Effects of chitosan on the alignment, morphology and shape of calcite crystals nucleating under Langmuir monolayers
Kyungil
Kim
,
Ahmet
Uysal
,
Sumit
Kewalramani†
,
Benjamin
Stripe
and
Pulak
Dutta
Department of Physics & Astronomy, Northwestern University, Evanston, IL 60208, USA
First published on 13th October 2008
Abstract
The growth of calcium carbonate crystals under Langmuir monolayers was investigated in the presence of chitosan, a soluble derivative of chitin added to the subphase to better simulate the polyelectrolyte-containing in vivo environment. Chitosan causes distinct concentration-dependent changes in the orientation, shape and morphology of the calcite crystals nucleating under acid and sulfate monolayers. Our results suggest that polyelectrolytes may play essential roles in controlling the growth of biogenic calcite crystals.
Introduction
Inorganic calcite crystals typically have a rhombohedral shape of which all six surfaces are electrostatically stable {104} planes. However, biogenic calcite grows in the c-axis direction such that calcium ion layers and carbonate layers alternate. The unstable charged (001) surfaces are covered by a protein and polysaccharide organic matrix.1 Floating (Langmuir) monolayers with acid head groups are extensively used as model organic templates for calcite crystal growth, but this biomimetic model, which reproduces the anionic effects of aspartic acid-rich proteins, does not exhibit the full range of the orientations and morphologies of biogenic calcites.2–7 The real organic matrix contains acidic proteins, hydrophobic silk fibroin, and β-chitin, all with aspects of polyelectrolytes. For example the silk fibroin, which is initially in a disordered state before mineral formation and inhibits calcium carbonate crystallizationin vitro, pairs with some of the acidic proteins involved in crystal growth.8 A better biomimetic system would incorporate the effects of some of these components in addition to the monolayer template.In our group's previous studies of non-biological mineral growth under Langmuir monolayers, we have observed oriented crystallization of BaF29 and 2(PbCO3) Pb(OH)210 under heneicosanoic acid monolayers. In the case of BaF2 both the monolayer and the inorganic crystal are strained in order to achieve a lattice match, while in 2(PbCO3) Pb(OH)2 superlattice structures form at the surface as a strain relaxation mechanism.11Growth of oriented calcite crystals under carboxylic acid monolayers has proved more challenging; only disordered (powder) growth is seen in situ using X-rays.6,12 However, the use of a bis-urea based carboxylate monolayer results in oriented growth.6 The orientation and shapes of calcite single crystals are also elegantly controlled by simple hydrocarbon chain self-assembled monolayers (SAM) when grown on various crystalline substrates to produce a range of lattice spacings.13–15
The nacre organic matrix contains chitin, an insoluble material whose role in biomineralization is poorly understood. Chitosan is obtained by the deacetylatation of chitin, and is soluble in acidic to neutral pH. It has both hydrophilic and hydrophobic sites, and the hydrophobicity can be adjusted by the degree of deacetylation.16,17 Important studies of nucleation at the surfaces of thin films of chitin and chitosan, in combination with acidic proteins and/or silk fibroins,18–21 have shown that one can control whether calcite, vaterite or aragonite crystals grow. In a non-biomimetic study of the effect of the polystyrenesulfonate on calcite mesocrystals,22 it was found that this polyelectrolyte caused the assembly of small crystals into a variety of larger shapes.
We have focused on calcite growth under Langmuir monolayers, a familiar and well-studied biomimetic process, and sought to determine whether the crystal alignment and morphology can be affected by dissolved polyelectrolytes. Chitosan in the subphase is a simple way to reproduce aspects of the polyelectrolyte environment of the in vivo organic matrix, while retaining the simplicity and flexibility of the Langmuir monolayer model system. There is also a practical benefit![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) chitosan partially segregates to the surface and makes the surface region viscous. This makes it easier to transfer crystals to substrates, and it makes it harder for the crystals to change their orientations during or after transfer.
chitosan partially segregates to the surface and makes the surface region viscous. This makes it easier to transfer crystals to substrates, and it makes it harder for the crystals to change their orientations during or after transfer.
