Distribution of guest molecules in Pluronic micelles studied by double electron electron spin resonance and small angle X-ray scattering†
Sharon
Ruthstein
a,
Arnold M.
Raitsimring
b,
Ronit
Bitton
c,
Veronica
Frydman
d,
Adelheid
Godt
e and
Daniella
Goldfarb
*a
aDepartment of Chemical Physics, Weizmann Institute of Science, Rehovot 76100, Israel
bDepartment of Chemistry, University of Arizona, Tucson, AZ 85721, USA
cDepartment of Chemical Engineering, Ben-Gurion University of the Negev, 84105, Beer Sheva, Israel
dChemical Research Support Unit, Weizmann Institute of Science, Rehovot 76100, Israel
eBielefeld University, Faculty of Chemistry, Organic Chemistry, Universitätsstr. 25, D-33615, Bielefeld, Germany
First published on 29th October 2008
Abstract
Pulse double electron–electron spin resonance (DEER) measurements were applied to characterize the distribution and average number of guest-molecules (in the form of spin-probes) in Pluronic P123 micelles. Two types of spin-probes were used, one of which is a spin-labeled P123 (P123-NO), which is similar to the micelles constituent molecules, and the other is spin-labeled Brij56 (Brij56-NO) which is significantly different. Qualitative information regarding the relative location of the spin-labels within the micelles was obtained from the isotropic hyperfine coupling and the correlation times, determined from continuous wave EPR measurements. In addition, complementary small angle X-ray scattering (SAXS) measurements on the P123 micellar solutions, with and without the spin-probes, were carried out for an independent determination of the size of the core and corona of the micelles and to ensure that the spin-probes do not alter the size or shape of the micelles. Two approaches were used for the analysis of the DEER data. The first is model free, which is based on the determination of the leveling off value of the DEER kinetics. This provided good estimates of the number of radicals per micelle (low limit) which, together with the known concentration of the P123 molecules, gave the aggregation number of the P123 micelles. In addition, it provided an average distance between radicals which is within the range expected from the micelles' size determined by SAXS. The second approach was to analyze the full kinetic form which is model dependent. This analysis showed that both spin-labels are not homogeneously distributed in either a sphere or a spherical shell, and that large distances are preferred. This analysis yielded a slightly larger occupation volume within the micelle for P123-NO than for Brij56-NO, consistent with their chemical character.
Introduction
Water-soluble triblock copolymers of poly(ethylene oxide) (PEO) and poly(propylene oxide) (PPO), often denoted as Pluronics PEOn–PPOx–PEOn, are commercially available non-ionic macromolecular surface active agents.1,2 Variation of the copolymer composition (PPO/PEO ratio) and molecular weight (PEO and PPO block length) produces molecules with optimized properties that often meet the specific requirements for various technological applications. Consequently, PEOn–PPOx–PEOn block copolymers comprise an important class of surfactants that find widespread industrial applications in detergency, dispersion stabilization, foaming, emulsification, lubrication, and formulation of cosmetics.1 Another important application of these block-copolymers is also their use as templates in the synthesis of ordered mesoporous materials.3–6The water solubility of the Pluronics is highly dependent on temperature. At low temperatures both the PEO and PPO blocks are significantly hydrated and, below a critical micellization temperature (CMT), the polymers are present mainly as soluble individual single chains (unimers). As the temperature increases, the PPO groups become increasingly hydrophobic and above the CMT, micelles are formed.1,7–10 The PPO block comprises the hydrophobic core of the micelles and the PEO blocks the hydrophilic corona.
One of the most useful properties of the Pluronic micelles is their ability to take up hydrophobic guest molecules, which are otherwise only sparingly soluble in water. The micelle’s core serves as a compatible micro-environment for the water-insoluble guest molecules, thereby enhancing their solubility in water. This process, referred to as solubilization,9,10 is important for applications such as drug delivery.11,12 For this purpose the knowledge of physico-chemical properties of the micelles such as CMT and CMC (critical micelles concentration), micelles dimensions, the effect of the guests on these properties, and the amount and partitioning of the guests within the micelles, is required. The solubilization characteristics of the Pluronics also allow introducing various probes, such as magnetic or fluorescence probes, to explore various local properties of the micelles13 and follow processes such as the formation mechanism of mesoporous materials.14,15 Several studies dealt with solubilization of hydrophobic guests in block copolymer micelles and their effect on the CMC, CMT and aggregation number using techniques such as SANS (small angle neutron scattering), SAXS (small angle X-ray scattering), dynamic light scattering (DLS), fluorescence and NMR spectroscopy.9,13,16–19
Traditionally, micelles' properties have also been investigated by EPR of nitroxide spin-probes, focusing mainly on the dynamic characteristics determined using lineshape analysis and polarity gradients obtained from the hyperfine coupling constant.20,21Electron spin echo envelope modulation (ESEEM) spectroscopy has also been applied to investigate structural properties of micelles such as the water penetration profile.22,23 Double electron–electron resonance (DEER) is a pulse EPR method which is capable of recovering dipolar couplings between electron spins, and thus it can provide distances in the nm range.24–28 Consequently, it has been applied to investigate the distribution of radicals in irradiated solids and of spin-probes in polymers for identifying and characterizing the formation of clusters.24–26,29,30 In a recent study we demonstrated that DEER can also be used to explore the properties of micelles of Pluronics31 and that it can detect variations in micelles’ dimensions during the formation of mesoporous materials prepared with Pluronic templates.32 In these early works the spin-probe used was 4-hydroxy-TEMPO-benzoate (4HTB), which is a small hydrophobic molecule localized in the hydrophobic core of the micelles.32,33 The DEER measurements gave estimates of the number of spin-probes in the core and the volume of the core of the micelles.31 In the present work we employ DEER to study the distribution of guest molecules in P123 micelles using different classes of spin-labeled guest molecules; specifically comparing Brij56-NO and P123-NO (see Fig. 1) which are considerably larger than 4HTB and located in different regions of the Pluronic micelles. P123-NO is a labeled Pluronic, which is highly similar to the molecules constituting the micelles and the label is located at the corona of the micelle. Brij56-NO has a highly hydrophobic alkyl chain, expected to locate the spin-label at the core–corona interface.
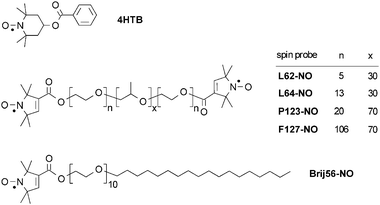 | ||
| Fig. 1 The spin-probes used in this study. | ||
The DEER technique works very well for distance determination of a pair of radicals with a narrow distribution of distances. In this case, the DEER kinetics exhibits modulation at a frequency characteristic of the dipolar interaction, which can be readily translated into the distance between the radicals. However, when the distance between the radicals has a large distribution and the number of interacting radicals becomes larger than two, as encountered in clusters and micelles, the DEER kinetics becomes featureless and the extraction of the distance distribution and the number of interacting spins becomes more involved and often model dependent. This essentially limits the data analysis that aims at providing the size of the cluster and the number of radicals in the cluster.
Here we present an experimental approach which allowed us to carry out a model free analysis of the DEER data to estimate the average number of spin-probes (guest molecules) in the micelles, the volume they occupy and the aggregation number of the micelle. This is achieved by performing a series of DEER measurements as a function of microwave power and pulse duration and of the concentration of the spin-probes. In addition to the DEER experiments, we present SAXS results which shows that the spin-probes added did not affect the micelles dimensions and provide an independent measure of the size of the core and corona of the micelles to validate and complement the DEER results.
