Molecular tectonics: modulation of size and shape of cuboid 3-D coordination networks†‡
Mei-Jin
Lin
,
Abdelaziz
Jouaiti
,
Nathalie
Kyritsakas
and
Mir Wais
Hosseini
*
Laboratoire de Chimie de Coordination Organique (UMR 7140), Université Louis Pasteur, Institut Le Bel, 4 rue Blaise Pascal, 67000, Strasbourg, France. E-mail: hosseini@chimie.u-strasbg.fr; Fax: +33390241325; Tel: +33 390241323
First published on 3rd November 2008
Abstract
The pore size and shape of cuboid 3-D coordination networks may be modulated by combining rigid neutral organic tectons of different length bearing two pyridines oriented in a divergent fashion with zinc dication and SiF6 dianion behaving as a metallic node and as an inorganic tecton, respectively.
Since the formal work by Wells on nets1 and the practical demonstration by Robson et al in 1991,2 coordination networks or metalloorganic frameworks (MOFs) are attracting considerable interest in terms of synthetic approaches, structural features (dimensionality, topology) and applications.3 These infinite architectures are hybrid species composed of organic moieties and metallic centers or complexes. The design, formation and description of this variety of assemblies may be explored by an approach called molecular tectonics.4 Indeed, these hybrid materials, i.e. periodic architectures for which the metal centres behave as structural nodes, may be analysed and designed as networks resulting from the mutual interconnection between organic and metallic tectons (construction units). The diamondoid and cubic type networks based on tetrahedral and octahedral nodes, respectively, form the two simplest geometrical categories. Let us focus on homonuclear cubic networks. Such architecture is defined by a single metal centre (M) as a structural node adopting an octahedral coordination geometry and three organic tectons (T) behaving as connectors (M,T1,T2,T3). The control of the metric parameters in cubic networks is pertinent as it allows to modulate the size and shape of the channels and the porosity of material (Fig. 1).
![Schematic representation of homonuclear cubic (a = b = c, left) and rectangular prismatic [(a = b,c), middle, (a,b,c), right] coordination networks.](/image/article/2009/CE/b815695f/b815695f-f1.gif) | ||
| Fig. 1 Schematic representation of homonuclear cubic (a = b = c, left) and rectangular prismatic [(a = b,c), middle, (a,b,c), right] coordination networks. | ||
As mentioned above, when three identical tectons (T) are used, geometrically the architecture is defined as a cube (M,3T) (Fig. 1 left). When the tectons are different, the assembly is of a cuboid or a rectangular prismatic type (M,2T1,T3) or (M,T1,T2,T3) (Fig. 1 middle and 1 right). The design of cubic networks, a two component system, is rather trivial and the majority of examples reported belong to that category.3,5 However, the design and controlled generation of cuboid architectures by self-assembly processes require a more sophisticated strategy. The simplest cuboid case (Fig. 1 middle), a three component system (M,2T1,T2) requires to combine a metal centre with two different tectons. In order to avoid homo self-assembly processes taking place, which leads to two cubic (M,3T1) and (M,3T2) networks, one needs to differentiate the two tectons through their mode of binding to the metal. The last case, not yet reported, is even more complicated to achieve since it requires a metal and three differentiated tectons (a four-component system).
For diamondoid type networks, we have previously reported an example of the control of geometry.6
Here, we describe the design and control of the metric parametres of cuboid 3-D networks based on the combination of Zn2+ cation adopting the octahedral coordination geometry, SiF62− dianion behaving as an inorganic linear tecton and three organic tectons of different length.
In order to generate cuboid architectures composed of three components, we used tectons 1–3 (Scheme 1). These three rigid and linear construction units are analogous in terms of their binding propensity (bismonodentate) and differ only by their length (dN–N of ca 7.1, 9.6 and 11.4 Å for 1, 2 and 3 respectively). Compound 1 is commercially available. Compounds 27 and 38 have been prepared following reported procedures.