This paper is a report of our studies of calcite nucleation under n-alkanoic acid and sulfate monolayers at various concentrations of chitosan in the subphase. We used optical microscope and scanning electron microscopy to look at transferred crystals, and in situgrazing-incidence X-ray diffraction (GID) at the air water interface to determine the structures of the crystals and the monolayers, averaged over a macroscopic X-ray footprint.
Results and discussion
1. Chitosan-CaCO3 solution without monolayer
Chitosan in the subphase inhibits CaCO3crystallization within the bulk solution and restricts it to the surface of water. Fig. 1 shows results for CaCO3growth at the surface from subphases with various concentrations of chitosan and 9mM CaCO3 (without a Langmuir monolayer). It can be seen from Fig. 1 (a), (b) and (d) that chitosan concentration has no visible effect on the crystals ultimately nucleated![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) unoriented rhombohedral calcite with {104} facets are seen. However, nucleation is slower at higher concentrations, and this allows us to better see the early stage (Fig 1(c)). Amorphous speculites are seen to be dispersed evenly on the surface of water at the early stage (2∼5hrs) and were visible even with the naked eye. No in situGID peaks were observed at this stage. After a 2–3 hour period when unoriented vaterites are dominant in the X-ray data, rhombohedral calcite finally prevails (Fig. 1(d)). Fig. 1(e) shows GID intensity contours, reconstructed from multiple horizontal-plane scans (ΔKxy = 0.005 A−1, ΔKz = 0.1 A−1) at the stage of Fig. 1(d). Faint gray lines indicate position of Scherrer rings; the peak intensities are distributed along the rings, showing that the crystals are randomly oriented. Calcite peaks are sharper and stronger than the broad vaterite peaks (denoted by ‘v’). Such unoriented growth has previously been observed at alcohol surfaces on SAMs.13
unoriented rhombohedral calcite with {104} facets are seen. However, nucleation is slower at higher concentrations, and this allows us to better see the early stage (Fig 1(c)). Amorphous speculites are seen to be dispersed evenly on the surface of water at the early stage (2∼5hrs) and were visible even with the naked eye. No in situGID peaks were observed at this stage. After a 2–3 hour period when unoriented vaterites are dominant in the X-ray data, rhombohedral calcite finally prevails (Fig. 1(d)). Fig. 1(e) shows GID intensity contours, reconstructed from multiple horizontal-plane scans (ΔKxy = 0.005 A−1, ΔKz = 0.1 A−1) at the stage of Fig. 1(d). Faint gray lines indicate position of Scherrer rings; the peak intensities are distributed along the rings, showing that the crystals are randomly oriented. Calcite peaks are sharper and stronger than the broad vaterite peaks (denoted by ‘v’). Such unoriented growth has previously been observed at alcohol surfaces on SAMs.13
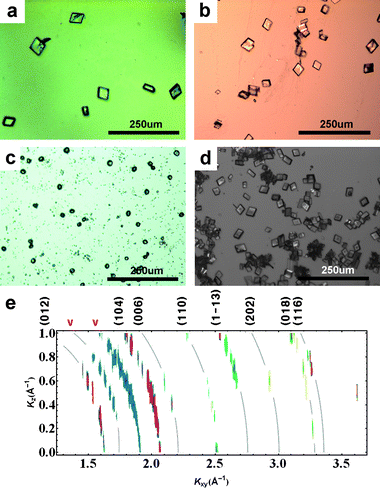 | ||
Fig. 1
Optical microscope images of crystals transferred to flat substrates, for calcium carbonate nucleation in the presence of chitosan but without a floating monolayer![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Chitosan concentrations are (a) 3 × 10−4% w/v; (b) 4.5 × 10−4% w/v; (c)–(d) Chitosan concentrations are (a) 3 × 10−4% w/v; (b) 4.5 × 10−4% w/v; (c)–(d)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 × 10−4% w/v, early and late stage. The larger number of crystals in (d) is an artifact of the increased viscosity near the surface at higher chitosan concentrations, which improves the transfer of crystals to substrates. In situ GID intensity contours for the case (d) are shown in (e). Curved lines have been added to indicate the positions of the “Debye rings” corresponding to the calcite peaks labeled along the top margin. 6 × 10−4% w/v, early and late stage. The larger number of crystals in (d) is an artifact of the increased viscosity near the surface at higher chitosan concentrations, which improves the transfer of crystals to substrates. In situ GID intensity contours for the case (d) are shown in (e). Curved lines have been added to indicate the positions of the “Debye rings” corresponding to the calcite peaks labeled along the top margin. | ||
