to offer reduced weight and volume.2 Separator plates for automotive fuel cells
The bipolar separator plates are one of the most important components of a PEMFC stack, as they distribute reactant gases to the gas diffusion electrodes, allow removal of water and heat, and provide mechanical support and electrical connection between the cells in the stack. Bipolar plates typically account for 80–85% of the weight and most of the volume of fuel cell stacks.3 Estimates of the cost contribution of bipolar plates vary widely with variations in the cost evaluation methodology, differences in material and processing costs and improvements in the technology. Estimates of the cost contribution of bipolar plates to overall stack costs include values from as low as 5%4 to as high as 90%5 (although this seems to be based on stainless steel plates of thickness 2–4 mm), with many intermediate values (15%,5 29%,6 45%,3 68%7). In spite of this variation, it is clear that low cost materials are needed which do not compromise the fuel cell stack performance and durability.The bipolar plates of a PEMFC must be able to operate in an acidic environment of pH 3–5 in the presence of SO42−, NO3−, Cl−, F− ions arising from the Nafion membrane while in direct contact with oxidizing gases such as pure O2 or air on the cathode side, and reducing gases such as H2 on the anode side. The PEMFC separator plates are also typically exposed to potentials of 0 to 1000 mV versusRHE by contact with the electrodes, and potentials as high as 1400 mV versusRHE can occur at the cathode.8 These working conditions greatly condition the selection of materials and determine the characteristics of an ideal separator plate material which should incorporate excellent corrosion resistance, minimum electrical resistivity and good mechanical strength. Additional constraints influencing material selection for bipolar plates arise from reliability and durability aspects, with a suggested maximum cell performance degradation of 0.1% over 1000 h required for projected operational lifetimes of 5000 h in transport applications.9 Furthermore, it should be possible to mass produce separator plates by integrating suitable flow fields, manifolds and seals at a low cost to enable PEMFCs to compete with the internal combustion engine. For transport applications the requirement to reduce cell cost and volume without compromising fuel cell performance is crucial; in the USA, cost and power density targets for automobiles have been quoted as $10 kW−1 and 1 kW dm−3 respectively.5 The US Department of Energy (DOE) has established specific targets for separator plates in PEMFC stacks for automotive applications to be achieved by 2010 (see Table 1).
| DOE Technical Targets: Bipolar Plates | |
|---|---|
| Cost/$ kW−1 | < 10 |
| System weight/kg kW−1 | < 1 |
| H2 permeation flux/cm3 s−1 cm−2 | < 2 × 10−6 |
| Corrosion/µA cm−2 | < 16 |
| Electrical conductivity/S cm−1 | > 100 |
| Durability with cycling/h | > 5000 |
| Interfacial contact resistance at 140 N cm−2/mΩ cm2 | < 10 |
In this review, materials currently used and being developed will be considered in the context of the DOE targets described in Table 1. The conductivity of materials can be measured by standard techniques, but the other key properties are associated with the fuel cell environment. Literature values of contact resistance between plate and gas diffusion layer (GDL) materials can vary significantly. This can be due to variations in any pretreatment and the GDL material used, but the most significant factor is the compression force. The DOE target in Table 1 is based on a standard technique with a compression force of 140 N m−2. In this review we will only compare contact resistance values made at this compression force. Measurements of corrosion rate and durability are typically carried out under a wide range of different conditions, which can make comparisons difficult.
Materials used for separator plates in PEMFC to date fall into three main categories: graphite, composites and metals (Fig. 1).
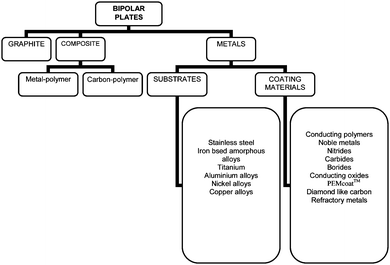 | ||
| Fig. 1 Materials used for bipolar separator plates for PEMFC. | ||
3 Graphite
Traditionally PEMFC bipolar plates have been constructed from graphite, both natural and synthetic, as it is resistant to corrosion in the fuel cell environment and has high electrical conductivity. Many studies have shown the benefits of using graphitic plates; Davies and co-authors12 presented long term data from Poco™ graphite plates tested in a single fuel cell with insignificant interfacial ohmnic losses relative to uncoated stainless steel and titanium plates; Cleghorn et al.13 also operated a single PEMFC continuously for three years with minimal power losses using Poco™ graphite plates. Although graphitic plates offer an adequate performance in stacks showing minimum degradation over time, graphite lacks mechanical strength increasing the size of the fuel cell stack and therefore limiting the volumetric power density. In addition, its high production costs remain a major problem with currently machined graphite plates accounting for 60% of the stack cost. For this reason graphite is unsuitable for automotive applications involving high volume manufacturing.144 Composites
Composites are an alternative to graphite offering a reduction in weight and an improvement in manufacture as they can be extruded or molded to any shape reducing manufacturing time and cost, and consequently they have gained much attention in the last few years. Cunningham stated that the cost of molding a polymer composite bipolar plate is 10–20 times less expensive than machining.15 Composite materials can be classified as carbon–carbon composites and carbon–polymer composites.Besmann16 proposed carbon–carbon composite plates prepared by slurry molding a chopped carbon fiber–phenolic resin preform, followed by sealing the material with chemically vapour infiltrated carbon, resulting in a hermetic graphitic carbon system. These materials have good conductivity (200–300 S cm−1) and adequate corrosion resistance however the complexity of the production process leads to high costs of fabrication. Hentall et al.2 recommended exfoliated graphite (Grafoil™) as an ideal plate material for PEMFC as it showed a significant increase in performance than standard graphite materials. This enhanced performance is due to its compressibility, enabling the material to form an intimate contact with the MEA thus minimizing interfacial resistance. This material is ideally suited for production as it is inexpensive and simple to cut into foils but it also suffers from impact damage and its compressibility could deter the manufacture of multiple cell stacks.
