DOI:
10.1039/B814158D
(Paper)
CrystEngComm, 2009,
11, 87-93
Destabilisation of a dual-synthon hydrogen bonding motif by packing effects and competing hydrogen bond donors†
Received
14th August 2008
, Accepted 30th September 2008
First published on 4th November 2008
Abstract
Several CuII complexes of carbamoyldicyanomethanide (cdm, C(CN)2(CONH2)−) with various bidentate co-ligands have been prepared and characterised to examine the persistence of a previously observed dual-synthon ‘heterotape’ hydrogen-bonding motif that incorporates both amide and nitrile functionalities. Using 2,2′-bipyridine or 1,10-phenanthroline as co-ligands yields dimeric complexes of the form [Cu(cdm)Cl(L)]2·2MeOH (L = 2,2′-bipy 1 or 1,10-phen 2). However, the bridging species in these two dimers are different; μ2 chloride bridging in the bipyridine dimer and η1(N):η1(N′) cdm bridging in the phenanthroline complex. The subtle change in co-ligand prompts a change in the bridging mode that prevents the desired motif from forming in the phenanthroline structure, an effect that appears to arise from the greater π-stacking of the phenanthroline groups. The dimeric complex [Cu(cdm)(acac)(H2O)]23 and the monomeric complexes [Cu(cdm)2(NH2(CH2)3NH2)] 4 and [Mn(cdm)Cl(1,10-phen)2] 5 also fail to display the expected tape motif, although other complicated hydrogen bonding is present, and their structures are discussed.
Introduction
The self-assembly of metals and ligands into either discrete or polymeric products is an area rife with both frustration and serendipity,1–5 although such systems have a wide range of potential applications.6 The ideal of delibrately designing supramolecular systems from first principles remains a significant challenge and the field of crystal engineering is still very much concerned with the elucidation of new structural motifs.7–10
Small polynitrile ligands, such as dicyanamide and tricyanomethanide, have attracted much attention in the past decade, mainly due to their abilty to facilitate magnetic coupling.11 In both cases the anions have three potential coordination sites to metals, all of which are nitrogen donors. We have recently turned our attention to other dinitrile anions that contain additional functionalities.12 In particular we have focussed on dicyanonitrosomethanide, C(CN)2(NO)−, and carbamoyldicyanomethanide, C(CN)2(CONH2)−, which incorporate nitroso and amide functionalies, respectively.13–18 The addition of different functional groups introduces the possibility of new bridging modes between metals or, in the case of carbamoyldicyanomethanide, the formation of intermolecular hydrogen bonds.
As part of our previous work with carbamoyldicyanomethanide, cdm, we reported the persistent formation of a ‘heterotape’ motif.15 In both a separated ion-pair structure and several CuII complexes a hydrogen-bonded tape was observed that incorporated both the amide group of the ligand and one of the nitrile arms, Scheme 1. Complexes in which the equatorial sites of octahedral CuII were occupied by bidentate co-ligands forced the cdm ligand to coordinate via a single nitrile arm thereby allowing the requisite groups for hydrogen bonding to be free. An exception to this motif was found for the diaminoethane complex, [Cu(cdm)2(NH2(CH2)2NH2)2], in which the hydrogen bond donor capability of the diamine co-ligand disrupted the tape. We now report several new transition metal complexes containing the cdm ligand. Of particular interest are the complexes [Cu(cdm)Cl(L)]2·2MeOH (L = 2,2′-bipy or 1,10-phen) in which the subtle change in co-ligand results in a change of the bridging moiety thought to be a consequence of the intermolecular interactions present.
 |
| | Scheme 1 The anionic, dinitrile ligand carbamoyldicyanomethanide, cdm, is able to form a 2D hydrogen-bonded sheet using the amide/nitrile ‘heterotape’ motif, when coordinated to an equatorially protected octahedral metal. The tape contains both an amide dimer (blue) and an amide–nitrile ring (red). | |
Results and discussion
In a continuation of our previous work on hydrogen-bonding motifs of the cdm anion,15 we have prepared complexes incorporating a variety of larger bidentate co-ligands yielding both monomeric and dimeric products. The expected heterotape motif that has previously been observed in a number of structures (cf.Scheme 1) is only found in one of these new complexes. A variety of reasons can be found for the absence of this arrangement, such as the cdm ligand adopting bridging binding modes or a cis arrangement of ligands around the metal. In all cases, however, the cdm ligands are involved in hydrogen bonding between the discrete complexes with a variety of interesting motifs occurring.
Dimeric complexes
The reaction of CuCl2 and K(cdm) in the presence of either 2,2′-bipyridine or 1,10-phenanthroline result in the formation of two constitutionally similar, yet structurally very different, dimeric complexes; [Cu(cdm)(2,2′-bipy)Cl]2·2MeOH 1 and [Cu(cdm)(1,10-phen)Cl]2·2MeOH 2. Despite the complexes having essentially the same composition, [Cu(cdm)(L)Cl]2·2MeOH (where L = bidentate co-ligand), each contains a different bridging environment between the two metals. The bipyridine complex contains two μ2 chlorides whereas the phenanthroline complex contains two bridging η1(N)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) η1(N′) cdm ligands, Fig. 1. In both cases the cdm ligand has not fully displaced the chloride during the reaction and the crystalline materials are the sole isolated products.