2. Heneicosanoic acid monolayers on CaCO3 subphase, with and without chitosan
Fatty acid monolayers are ordered but have all possible lattice orientations in the horizontal plane; thus the in-plane component of the momentum transfer, Kxy, cannot be further resolved into x- and y-components. The in-plane structure of the heneicosanoic acid monolayer on a 9 mM CaCO3 subphase, without chitosan, can be seen using in situGID (Fig. 2a). The carboxylate monolayer lattice structure is distorted-hexagonal with untilted molecules (the so called S phase) at close to zero surface pressure and room temperature. This structure is not what would be seen on pure water, but is known to appear in the presence of subphase ions. The monolayer peaks grow weaker and disappear when the calcite peaks appear, or even earlier.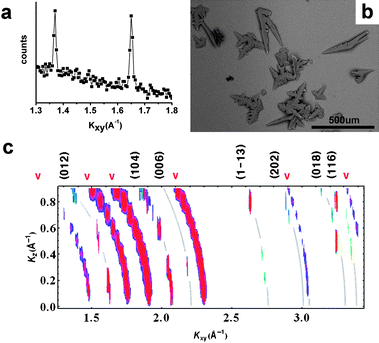 | ||
| Fig. 2 In situ GID intensity contours, and optical microscope images of transferred crystals, for CaCO3growth under heneicosanoic acid Langmuir monolayers without chitosan in the subphase. In (c), curved lines have been added to indicate the positions of the “Debye rings” corresponding to the calcite peaks labeled along the top margin. | ||
These monolayers nucleate sawtooth-like calcite polycrystals (Fig. 2b). Fig. 2c shows GID intensity contours, reconstructed from diffraction scans (ΔKxy = 0.005 A−1, ΔKz = 0.1 A−1). Again, because of the cylindrical symmetry about the surface normal, the nucleate is a powder in the plane and Kxy cannot be resolved into its x- and y-components. Calcite peaks are sharper and stronger than the broad vaterite peaks (denoted by ‘v’). Faint white lines indicate position of Scherrer rings. The peak intensities are distributed along the rings (see, especially, the strong calcite (104) peak) indicating that the nucleate is randomly oriented in all directions.
The appearance of calcite diffraction peaks can be slowed by adding chitosan to the subphase, and the organic peaks become broader. If ∼3 × 10−4% w/v chitosan is added to the CaCO3 subphase, the monolayer structure is undistorted hexagonal (LS phase) (Fig. 3a). This is the structure that would be seen at room teperature on a subphase without ions, and indicates that chitosan reduces the interaction of ions with the monolayer. (This is discussed further later in this paper.) The crystals that grow under these conditions (Fig. 3b) are very similar to those reported7 to grow under stearic acid, which is a shorter-chain acid and so may have a hexagonal LS structure even in the presence of ions. Thus chitosan modifies the acid monolayer template; this in turn changes the calcite growth characteristics. These crystals have previously been reported to be [110] oriented calcite.7 We see (Fig. 3c) that in the presence of chitosan, the peak intensities are not uniformly spread along the Scherrer rings, i.e. the calcite has some degree of alignment. However, there are more spots than would be visible in this range for a single orientation; the nucleate is still a mixture of multiple orientations.
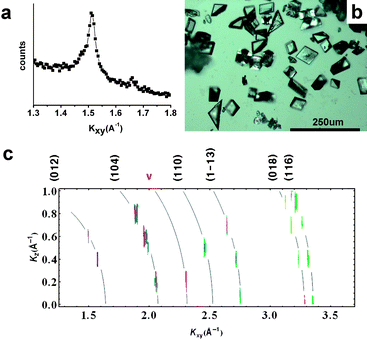 | ||
| Fig. 3 In situ GID data, and optical microscope images of transferred crystals, for CaCO3growth under heneicosanoic acid Langmuir monolayers with 3 × 10−4% w/v chitosan in the subphase. In (c), curved lines have been added to indicate the positions of the “Debye rings” corresponding to the calcite peaks labeled along the top margin. | ||
When chitosan (up to 6 × 10−4% w/v) is added only after the monolayer has been spread (by dropping it into the subphase behind the barrier of Langmuir trough), the monolayer structure does not change at all![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) it remains in the distorted-hexagonal phase. Calcite crystallization is slowed but is otherwise unaffected. It appears that any calcite-monolayer interaction and templating is immediate; chitosan introduced later cannot alter the final product. When the organic peaks have nearly disappeared but no calcite peak has yet appeared (∼2 h after starting the experiment), chitosan injection revives the organic peak intensities without changing their initial positions, and the polycrystalline calcite growth process restarts.