Carbon–polymer composite materials have been developed as an alternative to carbon–carbon composites. They consist of a polymer resin (thermosetting or thermoplastic), used as a binder and a conductive filler such as graphite, metal coated graphite or low melting metal alloys. A high loading of filler is required in order to increase the electrical conductivity, while insufficient binder will lead to poor mechanical properties. Compression or injection molding is used to generate the bipolar plates and the cost is reduced because the machining process is removed. This composite route has been the approach of SGL Technologies GmbH who presented a promising composite comprised of polypropylene and phenolic bonded graphite, which can be injection molded, shows good electrical conductivity, is thermally stable above 100 °C and can be produced cost effectively.17 Composite materials using liquid crystal polymer with a carbon content of around 85% have recently been developed.18 These materials have the advantage that they can be injection molded.
Until recently, carbon–polymer composite materials offered low bulk conductivity relative to carbon–carbon or graphite plates. In addition, these materials often exhibit lower through-plane conductivity than in-plane. Recently nanocomposite materials have been reported using fillers such as carbon nanotubes, graphitic powders and silver coated glass19,20 which can offer improved mechanical properties and in particular, increased conductivity. Laminated materials have also been developed which can improve the through-plane conductivity.21
Los Alamos National Laboratory has developed a metal-based composite bipolar plate based on porous graphite, polycarbonate plastic and stainless steel.22 Producing porous graphitic plates is not as expensive as non porous graphitic plates where the impermeability is provided by the steel and the polycarbonate. The stainless steel also provides rigidity to the structure while the polycarbonate contributes to the moldability and corrosion resistance. The layered plate appears to be a good alternative from stability and cost standpoints.
The main disadvantage of composite materials is their relatively low conductivity (typically 50–200 S cm−1 in-plane, 20–50 S cm−1 through-plane) compared to graphite (>500 S cm−1) and metal (>10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 S cm−1) plates. In addition they offer lower thermal conductivity and limited operating temperature range.
000 S cm−1) plates. In addition they offer lower thermal conductivity and limited operating temperature range.
5 Metals
Metals offer some advantages over graphite and composite materials as they can be manufactured as thin sheets at a lower cost. In addition, the electrical conductivity of metallic materials (e.g.iron alloys >5 × 106 S cm−1, titanium >2 × 106 S cm−1) is superior to graphite (∼103 S cm−1) and composites (1–300 S cm−1).9 Metallic plates also offer lower permeation rates for H2 than its competitors, in the order of 10−12 cm3 s−1 cm−2 for SS316 plates compared to values of 10−2 cm3 s−1 cm−2 for graphite plates (Table 2).10| Graphite | SS316 | |
|---|---|---|
| Cost/$ kg−1 | 75 | 7 |
| Density/g cm−3 | 2.25 | 8.02 |
| Plate thickness (mm) required to meet the DOE target of $10 per kW (assuming 0.6 W cm−2) | 0.35 | 1.0 |
| Electrical resistivity/Ω cm 10−6 | 6000 | 73 |
| Corrosion current/mA cm−2 | < 0.01 | < 0.1 |
| Gas permeability/cm3 s−1 cm−2 | 10−2–10−6 | <10−12 |
| Thickness of plate for same weight/mm | 2.5–4 | 0.16 |
| Modulus of elasticity/GPa | 10 | 193 |
| Tensile strength/MPa | 15.85 | 515 |
| Thermal conductivity/W m−1K−1 | 23.9 | 16.3 |
The primary cost of bipolar plates is the material cost. The material costs for a stainless steel plate, taking into account the bulk cost, density (Table 2) and typical plate thickness are significantly less than a graphite plate. It is possible to estimate the maximum viable plate thickness based on the DOE target plate cost of $10 per kW assuming a power density of 0.6 W cm−2. On this basis the bipolar plate material cost will exceed $10 per kW for a graphite thickness of only 0.4 mm. For stainless steel plates the raw material costs would be less than $10 per kW for a plate thickness of 1 mm. Of course in practice it would also be necessary to account for the processing and production costs.
5.1 Stainless steel
However, the performance of stainless steels as PEMFC plates is limited due to its inherent characteristics. Stainless steels typically contain small quantities of chromium, which forms a thin layer of oxide (Cr2O3) on the steel surface, inhibiting corrosion. The passive layer, however also leads to increased contact resistance between the plates and the gas diffusion layer. In a fuel cell, the contact resistance can become significant, particularly at the cathode25 where the oxidising environment leads to a thicker passive layer.