η1(N′) cdm ligands, Fig. 1. In both cases the cdm ligand has not fully displaced the chloride during the reaction and the crystalline materials are the sole isolated products.
![Structures of (a) the chloride bridged dimer [Cu(cdm)(2,2′-bipy)Cl]2·2MeOH 1 and (b) the cdm bridged dimer [Cu(cdm)(1,10-phen)Cl]2·2MeOH 2. Ellipsoids displayed at 50% probability. Symmetry equivalents used; †, −x, 2 − y, −z; ‡, 1 − x, 1 − y, 1 − z. Selected bond lengths (Å) and angles (°); 1, Cu(1)–N(2), 1.962(3); Cu(1)–N(4), 2.019(4); Cu(1)–N(5), 1.999(3); Cu(1)–Cl(1), 2.674(1); Cu(1)–Cl(1)†, 2.282(1); N(4)–Cu(1)–N(5), 80.94(11); Cu(1)–Cl(1)–Cu(1)†, 89.71(3). 2, Cu(1)–N(2), 1.965(3); Cu(1)–N(3)‡, 2.413(2); Cu(1)–N(4), 2.010(2); Cu(1)–N(5), 2.010(2); Cu(1)–Cl(1), 2.235(1); N(4)–Cu(1)–N(5), 82.14(9). Hydrogen bond lengths (D⋯A/Å); 1, O(2)⋯O(1), 2.799(3). 2, O(2)⋯O(1), 2.733(3); N(1)⋯Cl(1), 3.386(3).](/image/article/2009/CE/b814158d/b814158d-f1.gif) |
| | Fig. 1 Structures of (a) the chloride bridged dimer [Cu(cdm)(2,2′-bipy)Cl]2·2MeOH 1 and (b) the cdm bridged dimer [Cu(cdm)(1,10-phen)Cl]2·2MeOH 2. Ellipsoids displayed at 50% probability. Symmetry equivalents used; †, −x, 2 − y, −z; ‡, 1 − x, 1 − y, 1 − z. Selected bond lengths (Å) and angles (°); 1, Cu(1)–N(2), 1.962(3); Cu(1)–N(4), 2.019(4); Cu(1)–N(5), 1.999(3); Cu(1)–Cl(1), 2.674(1); Cu(1)–Cl(1)†, 2.282(1); N(4)–Cu(1)–N(5), 80.94(11); Cu(1)–Cl(1)–Cu(1)†, 89.71(3). 2, Cu(1)–N(2), 1.965(3); Cu(1)–N(3)‡, 2.413(2); Cu(1)–N(4), 2.010(2); Cu(1)–N(5), 2.010(2); Cu(1)–Cl(1), 2.235(1); N(4)–Cu(1)–N(5), 82.14(9). Hydrogen bond lengths (D⋯A/Å); 1, O(2)⋯O(1), 2.799(3). 2, O(2)⋯O(1), 2.733(3); N(1)⋯Cl(1), 3.386(3). | |
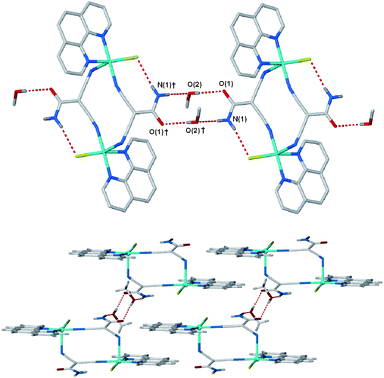
Complexes 1 and 2 both contain square pyramidal CuII and the bulk of the chelating co-ligands does not allow for two such ligands to occupy equatorial sites (unlike those we have previously studied, Scheme 1, and in the monomeric complex 4, vide infra).15 The bipyridine complex 1 is bridged by two μ2 chlorides at an angle of approximately 90° between the apical and in-plane coordination sites of the two CuII atoms, Fig. 1a. The coordination sphere of the copper is completed by the chelating bipyridine ligand and a monodentate cdm ligand that is coordinated through a single nitrile arm. The dimer is centrosymmetric with the cdm ligands orientated in opposing directions. This arrangement of the ligands is analagous to that in the previously observed monomeric complexes, Scheme 1,15 and allows for 2D sheets to assemble via the heterotape motif, Fig. 2. The lattice methanol molecules hydrogen bond to the amide oxygen atoms above and below the tape without interferring in the hydrogen bond motif. The sheets are supported by face-to-face π-interactions between the bipyridine ligands of adjacent dimeric complexes within the 2D network (inter-plane distance is 3.44 Å). The structure shows the sucessful extension of previous work to organise larger species (dimers instead of monomers) using a repeatable motif. However, a small change in the co-ligand, using phenanthroline instead of bipyridine, changes both the nature of the dimeric complex and the intermolecular interactions that are present.