it remains in the distorted-hexagonal phase. Calcite crystallization is slowed but is otherwise unaffected. It appears that any calcite-monolayer interaction and templating is immediate; chitosan introduced later cannot alter the final product. When the organic peaks have nearly disappeared but no calcite peak has yet appeared (∼2 h after starting the experiment), chitosan injection revives the organic peak intensities without changing their initial positions, and the polycrystalline calcite growth process restarts.
3. Arachidic sulfate monolayers on CaCO3 subphase, with and without chitosan
Vaterite peaks are not seen under sulfate monolayers. The calcite crystals grown under arachidic sulfate monolayers spread on a 9 mM CaCO3 subphase, without chitosan, are primarily oriented with their [001] axis perpendicular to the water surface as evidenced by the peak positions in Fig. 4(a). The spots are vertically extended because of the poor resolution of our apparatus in the vertical direction, but they are not smeared along Scherrer rings. Note, for example, that the (110) and (001) directions are perpendicular each other, so the presence of a strong (110) peak around Kz = 0, as well as the positions of the strong (102) and (1–13) spots in the Kxy–Kz plane, are all evidence of predominantly (001) growth. Not all spots consistent with (110) alignment; in other words there are some misoriented crystals.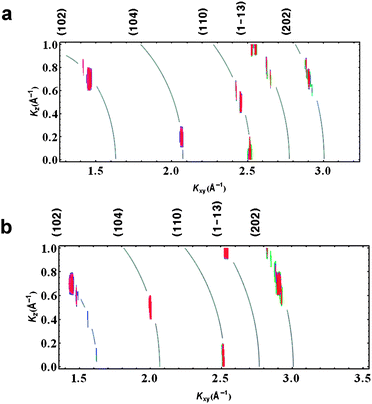 | ||
Fig. 4 In situ GID intensity contours for calcite growth under arachidic sulfate monolayers![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (a) without chitosan and (b) with 3 × 10−4% w/v dissolved chitosan. Curved lines have been added to indicate the positions of the “Debye rings” corresponding to the calcite peaks labeled along the top margin. (a) without chitosan and (b) with 3 × 10−4% w/v dissolved chitosan. Curved lines have been added to indicate the positions of the “Debye rings” corresponding to the calcite peaks labeled along the top margin. | ||
With 3 × 10−4% w/v chitosan added to the subphase (as before, but now with a sulfate monolayer rather than an acid monolayer), we found that the predominant (001) orientation remains (Fig. 4(b)). However, the dissolved chitosan has a strong effect on the shapes of the nucleated crystals, as discussed below.
Fig. 5 shows the shapes of nucleated crystals, both top and bottom views, at various chitosan concentrations. Top views are of crystals transferred to substrates in the usual way, i.e.via the Langmuir–Schaeffer method. We also lowered horizontal slides thinly coated with glue down into the surface in order to attach the upper surface of the crystals and allow us to look at the bottom surfaces. All larger images are from optical microscopes; small images (insets) are from a scanning electron microscope in environmental-SEM mode, except in the two cases when the crystal faces were unstable in the electron beam. These cases are noted below and the corresponding insets are optical images.