Experimental
Sample preparation
Pluronic P123 (EO20PO70EO20, Mav = 5800) was a gift from BASF Corp. (USA). The spin-probes L62-NO, L64-NO, P123-NO, F127-NO and Brij56-NO were synthesized as described in the literature34 and 4-hydroxy-TEMPO-benzoate (4HTB) was purchased from Aldrich. Fig. 1 depicts the structure of the spin-probes used. The triradical (Fig. S2b)† has been synthesized as described earlier35 and has been investigated by DEER.36 The biradical (Fig. S2a)† was kindly given to us by Prof. G. Jeschke.The samples for DEER and ESEEM measurements were prepared as follows![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) the spin-probe was dissolved in 4 wt% P123 solution in D2O at 50 °C; after stirring for approximately 1 h, a sample from the solution was placed in a 3 mm od Teflon tube (for X-band measurements) or in a thin wall, 2.9 mm od quartz tubes (for Ka-band measurements). The samples were kept at 50 °C in a hot water bath for 20 min and then frozen rapidly by placing the tubes in a 2-methyl butane bath cooled by liquid nitrogen. The samples for X-band continuous wave (CW) EPR were prepared similarly, except for the freezing part, and flat cells were used. The solutions made were of Brij56-NO or P123-NO dissolved in a 4 wt% P123 solution in D2O at three total spin concentrations, 0.3, 0.5 and 0.7 mM. The latter corresponds to a molar ratio of 10
the spin-probe was dissolved in 4 wt% P123 solution in D2O at 50 °C; after stirring for approximately 1 h, a sample from the solution was placed in a 3 mm od Teflon tube (for X-band measurements) or in a thin wall, 2.9 mm od quartz tubes (for Ka-band measurements). The samples were kept at 50 °C in a hot water bath for 20 min and then frozen rapidly by placing the tubes in a 2-methyl butane bath cooled by liquid nitrogen. The samples for X-band continuous wave (CW) EPR were prepared similarly, except for the freezing part, and flat cells were used. The solutions made were of Brij56-NO or P123-NO dissolved in a 4 wt% P123 solution in D2O at three total spin concentrations, 0.3, 0.5 and 0.7 mM. The latter corresponds to a molar ratio of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for P123
1 for P123![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Brij56-NO and 20
Brij56-NO and 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for P123-NO.
1 for P123-NO.
EPR spectroscopic measurements
The EPR measurements were performed at X (∼9.4 GHz) and Ka (∼30–34 GHz)-bands. CW EPR spectra were recorded using a X-band Bruker Elexsys 500 spectrometer. The pulse X-band EPR measurements, (ESEEM and DEER) were carried out on a Bruker Elexsys E580 spectrometer and the Ka-band DEER experiments were performed with the home-built spectrometer of the University of Arizona.37 The three-pulse ESEEM sequence, π/2-τ-π/2-T-π/2-τ-echo, was employed with a 4-step phase cycling.38 The π/2 pulse length was 20 ns, and τ was set to 224 ns, to maximize the 2H modulation depth and the field was set to maximum echo intensity (MI = 0 component). The modulation depth was taken as k = (a/(a + b)), where a + b is the interpolated echo intensity between the first and second maxima, and b is the echo intensity at the first minimum.14The triradical and biradical homogeneous solutions were measured at the X-band using the constant time four-pulse DEER sequence, (π/2)(νobs)-τ1-π(νobs)-t-π(νpump)-(τ1+τ2-t)-π(νobs)-τ2-echo.30 A two step phase cycle was employed on the first pulse, and averaging over 25 increments of τ1 (τ1 = 200 ns, Δτ1 = 8 ns) was carried out to suppress proton and deuterium modulations. The echo was measured as a function of t (Δt = 20 ns) while τ2 was kept constant to eliminate relaxation effects. The pump frequency, νpump, was set to the maximum intensity of the nitroxide spectrum, and the observer frequency, νobs, was 60 MHz higher. Both the π/2 and π pulses (observe and pump) had a length of 40 ns. Typical numbers of shots per point and accumulations are 30 and 10, respectively, and the measurement temperature was 50 K. The flip probability of the pump pulse was determined experimentally using a series of glassy solutions of 4HTB spin-probes in 50/50 methanol/toluene (more details are given below).
The micellar solutions were measured using the three-pulse DEER, (π/2)(νobs)-t-π(νpump)-(τ-t)-π(νobs)-τ-echo. This sequence was chosen because of its better sensitivity, which enables a larger acquisition time interval (at the same S/N) or a better S/N for the same time interval, as compared with the 4-pulse DEER. Due to the large distance distribution in micelles solutions, the DEER kinetics does not exhibit any features that could be lost due to the dead-time. For the X-band measurements the first value of t was 80 ns, Δt = 20 ns, τ = 1.6–2.0 μs and a two-step phase cycle on the first pulse was employed. The set up of the pump and observe frequencies were as above and so were all other experimental conditions except that pump pulse durations of 40 and 28 ns were used.
In the Ka-band DEER measurements the nominal flip angle of the pump pulse was varied between π and ∼π/6. This was achieved by variation of the pump pulse duration from ∼20 to 50 ns as well as of the pulse amplitude. The typical separation between pump and observed frequencies was ∼40–50 MHz, with the pump frequency set to the maximum intensity of the nitroxide spectrum. Here the time interval, τ, was ∼2.5 μs and the first data point collected was at time equal to the sum of the duration of the pump and observed pulses, typically ∼60–80 ns.
Because the evaluation of the magnitude of DEER kinetics becomes highly sensitive even to small background corrections when the decay is large (>e−3), an additional test for the presence of baseline background was made in the Ka-band measurements. This was achieved by performing the DEER measurements in absence of a signal and the trace obtained was used in final data processing to correct the DEER kinetics.