 |
| | Fig. 2 (a) Hydrogen-bonded 2D sheets in 1 are held together by the amide/nitrile ‘heterotape’ motif and (b) are supported by intra-sheet π-stacking between bipyridine ligands. Symmetry equivalents used; †, −x, 3 − y, 1 − z; ‡, 1 − x, 2 − y, 1 − z. Hydrogen bond lengths (D⋯A/Å); N(1)⋯N(3)†, 3.094(4); N(1)⋯O(1)‡, 2.970(4). | |
In the dimer [Cu(cdm)(1,10-phen)Cl]2·2MeOH 2 the positions of the cdm and chloride ligands are reversed relative to those in 1, so that there are terminal chlorides and bridging cdm ligands, Fig. 1b. The cdm ligand bridges via the two nitrile groups between in-plane and apical coordination sites of the two copper atoms (cf.1). The plane of the ligand binding to the apical site is almost perpendicular to the Cu–N bond, similar to the 90° Cu–Cl–Cu bridge in 1. The bridging geometry of the cdm ligand means that the heterotape motif is unable to form (as one of the nitrile arms must be non-coordinating) and the NH2group instead takes part in both intra- and intermolecular hydrogen bonds. Intramolecularly, the amine hydrogen bonds to the terminal chloride ligand that is coordinated to an adjacent position on the metal. N–H⋯Cl–M hydrogen bonding is often observed both inter- and intramolecularly.19,20 The remaining NH hydrogen bonds to a lattice methanol molecule, which in turn hydrogen bonds to the amide oxygen atom in an adjacent dimer, Fig. 3a. These interactions form an R44(12) hydrogen bond ring which can be viewed as an extended amide dimer synthon, in which the two methanol molecules are inserted into the ‘traditional’ R22(8) amide dimer ring.21 The extended amide dimers between adjacent complexes give rise to hydrogen-bonded 1D chains. The 1D chains pack via π-stacking between the phenanthroline groups with the mean-planes of the phenanthroline ligands separated by 3.30 Å (Fig. 3b).
 |
| | Fig. 3 (a) A portion of the 1D hydrogen-bonded chain in 2. Methanol molecules are incorporated into the ‘traditional’ amide dimer synthon. Symmetry equivalent used; †, −x, 2 − y, 1 − z. Hydrogen bond distances (D⋯A/Å); O(2)⋯O(1), 2.733(3); N(1)⋯O(2)†, 2.932(4). (b) Packing between 1D chains involves π-stacking between phenanthroline ligands. | |
It is perhaps surprising that a subtle change in the bulk of the co-ligand, with no substantial alteration to the metal coordination sphere, causes such a significant change in the complex that is obtained. In this instance it appears that the π-stacking interactions between the metal complexes may be responsible for this difference. The change in the bidentate co-ligand (i.e. from bipy to phen) may not directly influence the structure of the dinuclear complex, rather the supramolecular interactions between adjacent complexes actually determine the product that crystallises. Cu(II) is known to have a very adaptable coordination geometry and therefore the change in coordination mode between 1 and 2 may involve only a very small amount of energy which could readily be offset by favourable intermolecular interactions. The π-stacking interactions in 2 are closer than those in 1 (3.30 and 3.44 Å, respectively) and there is significantly more horizontal overlap between adjacent aromatic ligands in 2 due to the larger π-surface of 1,10-phenanthroline compared to 2,2′-bipyridine. 1,10-phenanthroline is well known to form strong π–π interactions, particulary the ‘phenyl embrace’ motif.22,23
A CuCl2/K(cdm) mixture was also reacted with 2,4-pentadione yielding the mixed ligand dimer [Cu(cdm)(acac)(H2O)]2·4H2O 3 (acac = acetylacetonate) containing an anionic chelating co-ligand (Fig. 4). From a methanolic solution of K(cdm), CuCl2 and 2,4-pentadione blue crystals of [Cu(acac)2] formed within two days (determined by unit cell analysis). However, after sitting for a further four weeks these redissolved and were replaced by green crystals of 3. The centrosymmetric dimer contains square pyramidal Cu(II) atoms (cf.1 and 2) bridged by cdm ligands and capped by both acac anions and aqua ligands. The cdm ligands are bridging via the two nitrile arms, both coordinated to in-plane positions of the copper atoms. The apical coordination site is occupied by the water ligand. Due to the cdm ligands bridging through the dinitrile group only the amide functionality is free to form hydrogen bonds, in a situation similar to that of 2. Unlike compound 2, however, the amide groups form the more common amide R22(8) ring motif with no solvent interference, Fig. 5, with the lattice water bridging both within and between complexes. Each dimer has two associated water molecules bridging between cdm and acac whereby the NH2 of the cdm ligand donates a hydrogen bond to the water which in turn donates a hydrogen bond to one of the acac oxygen atoms, Fig. 5. On the opposite side of the dimer the other acac oxygen atom and that of the amide are hydrogen-bond bridged by the aqua ligand from a neighbouring complex, Fig. 6a. Overall the hydrogen bonding in the structure forms a complicated 2D sheet, Fig. 6b, with intermittent water⋯water hydrogen bonds between these sheets.