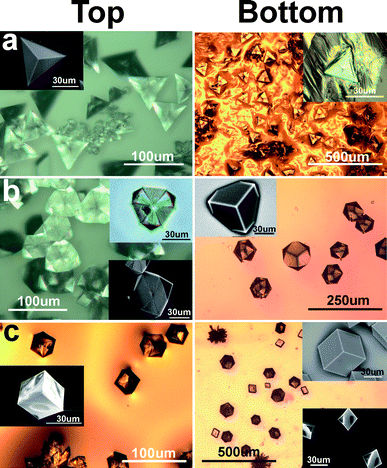 | ||
| Fig. 5 Shape and morphology of calcite crystals grown under arachidic sulfate monolayers, with (a) no chitosan in subphase; (b) ∼3 × 10−4% w/v chitosan in subphase, and (c) ∼4.5 × 10−4% w/v chitosan in subphase. | ||
In the absence of chitosan, the calcite crystals have shapes similar to those previously reported to grow over SAMs on Au substrates.15 Heywood and Mann5 suggested that the pyramidal calcite single crystals (Fig. 5a, left) grow downward from the air–water interface, with the monolayer tightly binding to the (001) basal plane, and rotate 180° when the crystals are transferred to substrates by the Langmuir–Schaeffer method (fig.4c of ref. 5). We found that the calcite crystals are triangular dipyramid shaped, with the lower pyramid rotated 60° and confined inside the triangle area of the basal (001) plane (Fig. 5a, right). These bottom faces are unstable upon drying or in electron beams. Thus the calcite crystals grow pyramidally in both directions, overcoming any monolayer binding to the (001) surface. In this system we do not see passivated (001) surfaces.
At 3 × 10−4% w/v chitosan concentration, we found an intermediate state of calcite single crystals (Fig. 5b) whose bottom shape is the same as the shapes on Au/Ag alloy substrates reported in ref. 15. The top view of the “intermediate” calcite crystals show well developed pinwheel-like planes. These planes can be various calcite faces as the crystals grow. The intermediate state calcites are unstable![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) drying or electron beam exposure changes the morphology; compare the optical image (upper inset) with the SEM image (lower inset). The bottom faces in Fig. 5b are stable and appear to be {104} faces.
drying or electron beam exposure changes the morphology; compare the optical image (upper inset) with the SEM image (lower inset). The bottom faces in Fig. 5b are stable and appear to be {104} faces.
When 4.5 × 10−4% w/v or more of chitosan is added, cubic shaped calcite crystals are formed and the bottom side also shows cubic shapes whose bottom shape is the same as the shapes on Ag substrates reported in ref. 15 (fig. 5(c)). There are also some trigonal trapezohedron shapes in which one corner is not developed. GID experiments confirm that the under all the conditions of Fig. 5, the crystals are predominantly oriented with the (001) direction normal to the water surface. When even more chitosan is added, the chitosan dominates the process entirely, and nucleation is then similar to that occurring in the absence of any monolayer (Fig. 1).
Experimental
9.6 g of chitosan (low density, Aldrich) was dissolved and stirred with a magnetic stirrer in 25 ml of acetic acid and 200 ml of 18.2 MΩ cm deionized water for 2 d in a closed bottle. More water was added slowly to dissolve the sticky chemical completely into water solution. This produces a base solution of 1.92% w/v chitosan. ∼9 mM CaCO3 supersaturated solutions were made by bubbling with CO2 (bone dry grade 2.8∼3) according to the Kitano method.23 The initial pH of the CaCO3 subphase is 6.0 ± 0.2. The chitosan solution is added using a syringe into 300 ml of CaCO3 solution and stirred briefly to make the subphase. The small amounts of chitosan we added do not change the pH significantly. Heneicosanoic acid (CH3(CH2)19COONa, Aldrich) is dissolved in chloroform to a concentration of 3 mM, and arachidic sulfate (CH3(CH2)19OSO3Na, Aldrich) is dissolved in a mixture of cyclohexane, methanol, and chloroform (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 vol.) to a concentration of 1 mM. 75 μl of heneicosanoic acid or 225 μl of arachidic sulfate is spread on the CaCO3 subphase to make acidic monolayers using syringes in the Langmuir trough (400 cm2 is the initial surface area). The monolayers on non-chitosan subphases are compressed slightly over zero surface pressure. The addition of chitosan increased the surface pressure. We performed calcite crystallization on the chitosan subphase at the same surface area as non-chitosan subphase case. All GID data in this paper were taken ∼12hrs after starting the experiment. SEM images are from a Hitachi S3400 used in its Environmental-SEM mode.