SAXS measurements
Small angle X-ray scattering (SAXS) were performed using a small angle diffractometer (The Molecular Metrology SAXS System) at the Technion-Israel Institute of Technology. Cu Kα radiation is generated by Osmic MicroMax®, an integrated microfocus sealed tube generator (powered at 45 kV and 0.9 mA) with Osmic™ Confocal Max-Flux® optics, 3 pinholes collimation. A 20 × 20 cm2 2D position-sensitive wire detector (1024 × 1024 pixels) was positioned 150 cm behind the examined samples, resulting in a q-range of 0.07 < q < 2.8 nm−1. The wave vector defined as, q = (4π/λ) sin(2θ/2), where 2θ is the scattering angle and λ is wave length of Cu Kα radiation (0.1542 nm). A total of 106 or more counts were collected in order to obtain a high signal to noise ratio. Conversion to absolute units was performed according a procedure described by Zemb et al.39 The 2D SAXS patterns were azimuthally averaged and the background subtracted using standard methods. Data analysis was based on fitting the scattering curve to an appropriate model using a least-squares method.DEER theoretical background
In spin echo based experiments, the evolution time of the spin echo signal for a single pair (i,k) of dipolar interacting spins is:40| V(t) = V0[1 −λk(1 − cos(ωikt))] | (1) |
| ωik = ω(ik)dd(3 cos2θik− 1) | (2) |
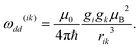 | (3) |
| V(t) = V0[1 −λk(1 −∫cos(ωikt)d cos θik)] | (4) |
In a multi-spin system, the echo intensity due to any A spin is given by:
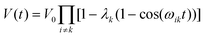 | (5) |
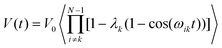 | (6) |
For a disordered system with an isotropic homogeneous distribution of spins, the dipolar time evolution exhibits a mono-exponential decay, which depends on the spin concentration, C, according to41
| V(t) = V0 exp(−t/Thom) | (7) |
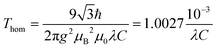 | (8) |
In a system consisting of aggregates (clusters) of radicals, where the average inter aggregate distance is larger than the average distance within the aggregate, the DEER kinetics can be described as the following product24,26,42,43
| V(t) = Vintra(t)Vinter(t) | (9) |
| Vinter(t) = V0 exp(−t/χThom). | (10) |
For a micellar solution with Mav average spins per micelle, Vintra(t) (eqn (9)) is:
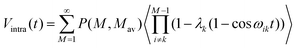 | (11) |
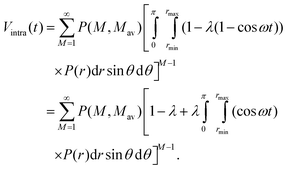 | (12) |
For long time intervals the integral over the cosine averages to zero and Vintra(t) levels off and converges to:
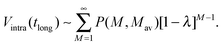 | (13) |
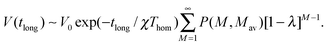 | (14) |
| V(tlong) ∼V0 exp(−tlong/χThom)[1 −λ]Mav−1 | (15) |
| ln(V(tlong)/V0) + tlong/χThom = ln(Vintra(tlong)) ∼ (Mav− 1)ln[1 −λ]. | (16) |
The time at which the echo levels off, ta, is related to a characteristic (average) pair distance, Ra according to:46
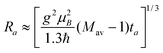 | (17) |
The determination of Mav requires the knowledge of λ. It can be calculated from the EPR lineshape according to:46
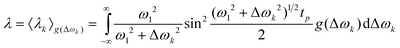 | (18) |
Eqns (15) and (16) show that to obtain a good evaluation of the number of guest radicals in the micelles leveling off of Vintra(t) should be reached. Limitations in S/N (due to a finite phase memory time) suggest an experimental strategy where measurements should begin with samples having a maximal radical load and a minimal number of micelles, and experimental conditions with the largest possible λ should be applied. If the DEER kinetics in such a sample shows features characteristic of radicals in a confined volume, manifested by leveling off of Vintra(t), measurements of other radicals/micelles concentrations can be gradually worked out. Results obtained using such an approach are presented in this work.
Modeling of small angle scattering intensities
The scattering intensity of a monodisperse system of particles of identical shape can be described as47,48| I(q) = NP(q)S(q) | (19) |
In dilute solutions, where the interactions between the objects can be neglected, S(q) equals one. The total intensity scattered from a polydisperse system with particles having an identical shape can be described by:
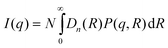 | (20) |
A form factor for a simple spherical core-shell model where the core and the shell have a uniform electron density is given by:
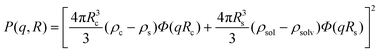 | (21) |
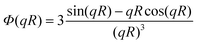 is the normalized amplitude scattered by a sphere.
is the normalized amplitude scattered by a sphere.
A Gaussian distribution is given by:
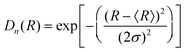 | (22) |
Results
SAXS measurements
The scattering curves of 4 wt% P123, with and without 0.7 mM Brij56-NO, at 50 °C are presented in Fig. 2. Both curves show a broad peak at the same position. This peak reflects the form factor of the micelles’ core49 indicating that the presence of the spin-probes does not affect the micelle’s core size. The influence of the structure factor is minor. Ideally, the scattering pattern would be fitted to a form factor of a spherical block copolymer micelle, which includes the self correlation of the PPO spherical core, the self correlation of the PEO chains, the cross term between the core and the chains and cross term between chains.50 However, due to the low contrast between PEO and water and the high degree of solvation, the electron density of the chains is very similar to that of the solvent. Therefore, it was difficult to obtain a clear signal at the larger scattering vector range, corresponding to the contribution of the individual PEO chains form factor. An attempt to fit the data to a simpler model describing only the spherical core failed, therefore we fitted the data to a spherical core-shell model given by eqn (21), where the fine structure from which the shell is made is ignored. A Gaussian distribution was chosen to account for the polydispersity of the micelles. The best fit parameters are presented in Table 1. We note, however, that the shell thickness and electron density could not be determined accurately. The core radius, Rc, was found to be 5.2 nm and the aggregation number, Nagg was calculated according to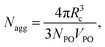 | (23) |
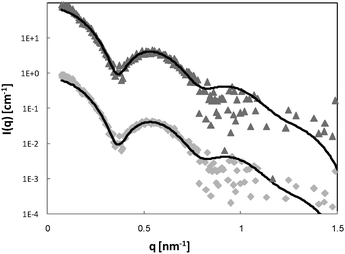 | ||
| Fig. 2 SAXS curves of 4 wt% P123 (◆) and 0.7 mM Brij56-NO in 4 wt% P123 (▲). The solid lines are fit to a polydisperse core-shell spherical model (eqn (21)) and the best-fit parameters are summarized in Table 1. The 0.7 mM Brij56-NO in 4 wt% P123 curves is vertically shifted by a factor of 10 for clarity. | ||
| R c (nm) ±0.1 | δ±0.01 | ρc (e−/nm3) | R s (nm)±0.1 | ρ s (e−/nm3) | ρ m (e−/nm3) | N agg±5 | R HS (nm) ±0.1 | ϕ | N a (nm−3) | |
|---|---|---|---|---|---|---|---|---|---|---|
| a Micelles density. | ||||||||||
| 4 wt% P123 | 5.2 | 0.19 | 335.9 | 4 | 328.9 | 333.4 | 88 | 1.2 × 10−4 | ||
| 0.7 mM Brij56-NO in 4 wt% P123 | 5.4 | 0.15 | 335.6 | 4 | 329.6 | 333.4 | 97 | 1.0 × 10−4 | ||
| 6 wt% P123 | 5.4 | 0.15 | 336.1 | 3.5 | 329.0 | 333.4 | 99 | 12.7 | 0.077 | 1.57 × 10−4 |
| 0.7 mM Brij56-NO in 6 wt% P123 | 5.5 | 0.14 | 336.2 | 3.6 | 329.0 | 333.4 | 104 | 12.7 | 0.077 | 1.54 × 10−4 |
In order to verify these results and the validity of the chosen model, we repeated the SAXS experiment with 6% P123 samples (Fig. 3). The curves were fitted to the spherical core-shell model. The structure factor cannot be neglected in this case; therefore an analysis described by Soni et al.52 using Percus–Yevic structure factor55 and Gaussian polydispersity was applied. The micellar volume fraction ϕ, and the hard sphere interaction distance RHS were variables in the S(q) calculation. The best fit parameters are presented in Table 1. As for the 4 wt% P123 systems, the fitted parameters are in good agreement with values reported in the literature and the addition of Brij56-NO did not change the size of the micelles. These results confirm that at the highest loading of spin-probe used in this work, 0.7 mM, the presence of the spin-probe does not alter the size or shape of the micelles for both 4% and 6 wt% P123 micellar solutions.