![The mixed ligand complex [Cu(cdm)(acac)(H2O)]23. Ellipsoids displayed at 50% probability. Symmetry equivalent used; †, −x, −y, −z. Selected bond lengths (Å) and angles (°); Cu(1)–N(2), 1.984(3); Cu(1)–N(3)†, 1.977(3); Cu(1)–O(2), 1.931(2); Cu(1)–O(3), 1.917(2); Cu(1)–O(4), 2.283(2); O(2)–Cu(1)–O(3), 93.81(9); N(2)–Cu(1)–N(3)†, 86.38(11).](/image/article/2009/CE/b814158d/b814158d-f4.gif) |
| | Fig. 4 The mixed ligand complex [Cu(cdm)(acac)(H2O)]23. Ellipsoids displayed at 50% probability. Symmetry equivalent used; †, −x, −y, −z. Selected bond lengths (Å) and angles (°); Cu(1)–N(2), 1.984(3); Cu(1)–N(3)†, 1.977(3); Cu(1)–O(2), 1.931(2); Cu(1)–O(3), 1.917(2); Cu(1)–O(4), 2.283(2); O(2)–Cu(1)–O(3), 93.81(9); N(2)–Cu(1)–N(3)†, 86.38(11). | |
 |
| | Fig. 5 The amide dimer synthon between amide groups in the structure of 3. Symmetry equivalents used; ‡, 1 − x, −y, 1 − z. Hydrogen bond distances (D⋯A/Å); N(1)⋯O(1)‡, 2.883(3); N(1)⋯O(6), 2.985(4); O(6)⋯O(3), 2.892(4); O(6)⋯O(5), 2.649(7). | |
 |
| | Fig. 6 (a) Hydrogen bonding involving the aqua ligands in 3 and (b) a complete hydrogen bonded sheet with one complex highlighted for clarity. Symmetry equivalents used; †, x, 1 + y, z; #, −x, 1 − y, −z. Hydrogen bond distances (D⋯A/Å); O(4)⋯O(1)†, 2.738(3); O(4)⋯O(2)#, 2.860(4). | |
Monomeric complexes
We previously reported that the complex [Cu(cdm)2(en)2] (en = diaminoethane), despite adopting the desired coordination geometry, did not assemble via the amide/nitrile heterotape motif.15 The diaminopropane complex [Cu(cdm)2(NH2(CH2)3NH2)2] 4 has since been prepared which also does not show the desired synthon but crystallises with a complicated 3D hydrogen bonding network in the non-centrosymmetric space groupFdd2.
The complex [Cu(cdm)2(NH2(CH2)3NH2)2] is similar to the diaminoethane analogue with equatorial chelating diamine ligands and axial cdm ligands. The cdm anions coordinate through a single nitrile arm leaving the remaining nitrile arm and the amide group free to participate in intermolecular interactions. Unlike the monomeric complexes we have previously observed, the cdm ligands are not co-planar, with the mean planes twisted by 31° with respect to each other. As with the diaminoethane complex, however, the presence of eight acidic hydrogen atoms per complex (i.e., potential hydrogen bond donors) in the co-ligands disrupts the heterotape motif. Hydrogen bonding in the structure of 4 involves only five of the six unique NH hydrogen atoms as one of those belonging to the cdm amide group faces towards the diamine co-ligands of the complex and is sterically unable to form an intermolecular interaction (Fig. 7). It is interesting that the cdm ligand does not partake in any hydrogen bonding interactions involving hydrogen bonded ring motifs, as seen for all other structures herein and in our previous studies.15–17 The oxygen atom of the amide group receives hydrogen bonds from three diaminopropane NH atoms (the fourth of which is involved in a weak NH⋯N interaction with a free nitrile arm). The available NH of the amide group forms a nearly linear hydrogen bond to a non-coordinating nitrile arm (177°). The amine⋯carbonyl hydrogen bonds form a sheet parallel to the ac plane and the amide⋯nitrile interactions link between these sheets, Fig. 8. The three NH interactions to the carbonyl groups lie within a narrow range (D⋯A = 2.98 – 3.04 Å) whilst the two hydrogen bonds to nitrile groups are also very similar to each other (D⋯A = 3.27 and 3.34 Å).