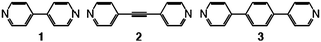 | ||
| Scheme 1 | ||
Owing to its propensity to adopt an octahedral coordination geometry, among others, required for the formation of 3-D cubic networks, Zn2+ was chosen as the metallic part. In the crystalline phase, for the combination of neutral tectons such as 1–3 with Zn2+ dication, one may use weakly or strongly coordinating anions for charge balance. In the latter case, one may select the anion for both charge neutralization and for directing the construction of the network.9 In order to obtain neutral structural nodes, we combined Zn2+ with the SiF62− dianion. Indeed, the latter was used because, in marked contrast with weakly coordinating monoanions of the type MF6− (M = P, Sb, As), SiF62− bridges metal centres and the interconnection takes place through the two fluorine atoms occupying the apical positions on the metal cation.10
Upon slow diffusion of an EtOH solution of ZnSiF6·xH2O into a CHCl3 solution of the organic tecton (1–3), colourless crystalline materials were obtained in crystallization tubes after several days. For all three combinations, the structural study was achieved by an X-ray diffraction technique on single crystals.¶ For all three tectons 1–3, isostructural crystals (tetragonal, space groupI4/mcm) are formed (Fig. 2). The Zn atoms are at sites with 42 symmetry and the Si atoms are at sites with 4/m symmetry. For all three cases, no interpenetration is observed. The channels are occupied by solvent molecules (H2O for 1, CHCl3 for 2 and a mixture of CHCl3 and H2O for 3). Some solvent molecules are disordered and unfortunately not all of them could be refined. For that reason, the quality of the structural determination is rather poor.
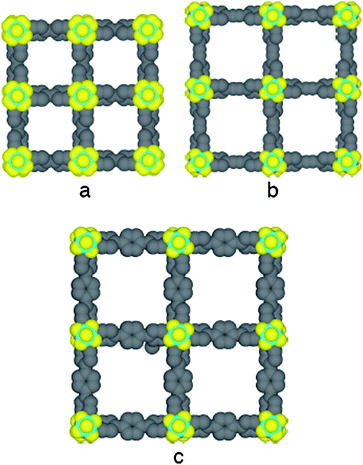 | ||
| Fig. 2 Portions of X-ray structures of the cuboid 3-D networks generated upon combining tectons 1 (a), 2 (b) and 3 (c) with Zn dication and SiF6 dianion showing the formation of channels along the c axis. H atoms and solvent molecules are omitted for clarity. | ||
As expected from the design principle mentioned above, the three component system composed of the organic tectons (1, 2 or 3), Zn2+ cation and SiF62− dianion (octahedral geometry with FSiF angles of 180 and 90°) behaving as a linear inorganic tecton, leads to the formation of neutral cuboid type architectures (Fig. 2). The combinations of zinc cations (octahedral geometry with FZnF angles of 180° and NZnN angles of 90°) with SiF62− anion (dSi–F = 1.59–1.67 Å for unbound fluorine atoms and dSi–F = 1.70–1.72 Å for bridging F atoms) leads to the formation of a neutral 1-D network along the c axis resulting from the bridging of cations by anions through Zn–F bonds (2.07–2.12 Å) (Fig. 3). The latter may be regarded as a pillar imposing the dimension of one of the three parametres of the cuboid architectures (dZn–Zn = 7.53–7.68 Å) (Fig. 4b, d and f). Each Zn2+ cation, offering four free coordination sites (2 positions occupied by F atoms), serves as a tetra-connecting node and by bridging four different organic tectons through the formation of Zn–N (dZn–N = 2.12–2.14 Å) bonds in the square base of the octahedron around the metal leads to the final cuboid 3-D network (Fig. 3).
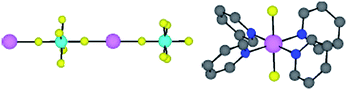 | ||
| Fig. 3 Portions of X-ray structures showing the bridging of consecutive Zn2+ cations by SiF62− anions (left) and the coordination sphere around the Zn(II) cation by two fluoride and four nitrogen atoms belonging to different tectons (right). H atoms and solvent molecules are omitted for clarity. | ||
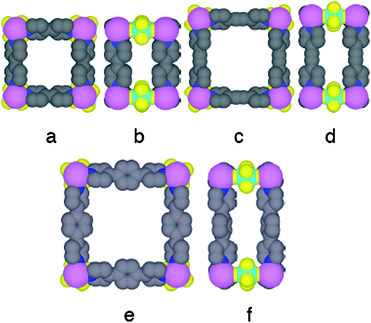 | ||
| Fig. 4 Portions of the X-ray structures of cuboid neutral 3-D networks obtained upon combining the bis-monodentate tectons 1 (a, b), 2 (c, d) and 3 (e, f) with Zn dication and SiF6 dianion showing the modulation of the size of the rectangular prisms by the length of the tecton. H atoms and solvent molecules are omitted for clarity. | ||
For the three tectons 1–3, the pyridine rings are parallel and coplanar (CCCC dihedral angle of 0.0°). In the case of 3, the central aromatic moiety is tilted by ca −37.8° with respect to the pyridine units.