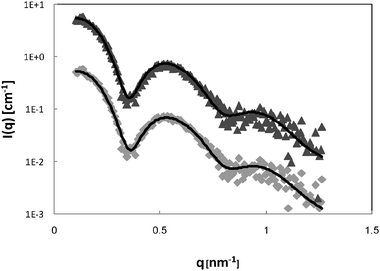 | ||
| Fig. 3 SAXS curves of 6 wt% P123 (◆) and 0.7 mm Brij56-NO in 6 wt% P123 (▲). The solid lines are fits to a polydisperse core-shell spherical model (eqn (21)) and best-fit parameters are summarized in Table 1. The 0.7 mM Brij56-NO in 6 wt% P123 curves is vertically shifted by a factor of 10 for clarity. | ||
The location of various spin-probes in Pluronic P123 micelles
DEER and ESEEM measurements are carried out at low temperatures to slow down the phase memory time such that echoes can be detected and to avoid motional averaging of dipole–dipole interactions in liquid samples. We have shown in a number of studies that upon rapid freezing the structure of the Pluronic micelles is preserved.1,14,31 The CMT of 3–4 wt% P123 is around 35 °C1,56 and therefore CW EPR measurements were carried out at 50 °C, which is well above the CMT, and samples for DEER and ESEEM were rapidly frozen from 50 °C.The depth of the nitroxide spin label of several Pluronic spin-probes and 4HTB in the P123 micelle was estimated previously by their relative hyperfine coupling, aN, and the correlation time τc, obtained from the CW EPR spectrum, and by the 2H modulation depth, k(2H), of three-pulse ESEEM traces of P123 micellar solutions in D2O.15,33k(2H) gives a qualitative measure of the amount of water around the spin-label and therefore, the lower k(2H) is, the deeper in the micelle is the nitroxide label. Similarly, the lower is aN and the higher is τc, the deeper is the label. Table 2 lists the aN, k(2H), and τc values for a number of spin probes in 3 wt% P123 micellar solutions at 50 °C and of L62-NO in a water solution for comparison. Fig. 4a presents the corresponding CW EPR spectra. 4HTB is the most hydrophobic spin-probe, it’s spectrum reveals the existence of two species. One is assigned to 4HTB dissolved in single chains (unimers) in solution (15%), and the other to 4HTB in the core of the micelle (85%). The nitroxide group of 4HTB is a TEMPO group (six-membered ring) as opposed the Pluronic spin-probe, which has a proxyl group (five-membered ring), therefore it’s aN value is larger than that of the Pluronic spin-probes and cannot be compared directly.57
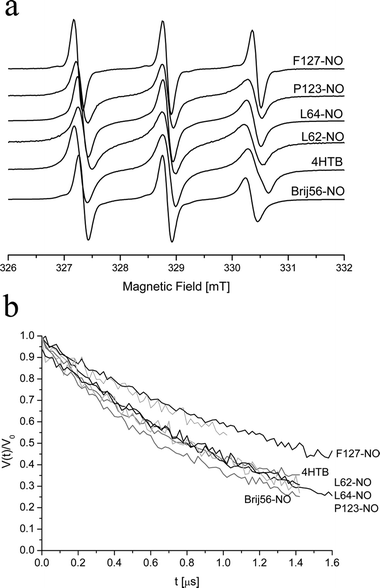 | ||
| Fig. 4 (a) CW-EPR spectra of 0.5 mM of various spin-probes in 3 wt% P123 at 50 °C. (b) DEER kinetics of 0.3 mM (spin concentration) of various spin-probes in 4 wt% P123 measured at 50 K and freeze quenched from 50 °C. | ||
| Spin-probe | a N [mT] ± 0.02 mT | k(2H) ± 0.005 | τ c 1010 s ± 0.1 |
|---|---|---|---|
a
4HTB and L64-NO are distributed at these conditions between two different environments![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) micelles and single chains.
b For a solution of 4 wt% P123. micelles and single chains.
b For a solution of 4 wt% P123.
|
|||
| L62-NO in water | 1.66 | 0.23 | 1.0 |
| F127-NO | 1.59 | 0.13 | 0.8 |
| P123-NO | 1.56 | 0.076 | 3.5 |
| 1.56b | 3.5b | ||
| L62-NO | 1.55 | 0.062 | 2.0 |
| L64-NO | 1.52/1.59a | 0.065 | 3.5/1.0 |
| Brij56-NO | 1.48 | 0.07 | 3.5 |
| 1.48b | 3.5b | ||
| 4HTB | 1.55/1.72a | 0.053 | 2.6/0.8 |
The results summarized in Table 2 show that the location of the spin-label of the Pluronic spin-probes in the micelles is mostly determined by the PEO length: F127-NO has the longest EO chain, and thus, on the average, the label is situated close to the corona-water interface. This is manifested by a large aN, close to that of spin-labeled Pluronic dissolved in water (aN = 1.6 mT), a large k(2H) and a low τc. P123-NO has lower aN and k(2H) values than F127-NO, and a larger τc =3.5 × 10−10 s owing to its deeper location in the P123 corona. L64-NO and L62-NO were found at two regions in the corona, where most of the spin-labels, 92%, are located at the core-corona interface, with aN = 1.52 mT and τc =3.5 × 10−10 s, and the others are closer to the corona-water interface, with aN = 1.59 mT, and τc decreases to 1.0 × 10−10 s. The PEO block length of Brij56-NO is very similar in length to that of L64-NO, but it has a different hydrophobic part. Its k(2H) is similar to that of L62-NO, but the aN of Brij56-NO is significantly smaller, 1.48 mT, than that of L64-NO, and also τc of Brij56-NO is higher than that of L64-NO. This suggests that the alkyl tail of Brij56-NO, which is more hydrophobic than the PPO block, pulls it into a pocket, where its motion is restricted and with very little water that can hydrogen bond to the NO group.58 The k(2H) value of L64-NO, P123-NO, L62-NO and Brij56-NO are close and show that the resolution of k(2H) in terms of depth within the micelles is lower than that of aN because of its longer range of interaction with water molecules. CWEPR measurements of 4HTB in P123 solutions with various concentrations (3–6 wt%) showed that the increase in the P123 concentration changed only the distribution of the spin-probes between unimers and micelles, where aN and τc values remained constant.31 Similarly, measurements of P123-NO and Brij56-NO in 3% and 4 wt% P123 gave the same aN and τc values (see Table 2).
Comparative DEER traces of 0.3 mM (radical concentration) spin-probe in 4 wt% P123, freeze-quenched from 50 °C, are shown in Fig. 4b. The following trend of the decay rates is observed F127-NO < 4HTB < L62-NO ∼ L64-NO ∼ P123-NO < Brij56-NO. This trend is expected because F127-NO, which is located at the corona/water, interface exhibits a slower decay as it is distributed over a larger radius within the micelle, compared to the other Pluronic spin-probes. The relatively lower decay rate of the 4HTB is attributed to its lower effective concentration within the micelles owing to the presence of 15% in solution (dissolved within unimers). Brij56-NO decays somewhat faster than L62-NO, probably because it is located in a smaller radius within the micelle, consistence with its smaller aN and larger τc.
DEER experiments
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif)
| ln(V(t)/V0) = ln(Vintra(t)) − (tλC/10−3χ)) | (24) |
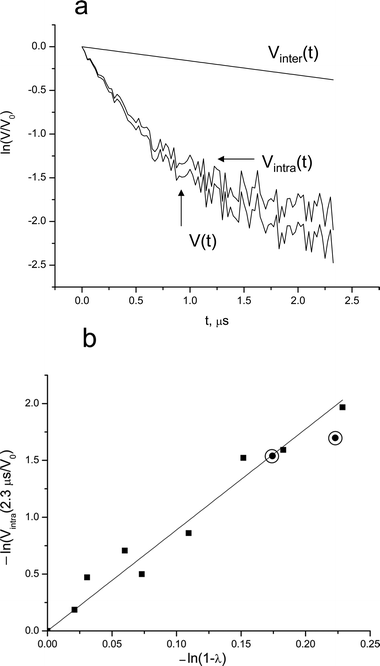 | ||
| Fig. 5 (a) The DEER kinetics of 0.7 mM Brij56-NO in 4 wt% P123: normalized raw data (V(t)), Vintra(t) and the calculated Vinter(t) as indicated on the figure. (b) The dependence of ln(Vintra(t)/V0), for t = 2.3 μs, on ln(1 −λ). (Ka-band measurements). The solid line represents a linear best fit. The circled points represent data obtained from P123-NO in the same solution and at t = 1.7 μs measured at X-band. | ||
For a more precise determination of Mav we performed a series of measurements at different λ values and Fig. 5b shows the dependence of ln Vintra(t = 2.3 μs) on ln(1 −λ) (eqn (16)). Here the λ-values were obtained by calculations and corrected according to the experimental calibration of λ (Fig. S1). The linear dependence yields a slope of Mav−1 = 8.9, from which we obtain Mav≥ 9.9 for 0.7 mM Brij56-NO in 4 wt% P123 at 50 °C.