![Structure of the monomeric complex [Cu(cdm)2(NH2(CH2)3NH2)2] 4. Ellipsoids displayed at 50% probability. Symmetry equivalent used: †, −x, 1 − y, z. Selected bond lengths (Å) and angles (°); Cu(1)–N(2), 2.599(2); Cu(1)–N(4), 2.025(3); Cu(1)–N(5), 2.021(3); N(4)–Cu(1)–N(5), 91.07(7); N(4)–Cu(1)–N(4)†, 87.94(14); N(5)–Cu(1)–N(5)†, 90.41(14).](/image/article/2009/CE/b814158d/b814158d-f7.gif) |
| | Fig. 7 Structure of the monomeric complex [Cu(cdm)2(NH2(CH2)3NH2)2] 4. Ellipsoids displayed at 50% probability. Symmetry equivalent used: †, −x, 1 − y, z. Selected bond lengths (Å) and angles (°); Cu(1)–N(2), 2.599(2); Cu(1)–N(4), 2.025(3); Cu(1)–N(5), 2.021(3); N(4)–Cu(1)–N(5), 91.07(7); N(4)–Cu(1)–N(4)†, 87.94(14); N(5)–Cu(1)–N(5)†, 90.41(14). | |
 |
| | Fig. 8 (a) A sheet within the structure of 4, viewed along the b-axis, is formed by amine⋯carbonyl interactions. (b) Packing of 4 viewed along the c-axis with sheets connected by NH⋯nitrile hydrogen bonds. CH hydrogen atoms omitted for clarity. | |
The monomeric complex [Mn(cdm)Cl(1,10-phen)2] 5 is different from previously observed complexes containing cdm, with the anion bound solely via the oxygen atom (Fig. 9a). The manganese adopts a distorted octahedral geometry, with the two phenanthroline ligands forming chelating rings with N–Mn–N angles of approximately 73°. The cdm ligand forms an intramolecular hydrogen bond to the chloride ligand making a six-membered hydrogen bond ring. The discrete complex forms hydrogen-bonded dimers utilising the amide group and one of the nitrile arms, the same R22(12) ring that is present in the heterotape motif (N–H⋯N = 2.182 Å). Due to the coordination of the ligands through the oxygen atom the full tape motif is unable to form. The second nitrile arm is involved in hydrogen bonding with a lattice methanol molecule. A partial occupancy water molecule is also present in the lattice although it does not appear to take part in any significant intermolecular interactions. Packing in the structure is dominated by face-to-face π-interactions between the phenanthroline ligands with the lattice solvent molecules playing no significant role in hydrogen bonding between complexes (Fig. 9b). The η1(O) coordination mode of the cdm anion, to the best of our knowledge, has not previously been reported.12 Binding through the oxygen atom is observed in bridging coordination modes, with both (N,O) and (N,N′,O) modes known.24–26
![(a) The centrosymmetic hydrogen-bonded dimer of [Mn(cdm)Cl(1,10-phen)2] 5. CH hydrogen atoms and lattice solvent are omitted for clarity. Selected bond lengths (Å) and angles (°); Mn(1)–O(1), 2.106; Mn(1)–Cl(1), 2.474; Mn(1)–N(4), 2.243; Mn(1)–N(5), 2.326; Mn(1)–N(6), 2.265; Mn(1)–N(7), 2.272; N(4)–Mn(1)–N(5), 72.98; N(6)–Mn(1)–N(7), 73.41. (b) Packing in the structure of 5, viewed along the c-axis, is dominated by π interactions with solvent molecules present in ‘channels’ between the complexes.](/image/article/2009/CE/b814158d/b814158d-f9.gif) |
| | Fig. 9 (a) The centrosymmetic hydrogen-bonded dimer of [Mn(cdm)Cl(1,10-phen)2] 5. CH hydrogen atoms and lattice solvent are omitted for clarity. Selected bond lengths (Å) and angles (°); Mn(1)–O(1), 2.106; Mn(1)–Cl(1), 2.474; Mn(1)–N(4), 2.243; Mn(1)–N(5), 2.326; Mn(1)–N(6), 2.265; Mn(1)–N(7), 2.272; N(4)–Mn(1)–N(5), 72.98; N(6)–Mn(1)–N(7), 73.41. (b) Packing in the structure of 5, viewed along the c-axis, is dominated by π interactions with solvent molecules present in ‘channels’ between the complexes. | |
Conclusions
A robust supramolecular motif, previously observed between monomeric complexes, has been used to arrange dimeric species into 2D hydrogen-bonded sheets. Surprisingly, 2,2′-bipyridine and 1,10-phenanthroline fail to form the same dimeric species and therefore the tape motif does not exist in the latter case. It appears that π-interactions involving the aromatic co-ligands are responsible for the structural change. Further examples of complexes where the hydrogen-bonding tape fails to form suggests that strong competition from other hydrogen-bond donor/acceptor groups interferes with the targeted motif or in a rare case where the cdm ligand coordinates via the oxygen atom.
Experimental
Synthesis
All reagents and solvents were purchased from standard commercial sources and used without further purification. Potassium carbamoyldicyanomethanide, K(cdm), was prepared according to literature.27ATR-IR spectra were collected using a Bruker Equinox 55 spectrometer. Elemental analyses were conducted at the Cambell Analytical Laboratories, University of Otago, New Zealand. Yields are estimated to be in the range 50–70% for all reactions except 3 (see below).