Owing to the difference in the length of the tectons 1–3, the three structures differ by the distance between consecutive zinc cations (11.35, 13.85 and 15.60 Å for 1, 2 an 3, respectively) (Fig. 4a,c and e). Consequently, by modulating the length of the organic moiety, one may control the other two parametres and thus the size of the cavities (cross sections along the c axis of 7.68 × 11.35 Å for 1, 7.53 × 13.85 Å for 2 and 7.55 × 15.60 for 3) generated upon the formation of the cuboid architecture of the type (a = b, c) (Fig. 4). Unfortunately, all three crystalline materials obtained are rather unstable and upon removal of the solvent molecules they irreversibly collapse in a few seconds. For that reason, we could not further characterise and study them, in particular, for uptake and exchange of substrates and solvent molecules.
In conclusion, we have demonstrated that upon combining a rigid bismonodentate organic tecton with zinc dication and SiF62− anion as an inorganic tecton, 3-D cuboid type architectures may be designed. The cross section of the faces as well as the size of the channels may be tuned by the length of the organic unit. The 3-D networks do not interpenetrate and the empty space is occupied by solvent molecules. In order to obtain robust crystals, the same strategy is currently applied using other metal cations.
We thank the Université Louis Pasteur, the Institut Universitaire de France, the Ministry of Education and Research the CNRS and Marie Curie Est Actions FUMASSEC Network (Contrat N° MEST-CT-2005-020992) for financial support.
References
- A. F. Wells, Three-dimensional Nets and Polyhedra, Wiley, New York, 1977 Search PubMed.
- B. F. Abrahams, B. F. Hoskins and R. Robson, J. Am. Chem. Soc., 1991, 113, 3606 CrossRef CAS.
- (a) S. R. Batten and R. Robson, Angew. Chem., Int. Ed., 1998, 37, 1460 CrossRef; (b) A. J. Blake, N. R. Champness, P. Hubberstey, W.-S. Li, M. A. Withersby and M. Schröder, Coord. Chem. Rev., 1999, 193, 117 CrossRef; (c) M. W. Hosseini, in NATO ASI Series, ed. D. Braga, F. Grepiono and G. Orpen, Serie c, Kluwer, Dordrecht, the Netherlands, 1999, 538, 181 Search PubMed; (d) B. Moulton and M. J. Zaworotko, Chem. Rev., 2001, 101, 1629 CrossRef CAS; (e) M. Eddaoui, D. B. Moler, H. Li, B. Chen, T. M. Reineke, M. O'Keeffe and O. M. Yaghi, Acc. Chem. Res., 2001, 34, 319 CrossRef CAS; (f) G. F. Swiergers and T. J. Malefetse, Chem. Rev., 2000, 100, 3483 CrossRef CAS; (g) C. Janiak, Dalton Trans., 2003, 2781 RSC; (h) S. Kitagawa, Angew. Chem., Int. Ed., 2004, 43, 2434; (i) G. Férey, C. Mellot-Draznieks, C. Serre and F. Millange, Acc. Chem. Res., 2005, 38, 218.
- (a) M. Simard, D. Su and J. D. Wuest, J. Am. Chem. Soc., 1991, 113, 4696 CrossRef; (b) S. Mann, Nature, 1993, 365, 499 CrossRef CAS; (c) M. W. Hosseini, Acc. Chem. Res., 2005, 38, 313 CrossRef CAS; (d) M. W. Hosseini, CrystEngComm, 2004, 6, 318 RSC.
- C. Klein, E. Graf, M. W. Hosseini and A. De Cian, New J. Chem., 2001, 25, 207 RSC.
- S. Ferlay, S. Koenig, M. W. Hosseini, J. Pansanel, A. De Cian and N. Kyritsakas, Chem. Commun., 2002, 218 RSC.
- (a) L. D. Ciana and A. Haim, J. Heterocycl. Chem., 1984, 21, 607 Search PubMed; (b) A.-C. Ribou, T. Wada and H. Sasabe, Inorg. Chim. Acta, 1999, 134 CrossRef CAS.
- K. Biradha and M. Fujita, J. CHem. Soc., Dalton Trans., 2000, 3805 RSC.