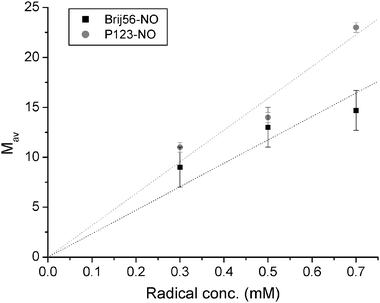
A decrease in the spin-probe concentration, while keeping all other conditions constant, should lead to a decrease in Mav as well. Such a decrease, however, will increase the acquisition time interval necessary to reach leveling off of Vintra(t) for the same size of micelles (see eqn (17)). Therefore, the time interval of the measurements should preferably be increased and scaled according to radical loading. Unfortunately, this was not possible due to S/N limitation. Fig. 6a and b present the dependence of ln Vintra(tlong) on ln(1 −λ) for 0.5 mM and 0.3 mM of Brij56-NO in 4 wt% P123 and the dependence of Mav on [Brij56-NO] is summarized in Fig. 6c. A linear fit of the latter with a forced zero-intercept, yields a slop of 15.6 ± 1.1 mM−1. The fit is not excellent, but reasonable and suggests that such a crude approach for estimating of Mav can be used with a high degree of confidence since all these estimates do not use any models or simulations.
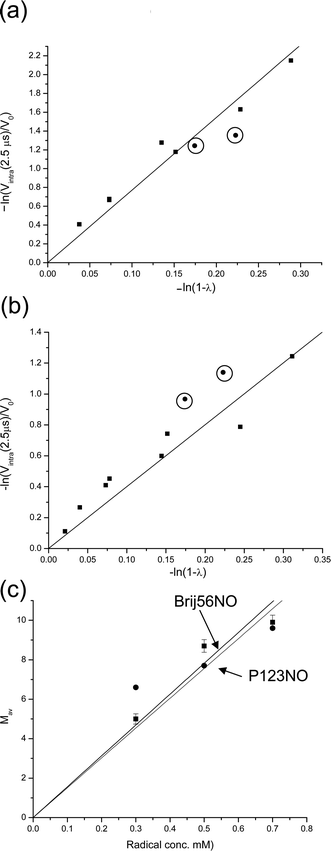 | ||
| Fig. 6 The dependence of ln(Vintra(t)/V0), for t = 2.5 μs, on ln(1 −λ) for (a) 0.5 mM and (b) 0.3 Brij56-NO in 4 wt% P123 (Ka-band measurements). In (a) and (b) the circled points represent data obtained from P123-NO for t = 1.7 μs in X-band. (c) The dependence of Mav on the total spin concentration for Brij56-NO and for P123-NO. The solid lines represent the linear best fit. | ||
For comparison, measurements were carried out with P123-NO, which is similar to the molecules constituting the micelles. Here the DEER measurements were carried out at X-band for two values of λ and the longest time interval attained was 1.7 μs. The dependence of ln Vintra(1.7 μs) on ln(1 −λ) for spin concentrations of 0.3, 0.5 and 0.7 mM were added to the plots of Brij56-NO at the same spin concentration in Fig. 5b and 6a,b. The data points (circled) fall quite close to those of Brij56-NO. The dependence of Mav on the radical concentration is depicted, together with the results of Brij56-NO in Fig. 6c, yielding a slope of 15.1 ± 1.8 mM−1 which is very close to the one determined for Brij56-NO. Interestingly, in both cases Mav for the high concentration, 0.7 mM, is somewhat underestimated and could suggest a saturation effect.
The slopes obtained from Fig. 6c provide the number of micelles per unit volume, Cm, according to Cm = [radical]/Mav = 0.067 ± 0.007 mM. The average aggregation number can be derived from the known concentration of the Pluronic in the solution (6.7 mM). This yields Nagg of 95 ± 7 and 100 ± 10 for the Brij56-NO and P123-NO data, respectively, in good agreement with the Nagg value that was calculated from the SAXS results (Table 1). In our calculations we neglected the dispersion in molecular weight and did not include the weight of the spin-probes. Furthermore, we assumed that the amounts of unimers in solution is negligible.
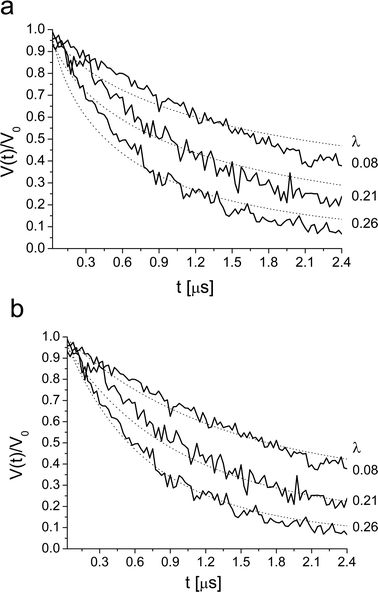 | ||
| Fig. 7 Ka-band DEER decay of 0.3 mM Brij56-NO in 4 wt% P123, measured at various λ. The dotted line is the best fit obtained with (a) homogenous distribution in a shell with rmax = 7.0 nm, rmin = 3.0 nm, Mav = 9.0. (c) A distribution of spins in a spherical with P(r) = r2, rmax = 9.0 nm, Mav = 9.0. Here rmax corresponds to the maximum distance between spins. | ||
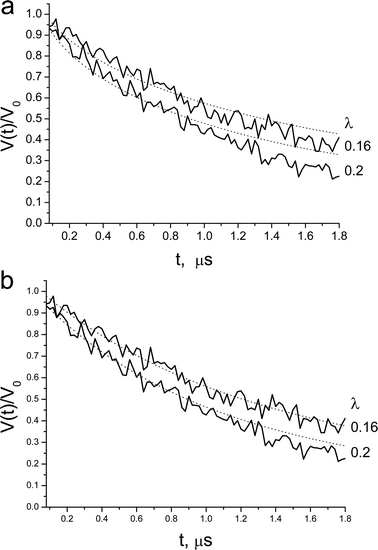 | ||
| Fig. 8 X-band DEER decay of 0.3 mM P123-NO in 4 wt% P123, measured at various λ. The dotted line is the best fit obtained with (a) homogenous distribution in a shell with rmax = 6.2 nm, rmin = 5.2 nm, Mav = 10.0. (b) A distribution of spins in a sphere, with P(r) = r2, rmax = 10.0 nm, Mav=11.0. Here rmax corresponds to the maximum distance between spins. | ||
The disagreement between the experimental DEER kinetics and the best-fit calculated kinetics suggests that the basic assumption that the positions of the spin-probes are independent is not valid. Therefore, we refined the model and included excluded volume. Namely, once a nitroxide is situated at a particular location, there is a volume around it that cannot be occupied by any other spin-label. Inclusion of an excluded volume of a sphere with a radius of 2.5 nm improved the fit for both spin-probes, but not enough. Extending the distribution within a shell to a sphere did not improve the fit either.