[Cu(cdm)Cl(2,2′-bipy)]2·2MeOH,1
K(cdm) (0.05 g, 0.34 mmol), CuCl2 (0.02 g, 0.17 mmol) and 2,2′-bipyridine (0.05 g, 0.34 mmol) were dissolved in MeOH (5 ml) giving a dark green solution. The solution was left at room temperature for one week during which time dark green crystals formed. Anal. calc. for C30H28N10Cl2Cu2O4: C, 45.58; H, 3.57; N, 17.72%. Found; C, 44.65; H, 3.27; N, 17.27% (with inclusion of one water per dimer calc. C, 44.56; H, 3.74; N, 17.32%).
[Cu(cdm)Cl(1,10-phen)]2·2MeOH,2
K(cdm) (0.05 g, 0.34 mmol), CuCl2 (0.02 g, 0.17 mmol) and 1,10-phenanthroline (0.06 g, 0.34 mmol) were dissolved in MeOH (5 ml) giving a dark green solution. The solution was left at room temperature for one week yielding dark green crystals. Anal. calc. for C17H14N5Cl1Cu1O2 (with loss of all MeOH): C, 48.69; H, 3.37; N, 16.70%. Found; C, 49.50; H, 2.99; N, 17.40%.
[Cu(cdm)(acac)(H2O)]2.4H2O,
3
K(cdm) (0.05 g, 0.34 mmol), CuCl2 (0.02 g, 0.17 mmol) and an excess of 2,4-pentadione (0.08 g, 0.8 mmol) were dissolved in MeOH (5 ml) giving a pale blue solution. Pale blue crystals formed within 24 h which were found to be [Cu(acac)2] by unit cell analysis. Over the course of one month these crystals were observed to redissolve and were replaced by a very small amount of pale green crystals of the mixed ligand complex. Sufficient compound could not be obtained for further analysis.
[Cu(cdm)2(NH2(CH2)3NH2)2],
4
K(cdm) (0.05 g, 0.34 mmol), CuCl2 (0.02 g, 0.17 mmol) and an excess of diaminopropane (0.1 g, 1.35 mmol) were dissolved in MeOH (5 ml) forming a deep blue solution. The solution was left for 3 months, yielding no solid product, and was then left to evaporate to dryness over the course of two weeks. Dark blue single crystals were hand-picked from the residue in the sample vial, although insufficient sample could be isolated in this manner for elemental analysis.
[Mn(cdm)Cl(1,10-phen)2]·MeOH·1/2H2O,
5
K(cdm) (0.05 g, 0.34 mmol), MnCl2·4H2O (0.03 g, 0.17 mmol) and 1,10-phenanthroline (0.06 g, 0.34 mmol) were dissolved in MeOH (5 ml). The solution was left at room temperature for two weeks yielding yellow crystals. Anal. calc. for C28H18N7O1Cl1Mn1 (with loss of all solvent): C, 60.17; H, 3.25; N, 17.54%. Found: C, 59.93; H, 3.31; N, 17.50%.
Crystals were mounted on fine glass fibres using viscous hydrocarbon oil. Data were collected using a Bruker X8 ApexII CCD diffractometer equipped with graphite monochromated Mo Kα radiation (λ = 0.71073 Å). Data collection temperatures were maintained at 123 K using an open-flow N2 cryostream. Initial data processing was carried out using the Apex II software suite.28 Structures were solved by direct methods using SHELXS-97 and refined using standard alternating least-squares cycles against F2 using SHELXL-97.29 The program X-Seed was used as a graphical interface.30Hydrogen atoms attached to carbon were placed in idealised positions and refined with a riding model. Hydrogen atoms attached to nitrogen or oxygen were located from the Fourier difference map and allowed to refine freely where possible. Crystallographic details are summarised in Table 1.