- (a) A. Jouaiti, M. W. Hosseini, A. De Cian and J. Fischer, Chem. Commun., 2000, 1863 RSC; (b) A. Jouaiti, V. Jullien, M. W. Hosseini, J.-M. Planeix and A. De Cian, Chem. Commun., 2001, 1114 RSC; (c) A. Jouaiti, M. W. Hosseini and N. Kyritsakas, Chem. Commun., 2002, 1898 RSC.
- (a) L. R. MacGillivray, S. Subramanian and M. J. Zaworotko, J. Chem. Soc., Chem. Commun., 1994, 1325 RSC; (b) S. Subramanian and M. J. Zaworotko, Angew. Chem., 1995, 34, 2127 CAS; (c) S.-I. Noro, S. Kitagawa, M. Kondo and K. Seki, Angew. Chem., 2000, 39, 2082 CAS; (d) S.-I. Noro, R. Kitaura, M. Kondo, S. Kitagawa, T. Ishii, H. Matsuzaka and M. Yamashita, J. Am. Chem. Soc., 2002, 124, 2568 CrossRef CAS; (e) M.-C. Suen and J.-C. Wang, Struct. Chem., 2006, 17, 315 CrossRef CAS.
- G. M. Sheldrick, Programs for the Refinement of Crystal Structures, University of Göttingen, Göttingen, Germany, 1996 Search PubMed.
Footnotes |
| † Dedicated to S. Shinkai on the occasion of his 65th birthday. |
| ‡ CCDC reference numbers 693608–693608. For crystallographic data in CIF or other electronic format see DOI: 10.1039/b815695f§ |
| § Tecton 1 was purchased from Aldrich, tectons 21,2 and 33 have been prepared according to published procedures.Crystallization conditions for 1·ZnSiF6, 2·ZnSiF6 and 3·ZnSiF6: in a crystallization tube (height = 15 cm, diameter = 0.4 cm), slow diffusion of an ethanol solution (ca. 1 mL, 5 mg) of ZnSiF6·xH2O into the chloroform solution (1 mL) of the tecton (3 mg) affords colourless crystals after a few days. Crystallography: data were collected at 173(2) K on a Bruker SMART CCD Diffractometer equipped with an Oxford Cryosystem liquid N2 device, using graphite-monochromated Mo Kα radiation. For all structures, diffraction data were corrected for absorption and structural determination was achieved using SHELXS97.11 |
| ¶ Crystal data for 1·ZnSiF6: 4(C20H16F6N4SiZn)·36H2O, M = 2727.89, tetragonal, space groupI4/mcm, a = 16.0491(8), b = 16.0491(8), c = 15.3683(16) Å, V = 3958.5(5) Å3, T = 173(2) K, Z = 1, Dc = 1.144 g cm−3, µ = 0.718 mm−1, 6674 collected reflections, 1229 independent (Rint = 0.0383), GooF = 1.065, R1 = 0.0886, wR2 = 0.2779 for I > 2σ(I) and R1 = 0.1091, wR2 = 0.3060 for all data. Crystal data for 2·ZnSiF6: C24H16F6N4SiZn.0.242(C4H4Cl12)·C2H2Cl6, M = 922.17, tetragonal, space groupI4/mcm, a = 19.5940(3), b = 19.5940(3), c = 15.0666(5) Å, V = 5784.4(2) Å3, T = 173(2) K, Z = 4, Dc = 1.063 g cm−3, µ = 0.898 mm−1, 22910 collected reflections, 1813 independent (Rint = 0.0852), GooF = 1.2139, R1 = 0.1088, wR2 = 0.2958 for I > 2σ(I) and R1 = 0.1601, wR2 = 0.3287 for all data. Crystal data for 3·ZnSiF6: C32H24F6N4SiZn, M = 672.01, tetragonal, space groupI4/mcm, a = 22.0593(13), b = 22.0593(13), c = 15.0937(18) Å, V = 7344.8(11) Å3, T = 173(2) K, Z = 4, Dc = 0.608 g cm−3, µ = 0.378 mm−1, 10236 collected reflections, 2262 independent (Rint = 0.1045), GooF = 1.013, R1 = 0.0704, wR2 = 0.1860 for I > 2σ(I) and R1 = 0.0931, wR2 = 0.1927 for all data. Because of the solvent disorder, the squeeze command was used. |
| This journal is © The Royal Society of Chemistry 2009 |