In our earlier study we showed that the DEER kinetics of 4HTB in P123 micelles31 could be fitted with a model based on eqn (12) with P(r) = r2. In this model the probability to find pairs with long distances is significantly higher than for the short distances. This implies that the excluded volume is large and that the nitroxides distribution prefers long distances. This model gave better fit than the homogeneous distribution model discussed above for both Brij56-NO and P123-NO. Here rmin was taken as 1.5 nm as well. Examples of the quality of the fit for both spin-probes are shown in Fig. 7b and 8b for the 0.3 mM samples and the best fit parameters are listed in Table 3. These were determined as follows: Initially, a least-squares fitting was done individually for each concentration (for all λ values), to find the best fit values of rmax and Mav. Then, from these rmax values, the average value was chosen and fixed and only Mav was fitted. The Mav values listed in Table 3 are averages over all DEER kinetics obtained with different λ-values.
| Brij56-NO | P123-NO | |
|---|---|---|
| 0.3/0.5/0.7 mM | 0.3/0.5/0.7 mM | |
| R a , model free | ∼10 nm | ∼11 |
| M av, model free | (5.0/8.8/9.9) ± 0.35 | (6.5/7.7/9.6) ± 0.5 |
| N agg, model free | 95 ± 7 | 100 ± 10 |
| M av, P(r) = r2 | (9.0/13/15) ± 1.5 | (11.0/13.0/22.0) ± 0.5 |
| r max [nm], P(r) = r2 | 9.0 ± 0.5 | 10.0 ± 0.2 |
| N agg, P(r) = r2 | 150 ± 15 | 210 ± 20 |
The rmax obtained for P123-NO is somewhat larger than for Brij56-NO as expected from the difference in aN. Here rmax provides the largest distances between two spins, which give the diameter of the spherical volume they occupy. Comparison of the values obtained (9–10 nm, Table 3) with a core diameter of 10.4–10.8 nm and a total micelle diameter of 18.4–18.8 nm determined from the SAXS measurements, suggests that this model underestimates the diameter of the volume with spin-probes.
Fig. 9 presents a plot of Mav obtained with the model with P(r) = r2, as a function of the spin-probe concentration. The Mav values are higher than those obtained from the model free analysis (see Table 3). This is expected since the model free analysis provides the lower limit of Mav due to difficulties in reaching the leveling off part of the DEER kinetics. However, the slopes obtained from Fig. 9 gave Nagg∼ 150–210 (see Table 3), which is 50–100% higher than obtained by the model free analysis and estimated from the core radius obtained by SAXS. The DEER experiments on 4HTB in 3 wt% P123, where unimers were detected also in the EPR spectrum, gave Nagg = 56 ± 4, and for 4HTB in 6 wt% P123 Nagg = 121 ± 7.27 The latter is higher by ∼20% with respect to the SAXS data. For 4HTB in 3 wt% P123 rmax = 7.3 ± 0.2 nm,31 which is smaller than the core volume. This is not surprising because of the hydrophobic nature of 4HTB. This is also smaller than the rmax of Brij56-NO and P123-NO as expected.
Discussion
In this work we have used DEER to study the number and distribution of guest molecules (spin-probes) in P123 micelles, as well as the volume they occupy and the aggregation number of the micelles. The SAXS measurements have shown that the addition of the spin-probes used (up to 0.7 mM) did not affect the dimensions of the micelles. The model free analysis of the DEER kinetics of P123-NO and Brij56-NO gave reasonable estimates of the volume occupied by the spin-probes, determined via the average distance between them, Ra, and average number of spins per micelle, Mav (see Tables 1 and 3) in spite of the limited acquisition time interval (2.3–2.5 μs for the Ka-band measurements and 1.7 μs for the X-band measurements). The aggregation number of the P123 molecules in a micelle was calculated from the dependence of Mav on the spin concentration at constant P123 concentration and is in good agreement with the value predicted from the core size determined by the SAXS measurements. Comparison of the results of Brij56-NO, which has one spin-label per polymer chain, and P123-NO, that has two labels, shows that the two spin-labels in P123-NO are completely uncorrelated and behave like spin-labels on two different chains.In the following we summarize essential points in the application of the model free approach:
1. The three-pulse sequence, as opposed to the four-pulse sequence, is recommended because it has a better S/N ratio and therefore longer acquisition times can be employed. The dead-time in this particular case is not critical because a fast decay at very short times, characteristic of pairs with short distances, is not expected.
2. Efforts to achieve long acquisition times, where the leveling off of the DEER decays can be reached, should be made. If possible, samples should be prepared in D2O to increase phase memory time. Measurements should begin with samples with the highest possible spin concentration, and with the largest possible λ value, to reach the leveling off value of Vintra(t). This single measurement will provide estimates of the parameters involved and supply good guidelines for the choice of the experimental parameters and samples compositions needed to substantiate the single point results.
3. Measurements on a series of samples with different spin concentrations and employing different λ values should be carried out.
Attempts to analyze the full DEER kinetics using rather simple models showed that the average distance between the spins in the micelle is considerably larger than expected from a homogenous distribution in micelles (in a shell or a sphere) with sizes compatible with those obtained from the SAXS measurements. A reasonable fit of the DEER kinetics was obtained with a model with a pair distance distribution function of r2. This gave the following trend for the diameter of the sphere the spin-probes occupy: 4HTB27 < Brij56-NO < P123-NO. This is in agreement with the location of the spin-label within the micelle as implied by the aN and τc values. However, the actual volumes occupied by the spin-probes seem to be underestimated for Brij56-NO and P123-NO in light of the SAXS results. Alternatively, these results could indicate that the spin-probes are localized within the core. While this may be possible for Brij56-NO, it is unlikely for P123-NO, based on the EPR results given in Table 2.
Remaining open questions are: What is the reason for the preferred large distances between the spin labels? Is this an inherent property of the P123 micelles, or is it a consequence of the freezing? This preference was observed for all spin-probes used, independent on their resemblance to the constituent molecules of the micelles and their sizes. Therefore this suggests that it reflects a general property of the micelles and not of the spin-probe. One possibility is that the high density of PPO chains in the core of the micelles does not allow guests such as 4HTB and the Brij56-NO to be close to one another, pushing them towards the core-corona interface. It is also possible that the freezing of the chain dynamics induces some local order and further expelling these foreign solutes outwards. Such an expulsion would be more facile for 4HTB, which is a small molecule compared to Brij56-NO. In contrast, P123-NO can be considered as a native constituent of the molecules forming the micelles and not as a solute molecule, as it differs only by the nitroxides at the ends of the PEO blocks. Therefore, its distribution should resemble that of the P123 end chains and therefore significant expulsion upon freezing is unlikely. Here one has to consider the character and size of the nitroxide, compared to the OH group. However, it is unlikely that it will be the reason for such a large excluded volume effect.
The large density in the core of the micelle was reported previously. The micelles of polystyrene/polyethylene oxide (PS/PEO) were studied by Monte Carlo simulations and SAXS.44,63 It was shown that the polymer density profile decays from the center of the micelle as (1/r)a, where a≥ 1 and it depends on the solvent quality. In addition, the excluded volume around each monomer in the polymer chain, named “blob size”, increases with r and decreases with an increase in the aggregation number. For instance, an average blob size diameter for a micelle with Nagg = 20 is 3.3 nm, whereas for Nagg = 100 is 1.66 nm. For Nagg = 65–80, the blob size at the corona of the micelle is 6.3 nm. Simulations for polymers in spherical volume also showed that the density at the core of the micelle is high and decreases from the center of the core.45,64 Calculations for Pluronic block copolymers showed that the density at the core of the micelle is fairly high (0.7–0.8 g cm−3) and spatially uniform. The density of the shell is appreciably lower (0.15–0.25 g cm−3) and decreases from the core/shell interface to the micelle periphery.65
In this work we have studied micelles of P123, which have a total diameter (core + corona) of 18 nm. Hence, relatively large intra-micelles distances between radicals are accommodated for relatively low loadings and long times are required for Vintra(t) to level off. Accordingly, we expect that the model free approach for DEER data analysis will be more easily applied to systems with smaller micelles, such as those formed by alkyl based cationic or anionic surfactants.