| |
1
|
2
|
3
|
4
|
5
|
|
Flack parameter = 0.000(15).31
|
| Formula |
C30H28Cl2Cu2N10O4 |
C34H28Cl2Cu2N10O4 |
C18H30Cu2N6O12 |
C14H24Cu1N10O2 |
C29H23Cl1Mn1N7O2.5 |
|
M
|
790.60 |
838.64 |
649.56 |
427.97 |
599.93 |
| Crystal system |
Triclinic |
Triclinic |
Triclinic |
Orthorhombic |
Triclinic |
| Space group |
P-1
|
P-1
|
P-1
|
Fdd2 a |
P-1
|
|
a/Å |
8.3016(8) |
7.4044(7) |
8.1154(10) |
17.8128(12) |
10.5476(7) |
|
b/Å |
8.8946(9) |
9.9340(9) |
8.9932(9) |
28.8740(30) |
11.5860(7) |
|
c/Å |
11.7703(13) |
12.0617(13) |
11.1540(11) |
7.1606(6) |
12.2153(7) |
|
α/° |
83.191(3) |
108.736(5) |
113.154(6) |
90 |
88.998(3) |
|
β/° |
81.216(3) |
91.509(5) |
101.507(6) |
90 |
67.186(2) |
|
γ/° |
67.746(3) |
99.329(5) |
101.847(6) |
90 |
84.951(3) |
|
V/Å3 |
793.19(14) |
826.15(14) |
696.12(13) |
3682.9(6) |
1370.42(15) |
|
Z
|
1 |
1 |
1 |
8 |
2 |
|
μ/mm−1 |
1.564 |
1.507 |
1.594 |
1.220 |
0.622 |
|
No. unique reflns |
3610 |
2864 |
3042 |
2092 |
6270 |
|
No. obs [I ≥ 2σI] |
2882 |
2465 |
2607 |
1866 |
5152 |
| Parameters |
230 |
248 |
182 |
213 |
381 |
|
R
int
|
0.0343 |
0.0230 |
0.0253 |
0.0399 |
0.0423 |
|
wR
2
(all data) |
0.1007 |
0.0759 |
0.1105 |
0.0650 |
0.1089 |
|
R [I ≥ 2σI] |
0.0438 |
0.0314 |
0.0430 |
0.0305 |
0.0422 |
Special refinement details
3.
Hydrogen atomss of the lattice water molecules could not be located from the Fourier difference map (presumably due to rotational disorder) and were therefore not included in the refinement. The hydrogen atoms of the NH2group would not refine satisfactorily and were placed in idealised positions (those of the aqua ligand were allowed to refine freely).
4.
Flack parameter = 0.000(15).31
5.
The partial occupancy water molecule was refined with a fixed occupancy of 50%. OH hydrogen atoms could not be located from the Fourier map and were not included in the refinement.
References
-
J. W. Steed, D. R. Turner and K. J. Wallace, Core Concepts in Supramolecular Chemistry and Nanochemistry, J. Wiley & Sons, Chichester, 2007 Search PubMed.
-
S. R. Batten, S. M. Neville and D. R. Turner, Coordination Polymers: Design, Analysis and Application, Royal Society of Chemistry, Cambridge, 2008 Search PubMed.
- M. J. Zaworotko, Cryst. Growth Des., 2007, 7, 4 CrossRef CAS.
- S. R. Batten, J. Solid State Chem., 2005, 178, 2475 CrossRef CAS.
- S. R. Batten and K. S. Murray, Aust. J. Chem., 2001, 54, 605 CrossRef.
- C. H. M. Amijs, G. P. M. van Klink and G. van Koten, Dalton Trans., 2006, 308 RSC and references threrein.
- D. Braga, L. Brammer and N. R. Champness, CrystEngComm, 2005, 7, 1 RSC.
- L. Brammer, Chem. Soc. Rev., 2004, 33, 476 RSC.
- D. Braga, Chem. Commun., 2003, 2751 RSC.
- K. Biradha, CrystEngComm, 2003, 5, 374 RSC.
- S. R. Batten and K. S. Murray, Coord. Chem. Rev., 2003, 246, 103 CrossRef CAS.
-
D. R. Turner and S. R. Batten, in Coordination Chemistry Research Progress, ed. T. W. Cartere and K. S. Verley, Nova Publishers, Hauppage, NY, 2008, p. 153 Search PubMed.
- A. S. R. Chesman, D. R. Turner, E. I. Izgorodina, G. B. Deacon and S. R. Batten, Dalton Trans., 2007, 1371 RSC.
- A. S. R. Chesman, D. R. Turner, D. J. Price, B. Moubaraki, K. S. Murray, G. B. Deacon and S. R. Batten, Chem. Commun., 2007, 3541 RSC.
- D. R. Turner, S. N. Pek and S. R. Batten, Chem.–Asian J., 2007, 2, 1534 CrossRef CAS.
- D. R. Turner and S. R. Batten, CrystEngComm, 2008, 10, 170 RSC.
- D. R. Turner, S. N. Pek and S. R. Batten, New J. Chem., 2008, 32, 719 RSC.
- D. R. Turner, S. N. Pek, J. D. Cashion, B. Moubaraki, K. S. Murray and S. R. Batten, Dalton Trans. 10.1039/b815084b.
- L. Brammer, E. A. Bruton and P. Sherwood, Cryst. Growth Des., 2001, 1, 277 CrossRef CAS.
- D. R. Turner, B. Smith, A. E. Goeta, I. Radosavljevic-Evans, D. A. Tocher, J. A. K. Howard and J. W. Steed, CrystEngComm, 2004, 6, 633 RSC.
- Using Graph set nomenclature, see J. Bernstein, R. E. Davis, L. Shimoni and N.-L. Chang, Angew. Chem., Int. Ed. Engl., 1995, 34, 1555 Search PubMed.
- I. Dance, New J. Chem., 2003, 27, 22–27 RSC.
- V. Russell, M. Scudder and I. Dance, J. Chem. Soc., Dalton Trans., 2001, 789–799 RSC.