In addition to the issue of contact resistance, corrosion can lead to the release of metal ions into the fuel cell which can lead to degradation of performance.26,27 While the presence of metal ions could influence the electrocatlayst performance, the main effect of these ions is on the membrane. Metal ions tend to exchange with protons in the membrane, binding with the associated SO3 sites and reducing the membrane conductivity.26 High valence chromium, iron and nickel ions have been found to cause the most significant effects. In addition, Cu2+ and Fe2+ ions can catalyse the degradation of the membrane by peroxide species generated at the cathode.27
Experiments carried out by Wind et al.28 in single cells at 75 °C, with a Nafion™ 115 membrane, revealed a voltage drop of up to 300 mV after 500 h of operation at 0.7 A cm−2 by using untreated stainless steel 316L. Mallant et al.29 found that PEMFC using stainless steel 316L bipolar plates showed about 10% degradation in performance over 1000 h. Furthermore, Tran et al.30 Hentall et al.,2 Makkus et al.,31Liet al.32 and Pozio et al.33 concluded that bare stainless steel SS316 is not suitable for application as a PEMFC plate material.
Research into steel alloys has shown that the elemental composition of these alloys and the nature of the surface have a significant influence on the fuel cell performance and durability. Lee et al.,34 discussed the importance of surface roughness that creates small potential differences in the PEMFC electrochemical environment leading to a more rapid dissolution of the metallic ions. The authors proposed an electrochemical process to smooth the surface roughness of SS316 improving the corrosion resistance and chemical stability of the plates. Davies et al.12 studied the composition of the superficial passive film on several types of austenitic stainless steel aiming to find the relation between the electrical resistance and the oxide film and found that the passive film thickness, determined by Auger Electron Spectroscopy analysis, decreased according to the sequence 321 > 304 > 347 > 316 > 310 > 904L > Incoloy 800 > Inconel 601, findings that corresponded to the trend observed in interfacial contact resistance. Despite exhibiting reduced interfacial resistance Incoloy and Inconel are prohibitively expensive to be used at large volumes.
In a later study, Davies et al.35 presented results from long term tests (over 3000 h) of alloys 904L and 310, showing that these alloys can produced higher fuel cell performance than 316. By means of AES, he showed that the oxide film is thicker in stainless steel containing less Ni and Cr and that increased performance is related to the decreased thickness of the oxide film. A thicker oxide film may offer a longer anti corrosion life but the surface conductivity will be lower, consequently a thin but dense oxide film is preferred. Wang et al.36 concluded that stainless steel 349™ exhibited the best behaviour over other stainless steel alloys such as 904L, 317L, 316L for both the cathode and anode environment.
The corrosion rate of austenitic/ferritic Duplex 2205 stainless steel was investigated by Wang et al.37 in a solution of 1M H2SO4 + 2ppm F− at 70 °C purged either with H2 or air to simulate the environment of a PEMFC plate. This material was found to have a corrosion rate similar to austenitic 349™ and ferritic AISI446.
Titanium must be therefore protected with coatings to preserve its conductivity. Hodgson et al.39 described the use of titanium coated with PEMcoat™ FC5, a proprietary coating developed by INEOS Chlor, showing that the contact resistance of this material is similar to graphite and far superior to uncoated stainless steel 316. Using this material, lifetimes in excess of 8000 h were achieved, with fuel cell power densities in a single cell of 1.8 kW dm−3 and >1 kW kg−1. Moore et al.40 also tested titanium coated with PEMcoat™ FC5 for over 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h with no significant drop in performance observed.
000 h with no significant drop in performance observed.
Overall titanium is a relatively expensive material better suited for aerospace applications rather than low cost automotive use.41
| Material | Cost/US$ kg−1 | Density/g cm−3 | Plate thickness for $10 kW−1 material cost/mm |
|---|---|---|---|
| Graphite | 105 | 1.79 | 0.25 |
| Aluminium | 8.8 | 2.7 | 2.5 |
Nevertheless, some researchers have expressed concerns about the stability of aluminium as a bipolar plate material. Hentall et al.2 stated that aluminium had proved reactive even when coated with gold.
Shanian and Savadogo45 reported the Ni–50Cr alloy as a very stable and electrically conductive material, with minimum corrosion rates and low electrical resistivity values when compared to other stainless steels alloys (Table 4), although cost could be an issue.