In this work we limited ourselves to spheroidal micelles, it is yet to be shown whether this approach can be applied to thread-like micelles as well. Because the model free approach is shape independent it cannot provide the shape of the micelles. Nonetheless, it does provide the average number radicals in a micelle and its dependence on the total spin concentration will provide the aggregation number. A transition from spherical-to thread-like micelles may be detected indirectly through the change in Nagg. Alternatively, the data could be analyzed using a model with cylinders to derive the size, however, because the exact form of P(r) is unknown such an analysis may be problematic.
Finally, we compare the information provided by the DEER experiments with that of SAXS or dynamic light scattering (DLS). While SAXS and DLS give information on size and shapes, from which the aggregation number is derived indirectly, they cannot give information regarding the local concentration and spatial distribution of guest molecules within the micelles. This, in turn can be determined by DEER measurements, which also give the aggregation number. Therefore, the techniques are complementary. When micelles properties are of interest the spin-probe concentration should be kept low to avoid changes in size or shape,31 but when the interest is the load of the micelles with guests and to follow their effects on the micelles properties this issue is no longer relevant. A disadvantage of the DEER technique is that it has to be carried out at low temperatures; therefore the issue of preserving the solution structure is crucial. In addition, the measurements are time consuming as series of measurements have to be carried out as a function of spin concentration and pump flip angle. When leveling off of Vintra(t) is not reached the data analysis becomes complicated and model dependent. Here an improvements in the fit of the experimental data can be obtained by introducing a more realistic model for the effect of the pump pulse as reported recently.66 In addition, the Poisson distribution may not be the most appropriate one to describe the distribution of the number of spin-probes in the micelles as it assumes non-interacting particles. The low temperature measurements may be advantageous for time resolved applications, such as the formation of templated mesoporous materials,67 because freeze quench measurements can be done.
Conclusions
DEER measurements were used to obtain the number of guest molecules (in the form of spin-probes), their distribution and the volume they occupy in P123 micelles and the aggregation number of the micelles. We showed that a simple model free approach for the analysis of the DEER data yields good estimates to the above properties, some we determined independently by SAXS measurements. The application of this approach, however, requires experimental efforts, namely a series of measurements as a function of microwave power and spin concentration are necessary. More importantly, it requires long acquisition times of the DEER kinetics, which are limited by the phase memory time, to reach the leveling off that is characteristic of distribution of radicals within a confined volume.Data analysis in terms of a simple model of spins distributed in a sphere or a shell of particular dimensions showed that for both P123-NO and Brij56-NO there is a preference for large distances between the nitroxide groups, situated at the polymer ends, suggesting the presence of a large excluded volume. It also showed that the volume the spin-labels occupy in P123 micelles varies according to P123-NO > Brij56-NO > 4HTB. This trend is consistent with the nitroxide depth in the micelles, determined by the hydrophophic/hydrophylic character of the spin-probe.
For full realization of the potential of DEER as a method to characterize micelles and guest molecules in micelles the data analysis of the complete DEER kinetics needs further improvement. This requires additional experimental results on solutions with different Pluronic concentrations and different types of Pluronics and spin probes.
Acknowledgements
Acknowledgment is given to the Donors of the American Chemical Society Petroleum Research Fund for partial support of this research. This research has been also supported by Binational USA-Israel Science Foundation (BSF) and the Ilse Katz Institute for Material Science and Magnetic Resonance Research. S.R. acknowledges the support of a grant from the Ministry of Science. D.G. holds the Erich Klieger Professorial chair in Chemical Physics. R.B. thanks the support of the Reimund Stadler Minerva Center for Mesoscale Macromolecular Engineering at Ben Gurion University. Minerva is funded through the BMBF. We thank Ciba-Geigy AG and Gunnar Jeschke (MPI for polymer research, Mainz) for providing the oligomethylene biradical and Rachel Yerushalmi-Rozen (Ben Gurion University) and Alexey Potapov (Weizmann Institute of Science) for very useful discussions.References
- P. Alexandridis and T. A. Hatton, Colloids Surf., A, 1995, 96, 1 CrossRef CAS.
- C. Booth and D. Attwood, Macromol. Rapid Commun., 2000, 21, 501 CrossRef CAS.
- D. Y. Zhao, J. L. Feng, Q. S. Huo, N. Melosh, G. H. Fredrickson, B. F. Chmelka and G. D. Stucky, Science, 1998, 279, 548 CrossRef CAS.
- D. Y. Zhao, Q. S. Huo, J. L. Feng, B. F. Chmelka and G. D. Stucky, J. Am. Chem. Soc., 1998, 120, 6024 CrossRef CAS.
- T. W. Kim, F. Kleitz, B. Paul and R. Ryoo, J. Am. Chem. Soc., 2005, 127, 7601 CrossRef CAS.
- F. Kleitz, S. H. Choi and R. Ryoo, Chem. Commun., 2003, 2136 RSC.
- P. Alexandridis, D. L. Zhou and A. Khan, Langmuir, 1996, 12, 2690 CrossRef CAS.
- L. Guo, R. H. Colby, M. Y. Lin and G. P. Dado, J. Rheol., 2001, 45, 1223 CrossRef CAS.
- R. Nagarajan, Polym. Adv. Technol., 2001, 12, 23 CrossRef CAS.
- R. Nagarajan, Colloids Surf., B, 1999, 16, 55 CrossRef CAS.
- S. R. Croy and G. S. Kwon, Curr. Pharm. Des., 2006, 12, 4669 CrossRef CAS.
- R. Liu, R.-M. Pannefelser and L. Shounfeng, in Water-Insoluble Drug Formulation, ed. R. Liu, CRC press, Taylor & Francis group, Boca Raton, FL., 2nd edn, 2008, pp. 205–306 Search PubMed.
- A. V. Kabanov, I. R. Nazarova, I. V. Astafieva, E. V. Batrakova, V. Y. Alakhov, A. A. Yaroslavov and V. A. Kabanov, Macromolecules, 1995, 28, 2303 CrossRef CAS.
- S. Ruthstein, V. Frydman and D. Goldfarb, J. Phys. Chem. B, 2004, 108, 9016 CrossRef CAS.
- S. Ruthstein, V. Frydman, S. Kababya, M. Landau and D. Goldfarb, J. Phys. Chem. B, 2003, 107, 1739 CrossRef CAS.
- C. A. Steinbeck, N. Hedin and B. F. Chmelka, Langmuir, 2004, 20, 10399 CrossRef CAS.
- V. K. Aswal and J. Kohlbrecher, Chem. Phys. Lett., 2006, 425, 118 CrossRef CAS.
- M. A. R. Meier, S. W. H. Aertz, B. B. P. Staal, M. Rasa and U. S. Schubert, Macromol. Rapid Commun., 2005, 26, 1918 CrossRef CAS.
- S. Ghosh, S. Dey, A. Adhikari, U. Mandal and K. Bhattacharyya, J. Phys. Chem. B, 2007, 111, 7085 CrossRef CAS.