- J.-M. Shi, H.-K. Xu, C.-J. Wu, B. Zhao and P. Cheng, Transition Met. Chem., 2004, 29, 586 CrossRef CAS.
- C.-J. Wu and J.-M. Shi, Chin. J. Struct. Chem., 2004, 23, 1161 Search PubMed.
- J.-M. Shi, W. Xu, Q.-Y. Liu, F.-L. Liu, Z.-L. Huang, H. Lei, W.-T. Yu and Q. Fang, Chem. Commun., 2002, 756 RSC.
- J. A. Schlueter and U. Geiser, Acta Crystallogr., Sect. C, 2004, 60, m10.
-
Apex
II Suite, Bruker AXS Ltd., 2006, Madison, WS Search PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. A, 2008, 64, 112.
- L. J. Barbour, J. Supramol. Chem., 2001, 1, 189–191 CrossRef CAS.
- H. D. Flack, Acta Crystallogr., Sect. A, 1983, 39, 876 CrossRef.
|
| This journal is © The Royal Society of Chemistry 2009 |
Click here to see how this site uses Cookies. View our privacy policy here. 
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) η1(N′) cdm ligands, Fig. 1. In both cases the cdm ligand has not fully displaced the chloride during the reaction and the crystalline materials are the sole isolated products.
η1(N′) cdm ligands, Fig. 1. In both cases the cdm ligand has not fully displaced the chloride during the reaction and the crystalline materials are the sole isolated products.
![Structures of (a) the chloride bridged dimer [Cu(cdm)(2,2′-bipy)Cl]2·2MeOH 1 and (b) the cdm bridged dimer [Cu(cdm)(1,10-phen)Cl]2·2MeOH 2. Ellipsoids displayed at 50% probability. Symmetry equivalents used; †, −x, 2 − y, −z; ‡, 1 − x, 1 − y, 1 − z. Selected bond lengths (Å) and angles (°); 1, Cu(1)–N(2), 1.962(3); Cu(1)–N(4), 2.019(4); Cu(1)–N(5), 1.999(3); Cu(1)–Cl(1), 2.674(1); Cu(1)–Cl(1)†, 2.282(1); N(4)–Cu(1)–N(5), 80.94(11); Cu(1)–Cl(1)–Cu(1)†, 89.71(3). 2, Cu(1)–N(2), 1.965(3); Cu(1)–N(3)‡, 2.413(2); Cu(1)–N(4), 2.010(2); Cu(1)–N(5), 2.010(2); Cu(1)–Cl(1), 2.235(1); N(4)–Cu(1)–N(5), 82.14(9). Hydrogen bond lengths (D⋯A/Å); 1, O(2)⋯O(1), 2.799(3). 2, O(2)⋯O(1), 2.733(3); N(1)⋯Cl(1), 3.386(3).](/image/article/2009/CE/b814158d/b814158d-f1.gif)


![The mixed ligand complex [Cu(cdm)(acac)(H2O)]23. Ellipsoids displayed at 50% probability. Symmetry equivalent used; †, −x, −y, −z. Selected bond lengths (Å) and angles (°); Cu(1)–N(2), 1.984(3); Cu(1)–N(3)†, 1.977(3); Cu(1)–O(2), 1.931(2); Cu(1)–O(3), 1.917(2); Cu(1)–O(4), 2.283(2); O(2)–Cu(1)–O(3), 93.81(9); N(2)–Cu(1)–N(3)†, 86.38(11).](/image/article/2009/CE/b814158d/b814158d-f4.gif)


![Structure of the monomeric complex [Cu(cdm)2(NH2(CH2)3NH2)2] 4. Ellipsoids displayed at 50% probability. Symmetry equivalent used: †, −x, 1 − y, z. Selected bond lengths (Å) and angles (°); Cu(1)–N(2), 2.599(2); Cu(1)–N(4), 2.025(3); Cu(1)–N(5), 2.021(3); N(4)–Cu(1)–N(5), 91.07(7); N(4)–Cu(1)–N(4)†, 87.94(14); N(5)–Cu(1)–N(5)†, 90.41(14).](/image/article/2009/CE/b814158d/b814158d-f7.gif)

![(a) The centrosymmetic hydrogen-bonded dimer of [Mn(cdm)Cl(1,10-phen)2] 5. CH hydrogen atoms and lattice solvent are omitted for clarity. Selected bond lengths (Å) and angles (°); Mn(1)–O(1), 2.106; Mn(1)–Cl(1), 2.474; Mn(1)–N(4), 2.243; Mn(1)–N(5), 2.326; Mn(1)–N(6), 2.265; Mn(1)–N(7), 2.272; N(4)–Mn(1)–N(5), 72.98; N(6)–Mn(1)–N(7), 73.41. (b) Packing in the structure of 5, viewed along the c-axis, is dominated by π interactions with solvent molecules present in ‘channels’ between the complexes.](/image/article/2009/CE/b814158d/b814158d-f9.gif)