| Cost/C$ kg−1 | Corrosion rate/yr−1 | Electrical resistivity/µΩ cm | H2 permeability | |
|---|---|---|---|---|
| 316 | 5.089 | 0.081 | 71 | 5.1 |
| 316L | 5.184 | 0.081 | 69 | 2.2 |
| 304 | 5.99 | 0.081 | 77 | 5.4 |
| 317L | 7.142 | 0.23 | 74 | 5.3 |
| 310 | 10.83 | 0.081 | 80 | 5.4 |
| 446 | 4.954 | 0.105 | 65 | 0.69 |
| 444 | 5.53 | 0.105 | 57 | 0.69 |
| 436 | 5.69 | 0.105 | 55 | 0.69 |
| 434 | 5.76 | 0.105 | 62 | 0.69 |
| Ni–50Cr | 10.37 | 0.005 | 40 | 4.2 |
| TiN | 34.56 | 0.061 | 60.3 | 0.32 |
| AuAl | 50 | 2 | 3.9 | 160 |
5.2 Coatings
To overcome oxide formation and ion dissolution for metal plate materials such as stainless steel, aluminium and titanium, various protective coatings have been employed. These coatings aim to be impermeable to the reactant gases and chemically inert, providing low contact resistance and offering good corrosion resistance in order to withstand the aggressive fuel cell environment. In many cases the integrity of the coating can be a key issue, as cracks or pinoles can lead to accelerated corrosion of the substrate. The various coatings that have been reported in the literature are discussed below.In spite of this concern, gold, which is a chemically stable and very conductive material (45 × 106 S cm−1), has been successfully used as a coating for PEMFC bipolar plates. It has been found to decrease the contact resistance of passive metals such as stainless steel, aluminium, titanium and their alloys. Lu and Wang56 used stainless steel bipolar plates with a gold coating of 0.5 µm in direct methanol fuel cells and obtained excellent results. Hentall et al.2 reported a superior performance of gold coated stainless steel 316L plates compared to that of Poco™ graphite when being tested in a single PEMFC. Wind et al.28 has also reported no deterioration of the cell voltage from gold coated stainless steel 316L plates after 1000 h of operation, with no difference in performance compared to PEMFC using graphite plates. Several patent applications have claimed low corrosion currents from gold coated stainless steel with thicknesses in the range of 10–20 nm.57,58 Hornung and Kappelt59 found that gold coated nickel bipolar plates offered contact resistance values low enough (2 mΩ cm2 at 21 bar) to perform in fuel cell applications and achieving the DOE target for contact resistance (<10 mΩ cm2 at 140 N cm−2).
Hentall et al.2 reported similar initial performance of PEMFC using gold coated aluminium and graphite plates (1.2 A cm−2 at 0.5 V) although the performance of the cells using the gold coated aluminium plates deteriorated very quickly. It was observed that some of the gold layer lifted from the plate and the membrane became contaminated with aluminium. Woodman et al.41 also studied aluminium plates coated with gold by electrodeposition, with thicknesses of 1 to 10 µm. The performance of these plates was equivalent to that of graphite when pulse current deposition was used but poorer performance was obtained when continuous current deposition was employed.
All the reported studies carried out with gold coatings showed that a very thin layer of gold is sufficient to prevent corrosion and to improve electrical conductivity. However, despite the fact that gold coatings offer excellent results when performing in the fuel cell environment, its high price is a major disadvantage for economies of mass production. Based on a power density of 0.6 W cm−2, the material cost of the gold coating alone amounts to more than the DOE target of $10 per kW for a coating thickness of 0.4 µm. It is clear that the very thin (of order 10–50 nm) coatings are most likely to offer a viable material.
The feasibility of using thermally nitride coated AISI 446 was studied by Wang et al.61 The nitrification process created a modification of the native oxide layer increasing the corrosion resistance, however the interfacial contact resistance was 40 mΩ cm2 at 150 N cm−2, above the DOE targets of 10 mΩ cm2 at 140 N cm−2 (Table 1).
Brady et al.62 at the Oak Ridge National Laboratory have recently developed a thermal nitridation process to form pinhole free CrN/CrN2 coatings on a Ni–Cr alloy. The process is achieved by diffusing nitrogen into the substrate and has the advantage that the layer does not peel off mechanically. The result is a chromium nitride surface layer which exhibits excellent corrosion resistance, little metal dissolution and negligible contact resistance increase over the course of a 4100 h exposure in a 80 °C sulfuric acid environment. In the same line of research, Brady et al.63 studied the nitration of several nickel chromium alloys such as Ni–50Cr and Ni–30Cr with promising results. These alloys are however very expensive for PEM applications and research is being directed towards developing Fe–Ni bases that can form similar protective chromium nitride surfaces.
Similarly, sputtered coatings of CrB2 have been reported by Dahm et al.,68 who observed excellent wear and corrosion resistance. There has also been recent work on ZrB2 thin films obtained by chemical vapour deposition.69
Another interesting boride alloy NiCoB has been investigated by Gamboa et al.70 They studied the corrosion behaviour of a NiCoB coating on carbon steel 1018, aluminium A6061 and stainless steel 304 in simulated PEMFC conditions of 0.5M H2SO4 at 60 °C using polarization curves, LPR, EIS and OCP measurements. In all cases the corrosion resistance of NiCoB coated alloys was superior to the uncoated alloy, with up to two orders of magnitude improvement for the carbon steel and one order of magnitude for the stainless steel.
Futhermore, researchers at Sumitomo Ltd71 have developed and patented stainless steel bipolar plates with metallic inclusions of carbide and boride protruding through the outer surface of the passive film for use in PEMFC, claiming superior electrical conductivity.
Although these materials may offer durability, they add significant cost to prepare and increase contact resistance respect to the bare substrate. The cost of diamond coatings has been estimated77,78 to be around $10 cm−2, around three orders of magnitude higher than the DOE target.