- S. Ristori, G. Martini and S. Schlick, Adv. Colloid Interface Sci., 1995, 57, 65–122 CrossRef CAS.
- M. Vasilescu, A. Caragheorgheopol and H. Caldararu, Adv. Colloid Interface Sci., 2001, 89–90, 169 CrossRef CAS.
- P. Baglioni, H. Nakamura and L. Kevan, J. Phys. Chem., 1991, 95, 3856 CrossRef CAS.
- P. Baglioni and L. Kevan, J. Phys. Chem., 1987, 91, 1516 CrossRef CAS.
- A. D. Milov and Y. D. Tsvetkov, Appl. Magn. Reson., 1997, 12, 495 CrossRef CAS.
- M. Pannier, M. Schops, V. Schadler, U. Wiesner, G. Jeschke and H. W. Spiess, Macromolecules, 2001, 34, 5555 CrossRef CAS.
- A. D. Milov, A. G. Maryasov and Y. D. Tsvetkov, Appl. Magn. Reson., 1998, 15, 107 CrossRef CAS.
- P. K. Borbat and J. H. Freed, Meth. Enzymol., 2007, 423, 52 CAS.
- G. Jeschke and Y. Polyhach, Phys. Chem. Chem. Phys., 2007, 9, 1895 RSC.
- M. K. Bowman, B. David, D. S. Michael and J. D. Zimbrick, Radiat. Res., 2005, 163, 447 CrossRef CAS.
- M. Pannier, V. Schadler, M. Schops, U. Wiesner, G. Jeschke and H. W. Spiess, Macromolecules, 2000, 33, 7812 CrossRef CAS.
- S. Ruthstein, A. Potapov, A. M. Raitsimring and D. Goldfarb, J. Phys. Chem. B, 2005, 109, 22843 CrossRef CAS.
- S. Ruthstein and D. Goldfarb, J. Phys. Chem. C, 2008, 112, 7102 CrossRef CAS.
- S. Ruthstein, J. Schmidt, E. Kesselman, R. Popovitz-Biro, L. Omer, V. Frydman, Y. Talmon and D. Goldfarb, Chem. Mater., 2008, 20, 2779 CrossRef CAS.
- A. Caragheorgheopol, H. Caldararu, I. Dragutan, H. Joela and W. Brown, Langmuir, 1997, 13, 6912 CrossRef CAS.
- A. Godt, C. Franzen, S. Veit, V. Enkelmann, M. Pannier and G. Jeschke, J. Org. Chem., 2000, 65, 7575 CrossRef CAS.
- Y. Polyhach, A. Godt, C. Bauer and G. Jeschke, J. Magn. Reson., 2007, 185, 118 CrossRef CAS.
- A. V. Astashkin, J. H. Enemark and A. M. Raitsimring, Concepts Magn. Reson., B, 2006, 29, 125 CrossRef.
- J. M. Fauth, A. Schweiger, L. Braunschweiler, J. Forrer and R. R. Ernst, J. Magn. Reson., 1986, 66, 74 CAS.
- T. Zemb, O. Tache, F. Ne and O. Spalla, J. Appl. Crystall., 2003, 36, 800 Search PubMed.
- K. M. Salikhov, S. A. Dzuba and A. M. Raitsimring, J. Magn. Reson., 1981, 42, 255 CAS.
- A. M. Raitsimring, K. M. Salikhov, B. A. Umanskii and Y. D. Tsvetkov, Fizika Tverdogo Tela, 1974, 16, 756 Search PubMed.
- A. D. Milov, Y. D. Tsvetkov, F. Formaggio, M. Crisma, C. Toniolo and J. Raap, J. Am. Chem. Soc., 2001, 123, 3784 CrossRef CAS.
- A. D. Milov, A. G. Maryasov, Y. D. Tsvetkov and J. Raap, Chem. Phys. Lett., 1999, 303, 135 CrossRef CAS.
- A. D. Milov, A. B. Ponomarev and Y. D. Tsvetkov, Chem. Phys. Lett., 1984, 110, 67 CrossRef CAS.
- G. Jeschke, G. Panek, S. Schleidt and U. Jonas, Polym. Eng. Sci., 2004, 44, 1112 CrossRef CAS.
- A. M. Raitsimring, Biol. Magn. Reson, 19, ed. G. R. Eaton, S. S. Eaton and L. J. Berliner, New York, 2000, ch. 10 Search PubMed.
- O. Glatter and O. Kratky, Small Angle X-ray Scattering, Academic Press, London, UK, 1982 Search PubMed.
- H. Bianco, M. Narkis and Y. Cohen, J. Polym. Sci. B, 1996, 34, 2775 CrossRef CAS.
- K. Mortensen, Polym. Adv. Technol., 2001, 12, 2 CrossRef CAS.
- J. S. Pedersen and M. C. Gerstenberg, Macromolecules, 1996, 29, 1363 CrossRef CAS.
- K. Mortensen, J. Phys.: Condens. Matter, 1996, 8, A103 CrossRef CAS.
- S. S. Soni, G. Brotons, M. Bellour, T. Narayanan and A. Gibaud, J. Phys. Chem. B, 2006, 110, 15157 CrossRef CAS.
- R. Ganguly, V. K. Aswal, P. A. Hassan, I. K. Gopalakrishnan and J. V. Yakhmi, J. Phys. Chem. B, 2005, 109, 5653 CrossRef CAS.
- J. Jansson, K. Schillen, M. Nilsson, O. Soederman, G. Fritz, A. Bergmann and O. Glatter, J. Phys. Chem. B, 2005, 109, 7073 CrossRef CAS.
- J. K. Percus and G. J. Yevick, Phys. Rev. E, 1958, 110, 1 Search PubMed.
- G. Wanka, H. Hoffmann and W. Ulbricht, Macromolecules, 1994, 27, 4145 CrossRef.
- D. Marsh and C. Toniolo, J. Magn. Reson., 2008, 190, 211 CrossRef CAS.
- J. D. Morrisett, Spin Labeling, Theory and Apllications, ed. L. J. Berliner, Academic Press, INC., 1976 Search PubMed.
- G. Jeschke, A. Bender, H. Paulsen, H. Zimmermann and A. Godt, J. Magn. Reson., 2004, 169, 1 CrossRef CAS.
- G. Jeschke, Macromol. Rapid Commun., 2002, 23, 227 CrossRef CAS.
- G. Jeschke, A. Koch, U. Jonas and A. Godt, J. Magn. Reson., 2002, 155, 72 CrossRef CAS.
- A. Schweiger and G. Jeschke, Principles of Electron Paramgnetic Resonance, University Press, 2001 Search PubMed.
- A. P. Gast, Langmuir, 1996, 12, 4060 CrossRef CAS.
- R. Toral and A. Chakrabarti, Phys. Rev. E, 1993, 47, 4240 Search PubMed.
- K. Prochazka, J. Phys. Chem., 1995, 99, 14108 CrossRef CAS.
- J. E. Banham, C. M. Baker, S. Ceola, I. J. Day, G. H. Grant, E. J. J. Groenen, C. T. Rodgers, G. Jeschke and C. R. Timmel, J. Magn. Reson., 2008, 191, 202 CrossRef CAS.
- S. Ruthstein and D. Goldfarb, J. Phys. Chem. C, 2008, 112, 7102 CrossRef CAS.
Footnote |
† Electronic supplementary information (ESI) available: Experimental calibration of λ at Ka-band and four-pulse DEER measurements of a triradical and a biradical as a function of radical concentration. See DOI![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10.1039/b812475b 10.1039/b812475b |
| This journal is © the Owner Societies 2009 |