Huang et al.11 evaluated SS316 coated with Zr, ZrN, ZrNb and ZrNAu. Experimental results showed that only the Zr/SS316 satisfied the corrosion DOE goals at cathode conditions and only the ZrNAu/SS316 satisfied the DOE contact resistance targets. Despite their benefits one of the main disadvantages of these materials for commercial applications is their relatively high cost.26
6 Conclusions
Of the DOE targets in Table 1, the corrosion rate, durability and contact resistance are the key performance parameters for bipolar plate materials. Table 4 shows these properties for all of the materials discussed in this paper for which data is available, compared against the DOE targets. The contact resistance data in Table 4 were all reported at a pressure of 140 N cm−2. The conditions used for corrosion testing varied and the data should therefore be considered to be indicative only. Measurements were typically carried out in sulfuric acid solution (0.1 to 1 M) and in some cases a few ppm of fluoride ions was added. Although the conditions do vary slightly, the corrosion data shown in Table 5 is useful in giving an indicative comparison of alternative materials relative to the DOE targets.| Plate material | Corrosion | Durability | Contact resistance | References | |||
|---|---|---|---|---|---|---|---|
| a indicates that there is insufficient or no data available. | |||||||
| DOE targets 2010(6,35) | <16 µA cm−2 | <95 µm year−1 | 5000 h (<10% drop in power) | <10 mΩ cm2 at 140 N cm2 | 10,46 | ||
| Graphite | Poco graphite | <10 µA cm−2 | <15 µm year−1 | 2.9% after 1300 h ∼2% after 5000 h (mainly due to membrane degradation ) | 15 mΩ cm2 | 10,12,41,13 | |
| Metals | Austenitic SS | SS316 | 100–500 µA cm−2 | <100 µm year−1 | 10% after 1000 ha | 50–150 mΩ cm 2 | 12,29,25,35,36,41,49,60 |
| SS304 | 10 µA cm−2 | a | 100 mΩ cm2 | 12,49 | |||
| SS310 | a | a | 1.5% after 1300 ha | 42 mΩ cm2 | 12 | ||
| SS317 | 20–40 µA cm−2 | a | 147 mΩ cm2 | 36 | |||
| SS321 | a | a | a | 190 mΩ cm2 | 12 | ||
| SS347 | a | a | a | 100 mΩ cm2 | 12 | ||
| 904L | 10–20 µA cm−2 | a | 40–135 mΩ cm 2 | 12,36 | |||
| 349™ | 10 µA cm−2 | a | 110 mΩ cm2 | 36 | |||
| Ferritic SS | AISI434 | 100–200 µA cm−2 | a | 150 mΩ cm2 | 25 | ||
| AISI436 | 20–60 µA cm−2 | a | 100 mΩ cm2 | 25 | |||
| AISI441 | 60–300 µA cm−2 | a | 100 mΩ cm2 | 25 | |||
| AISI444 | 20–50 µA cm−2 | a | 100 mΩ cm2 | 25 | |||
| AISI446 | 8–20 µA cm−2 | a | 200 mΩ cm2 | 25 | |||
| Duplex 2205 | a | a | a | 130 mΩ cm 2 | 84 | ||
| Incoloy 800 | a | a | a | 37 mΩ cm 2 | 12 | ||
| Inconel 601 | a | a | a | 20 mΩ cm2 | 12 | ||
| Amorph. alloys | Fe41Cr18Mo14Y2C15B6N4 | 5–36 µA cm−2 | a | 10 mΩ cm2 | 38 | ||
| Fe50Cr18Mo8Y2C14B6Al2 | 100–182 µA cm −2 | a | 15mΩ cm 2 | 38 | |||
| Titanium | Ti | <100 µm year−1 | 32% after 1300 ha | 50 mΩ cm2 | 12,41 | ||
| Aluminium | Al | 250 µm year−1 | a | a | 41 | ||
| Aluminium alloy | Al 5052 | 1100 µm year−1 | a | a | 85 | ||
| Nickel alloys | P–Ni | <30 µm year−1 | a | a | 41 | ||
| Ni–50Cr | a | a | a | 50 mΩ cm2 | 61 | ||
| Copper alloys | C-17200 | 0.3 µA cm−2 | a | a | 47 | ||
| Coatings | Polymers | PPY/304 | 1 µA cm−2 | a | 800 mΩ cm2 | 49 | |
| PANI/304 | 0.1 µA cm−2 | a | 800 mΩ cm2 | 49 | |||
| Gold | 316L/Au | 5.65 µA cm−2 | 0% after 1000 ha | 5 mΩ cm2 (>10 nm Au) | 11,28 | ||
| Nitrides | Ni–50Cr/CrNCr2N | a | a | a | <20 mΩ cm2 | 61 | |
| SS349™/CrNCr2N | a | a | a | 10 mΩ cm2 | 61 | ||
| SS446/CrNCr2N | a | a | a | 6–40 mΩ cm 2 | 61,84 | ||
| SS316L/CrNCr2N | <10 µA cm−2 | a | 10 mΩ cm2 | 60 | |||
| SS316/TiN | a | a | 11% after 1000 h (stack)a | 40 mΩ cm2 | 14 | ||
| Refractory metals | Zr/SS316 & ZrN/SS316 | a | a | a | 150–1000 mΩ cm2 | 11 | |
Little data is available regarding the durability of materials. Graphite and gold coated electrodes have been shown to meet the DOE target.13,83 However, none of the other metal plate materials have been tested for the 5000 h timescale specified in the DOE targets.
Only a few materials meet the target for contact resistance and of these, the only material which has been shown to meet the corrosion target is gold coated SS316. The aggressive fuel cell environment severely restricts the choice of materials for PEMFC bipolar plates. It is likely only carbon–polymer composites and coated metal systems will meet in the future the long term cost requirements needed for automotive fuel cells.
Companies and institutions such as The Oak Ridge National Laboratory, Da Lian Institute of Physical Chemistry, Denora, Intelligent Energy Ltd, Sarnoff Corporation and GenCell Corporation have made substantial efforts towards developing metallic bipolar plates,26 while polymer composites bipolar plates are being sold by companies such as DuPont, H2 Economy, ICM Plastics, NedStack, etc evidence that both routes are developing new materials and maturing. Although significant advances have been made to date, there is still a need for further research and development in order to overcome the significant deficiencies of currently available materials.
References
- G. J. K. Acres, J. Power Sources, 2001, 100, 60–66 CrossRef CAS.
- P. L. Hentall, J. B. Lakeman, G. O. Mepsted, P. L. Adcock and J. M. Moore, J. Power Sources, 1999, 80, 235–241 CrossRef CAS.
- H. Tsuchiya and O. Kobayashi, Int. J. Hydrogen Energy, 2004, 29, 985–990 CrossRef CAS.
- E. Carlsson, P. Kopf, J. Sinha, S. Sriramul and Y. S. Yang, Fuel Cell Seminar, 2005, Palm Springs, CA Search PubMed.
- I. Bar-On, R. Kirchain and R. Roth, J. Power Sources, 2002, 109, 71–75 CrossRef CAS.
- F. D. Lomax Jr, B. D. James, G. N. Baum and C. E. Thomas, Detailed Manufacturing Cost Estimates for Polymer Electrolyte Membrane (PEM) Fuel Cells for Light Duty Vehicles, Directed Technologies Inc.Arlington, VA 1998 Search PubMed.
- K. S. Jeong and B. S. Oh, J. Power Sources, 2002, 105, 58–61 CrossRef CAS.
- C. A. Reiser, L. Bregoli, T. W. Patterson, J. S. Yi, J. D. L. Yang, M. L. Perry and T. D. Jarvi, Electrochem. Solid State Lett., 2005, 8, A273–A276 CrossRef CAS.
- B. C. H. Steele and A. Heinzel, Nature, 2001, 414, 345–352 CrossRef CAS.
- B. R. Padhy and R. G. Reddy, J. Power Sources, 2006, 153, 125–129 CrossRef CAS.
- W. Yoon, X. Huang, P. Fazzino, K. L. Reifsnider and M. A. Akkaoui, J. Power Sources, 2008, 179, 265–273 CrossRef CAS.
- D. P. Davies, P. L. Adcock, M. T. Turpin and S. J. Rowen, J. Appl. Electrochem., 2000, 30, 101 CrossRef CAS.
- S. J. C. Cleghorn, D. K. Mayfield, D. A. Moore, J. C. Moore, G. Rusch, T. W. Sherman, N. T. Sisofo and U. Beuscher, J. Power Sources, 2006, 158, 446–454 CrossRef CAS.
- E. A. Cho, I. H. Oh, U. S. Jeon, S. A. Hong and S. G. Kang, J. Power Sources, 2005, 142, 177–183 CrossRef CAS.
- B. Cunningham and D. G. Baird, J. Mater. Chem., 2006, 16, 4385–4388 RSC.
- T. M. Besmann, J. W. Klett, J. J. Henry and E. Lara-Curzio, J. Electrochem. Soc., 2000, 147(11), 4083–4086 CAS.
- A. Müller, P. Kauranen, A. von Ganski and B. Hell, J. Power Sources, 2006, 154, 467–471 CrossRef.
- S. H. Liao, C. Y. Yen, C. H. Hung, C. C. Weng, M. C. Tsai, Y. F. Lin, C. C. M. Ma, C. Pan and A. Su, J. Mater. Chem., 2008, 18, 3993–4002 RSC.
- M. G. Miller, J. M. Keith, J. A. King, B. J. Edwards and N. Klinkenberg, Polym. Compos., 2006, 27, 388–394 Search PubMed.
- S. Bouatia, F. Mighri and M. Bousmina, Fuel Cells, 2008, 8, 120–128 CrossRef CAS.
- B. D. Cunningham and D. G. Baird, J. Power Sources, 2007, 168, 418–425 CrossRef CAS.
- A. Hermann, T. Chaudhuri and P. Spagnol, Int. J. Hydrogen Energy, 2005, 30, 1297–1302 CrossRef CAS.
- J. Adams, Fuel cells for transportation programme, US DOE's Fuel Cells for Transportation Programme. Contractors Annual Progress Report, 1998 Search PubMed.
- W. M. Yang, S. K. Chou and C. Shu, J. Power Sources, 2007, 164, 549–554 CrossRef CAS.
- H. Wang and and J. A. Turner, J. Power Sources, 2004, 128, 193–200 CrossRef.
- L. Ma, S. Warthesen and D. A. Shores, J. New Mater. Electrochem. Syst., 2000, 3, 221–228 Search PubMed.
- A. B. LaConti, M. Hamdan and R. C. McDonaldin Handbook of Fuel Cells - Fundamentals, Technology and Applications, ed. W. Vielstich, H. A. Gasteiger and A. Lamm, Wiley, New York, 2004, vol. 3, 647–662 Search PubMed.
- J. Wind, R. Späh, W. Kaiser and G. Böhm, J. Power Sources, 2002, 105, 256–260 CrossRef CAS.
- R. K. A. M. Mallant, F. G. H. Koene, C. W. G. Verhoeve and A. Ruiter, Fuel Cell Seminar, San Diego, CA, 1994 Search PubMed.
- D. T. Tran, O. A. Velev, I. J. Kakwan, S. Gamburzev, F. Simoneaux, T. R. Lalk and S. Srinivasan, Electrochem. Soc. Abstr. 190th Fall Meeting, San Antonio, TX, 1996 Search PubMed.
- R. C. Makkus, A. H. H. Janssen, F. A. de Brujin and R. K. A. M. Mallant, J. Power Sources, 2000, 86, 274–282 CrossRef CAS.
- M. C. Li, C. L. Zeng, S. Z. Luo, J. N. Shen, H. C. Lin and C. N. Cao, Electrochim. Acta, 2003, 48, 1735–1741 CrossRef CAS.
- A. Pozio, R. F. Silva, M. de Francesco and L. Giorgi, Electrochim. Acta, 2003, 48, 1543–1549 CrossRef CAS.
- S. J. Lee, J. J. Lai and C. H. Huang, J. Power Sources, 2005, 145, 362–368 CrossRef CAS.
- D. P. Davies, P. L. Adcock, M. Turpin and S. J. Rowen, J. Power Sources, 2000, 86, 237–242 CrossRef CAS.
- H. L. Wang, M. A. Sweikart and J. A. Turner, J. Power Sources, 2003, 115, 243–251 CrossRef CAS.
- H. L. Wang, G. Teeter and J. A. Turner, J. Electrochem. Soc., 2005, 152(3), B99–B104 CrossRef CAS.
- E. Fleury, J. Jayaraj, Y. C. Kim, H. K. Seok, K. Y. Kim and K. B. Kim, J. Power Sources, 2006, 159, 34–37 CrossRef CAS.
- D. R. Hodgson, B. May, P. L. Adcock and D. P. Davies, J. Power Sources, 2001, 96, 233–235 CrossRef CAS.
- J. M. Moore, J. B. Lakeman and G. O. Mepsted, J. Power Sources, 2002, 106, 16–20 CrossRef CAS.
- A. S. Woodman, E. B. Anderson, K. D. Jayne and M. C. Kimble, American Electroplaters and Surface Finishers Society, AESF SUR/FIN ′99 Proc., 1999, 6, 21–24 Search PubMed.
- H. Tawfik, Y. Hung and D. Mahajan, J. Power Sources, 2007, 163, 755–767 CrossRef CAS.
- S. Frangini and A. Masci, Surf. Coat. Technol., 2004, 184, 31–39 CrossRef CAS.
- H. Shimada and K. Ebihara, Jpn. Pat., JP2005302669-A, 2005 Search PubMed.
- A. Shanian and O. Savadogo, J. Power Sources, 2006, 159, 1095–1104 CrossRef CAS.
- R. G. Reddy and V. V. Nikam, J. Power Sources, 2005, 152, 146–155 CrossRef.
- V. V. Nikam and R. G. Reddy, Int. J. Hydrogen Energy, 2006, 31, 1863–1873 CrossRef CAS.
- R. G. Reddy and V. V. Nikam, Electrochim. Acta, 2006, 51, 6338–6345 CrossRef.
- S. Joseph, J. C. McClure, R. Chianelli, P. Pich and P. J. Sebastian, Int. J. Hydrogen Energy, 2005, 30, 1339–1344 CrossRef CAS.
- T. Tüken, G. Arslan, B. Yazini and M. Erbil, Corros. Sci., 2004, 46, 2743–2754 CrossRef CAS.
- J. E. P. da Silva, S. I. C. de Torresi and R. M. Torresi, Corros. Sci., 2005, 47, 811–822 CrossRef.
- K. Uehara, T. Ichikawa, T. Serikawa, S. Yoshikawa, S. Ehara and M. Tsunooka, Thin Solid Films, 1998, 322, 198–205 CrossRef CAS.
- D. E. Tallman, Y. Pae and G. P. Bierwagen, Corrosion, 2000, 56, 401–410 Search PubMed.
- D. E. Tallman, C. Vang, D. D. Wallace and G. P. Bierwagen, J. Electrochem. Soc., 2002, 149, C173–C179 CrossRef CAS.
- V. Brusic, M. Angelopulos and T. Graham, J. Electrochem. Soc., 1997, 144, 436–442 CrossRef.
- G. Q. Lu and C. Y. Wang, J. Power Sources, 2005, 144, 141–145 CrossRef CAS.
- M. Utsunomiya, M. Tsuji, T. Kuwayama and T. Ohtani, US Pat., US7,214,440, Honda Motors Co, 2004 Search PubMed.
- G. Vyas, Y. T. Cheng, M. H. Abd Elhamid and Y. M. Mikhail, US Pat., US6,866,958, 2003 Search PubMed.
- R. Hornung and G. Kappelt, J. Power Sources, 1998, 72, 20–21 CrossRef CAS.
- R. J. Tian, J. C. Sun and L. Wang, Int. J. Hydrogen Energy, 2006, 31, 1874–1878 CrossRef CAS.
- H. Wang, M. P. Brady, G. Teeter and J. A. Turner, J. Power Sources, 2004, 138, 86 CrossRef CAS.
- M. P. Brady, B. Yang, H. Wang, J. A. Turner, K. L. More, M. Wilson and F. Garzon, J. Min. Metals. Mater. Soc., 2006, 58, 50–57 Search PubMed.
- M. P. Brady, B. Yang, P. F. Tortorelly and K. L. More, FY2005 Progress Report, DOE Hydrogen Program, 2005 Search PubMed.
- J. Castaing and P. Costa. in Boron and Refractory Borides. ed. V. I. Matkovich, Springer, Berlin, 1977 Search PubMed.
- C. Mitterer, J. Solid State Chem., 1997, 133, 279–291 CrossRef CAS.
- A. B. LaConti, A. E. Griffith, C. C. Cropley and J. A. Kosek, US Pat., US6,083,641, 2000 Search PubMed.
- J. K. Lee, H. J. Kweon and J. W. Suh, Jpn. Pat. Appl., JP2006156386, 2006 Search PubMed.
- K. L. Dahm, L. R. Jordan, J. Haase and P. A. Dearnley, Surf. Coat. Technol., 1998, 109, 413–418 CrossRef.
- S. Jayaraman, E. J. Klein, Y. Yang, D. Y. Kim, G. S. Girolami and J. R. Abelson, J. Vac. Sci. Technol., A, 2005, 23, 631–633 CrossRef CAS.
- S. A. Gamboa, J. G. Gonzalez-Rodriguez, E. Valenzuela, B. Campillo, P. J. Sebastian and A. Reyes-Rojas, Electrochim. Acta, 2006, 51, 4045–4051 CrossRef CAS.
- Y. Tarutani, T. Doi, A. Seki and S. Fukuta, US Pat., US6,379,476, 2002 Search PubMed.
- H. Wang, J. A. Turner, X. Li and R. Bhattacharya, J. Power Sources, 2007, 171, 567–574 CrossRef CAS.
- M. H. A. Elhamid, G. Vyas, Y. T. Cheng and R. H. Blunk, Wld. Pat. Appl., WO2006055146, 2006 Search PubMed.
- M. M. Morshed, B. P. McNamara, D. C. Cameron and M. S. J. Hashmi, J. Mater. Process. Technol., 2003, 141, 127–131 CrossRef CAS.
- T. Iijima, M. Okada and K. Kubomura. Jpn. Pat., JP2005093172, 2005 Search PubMed.
- S. J. Lee, C. H. Huang and Y. P. Chen, J. Mater. Process. Technol., 2003, 140, 688–693 CrossRef CAS.
- J. V. Busch and J. P. Dismukes, Diamond Relat. Mater., 1994, 3, 295–302 CrossRef.
- I. A. Martorell, W. D. Partlow, R. M. Young, J. J. Schreurs and H. E. Saunders, Diamond Relat. Mater., 1999, 8, 29–36 CrossRef CAS.
- A. B. LaConti, A. R. Fragala and J. R. Boyack, J. Electrochem. Soc., 1977, 124, C132–C132.
- P. Costamagna and S. Srinivasan, J. Power Sources, 2001, 102, 253–269 CrossRef CAS.
- L. Gladczuk, C. Joshi, A. Patel, J. Guiheen, Z. Iqbal and M. Sosnowski, Mater. Res. Soc. Symp. Proc., 2003, 756, 423–428 CAS.
- W. Qu, L. Jian, D. G. Ivey and J. M. Hill, J. Power Sources, 2006, 157, 335–350 CrossRef CAS.
- S. Yoshioka, A. Yoshimura, H. Fukumoto, O. Hiroi and H. Yoshiyasu, J. Power Sources, 2005, 144, 146–151 CrossRef CAS.
- M. S. Wilson, C. Zawadzinski, S. Moller-Holst, D. N. Busick, F. A. Uribe and T. Zawodzinski, Proc. 1999 US DOE Hydrogen Program Annu. Rev., 1999 Search PubMed.
- S. J. Lee, C. H. Huang, Y. P. Chen and Y. M. Chen, J. Fuel Cell Sci. Technol., 2005, 2, 290–294 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2009 |