1 vol.) to a concentration of 1 mM. 75 μl of heneicosanoic acid or 225 μl of arachidic sulfate is spread on the CaCO3 subphase to make acidic monolayers using syringes in the Langmuir trough (400 cm2 is the initial surface area). The monolayers on non-chitosan subphases are compressed slightly over zero surface pressure. The addition of chitosan increased the surface pressure. We performed calcite crystallization on the chitosan subphase at the same surface area as non-chitosan subphase case. All GID data in this paper were taken ∼12hrs after starting the experiment. SEM images are from a Hitachi S3400 used in its Environmental-SEM mode.
Conclusions
Our studies show that the presence of dissolved chitosan has multiple effects. First, it acts as an inhibitor of calcite growth. This function is similar to that of polyaspartic acid and polyacrylic acid.24 When introduced after crystallization has begun, it even has the effect of reversing the process. These results suggest that this polyelectrolyte has the ability to store calcium carbonate, perhaps in amorphous form, and deliver it slowly.Second, increasing the concentration of chitosan under acid monolayers, while not producing perfectly aligned crystals, creates some degree of alignment whereas acid monolayers by themselves nucleate only uniform powders. This is probably because chitosan modifies the acid template. This result suggests an explanation for a puzzling result: why should SAM monolayers, composed of tethered molecules with limited flexibility, form better templates13–15 than laterally-untethered and thus ‘soft’ carboxylic acid Langmuir monolayers? It is well known that the presence of ions, including calcium ions, modifies carboxylic acid Langmuir monolayer structures. We suggest that such changes do not lead to the right structures for templating: rather, a separate component (such as chitosan in our experiments) is needed to play a role analogous to the SAM substrate. This material would pre-determine and stabilize the template structure against which calcium carbonate is nucleated.
Third, the chitosan concentration affects the shapes of the crystals grown under sulfate monolayers. Sulfate monolayers are much more weakly ordered than acid monolayers (only one small diffraction peak at Kxy = 1.445 A−1 is seen at high surface pressure,25 implying a hexagonal lattice), but calcite crystals grown under sulfate monolayers are (001) ordered25 just as in many biominerals. Remarkably, the shapes in our Fig. 5 reproduce those reported in Fig. 4 of ref. 15, where very different templates (self-assembled monolayers on Au, Ag and an Au–Ag alloy substrates) were used. In that paper it was postulated that increasing lattice mismatch caused increasing strain, which led to each of these shapes being favored. In our experiments we have no rigid substrate to which the template molecules are tethered. However, our results can still be related to those in ref. 15 by postulating that the driving force is the organic–inorganic interface tension (energy).26 In the system of ref. 15, increasing strain will increase the interface energy. In our case, it is reasonable that the interface energy increases with chitosan concentration (as described earlier, chitosan tends to ‘detach’ calcium ions from the monolayer). Thus the underlying physical mechanism is probably the same in both cases.
Floating monolayer studies do not reproduce the biological environment insofar as the crystals are free to grow in both directions, either by penetrating the monolayer or by sinking slightly into the water. Nonetheless, the addition of polyelectrolytes incorporates additional aspects of the biological situation into this class of model systems, and our studies suggest that the polyelectrolytes in the organic matrix may have shape-controlling and template-modifying roles in shells as well as a role in calcite storage.
Acknowledgements
We thank Jianming Bai, Evguenia Karapetrova and Paul Zschack for their advice and assistance with this project. This work was supported by the US Department of Energy under grant no. DE-FG02-84ER45125. Grazing-incidence X-ray scattering experiments were performed at Beam Line X-14A of the National Synchrotron Light Source and Sector 33-BM-C of the Advanced Photon Source, both of which are supported by the US Department of Energy.References
- S. Mann, Biomineralization: principles and concepts in bioinorganic materials chemistry, Oxford University Press, Oxford, New York, 2001 Search PubMed.
- D. Volkmer, N. Mayr and M. Fricke, Dalton Trans., 2006, 4889–4895 RSC.
- S. Mann, B. R. Heywood, S. Rajam and J. D. Birchall, Nature, 1988, 334, 692–695 CrossRef CAS.
- S. Mann, B. R. Heywood, S. Rajam and J. D. Birchall, Proc. R. Soc. London, Ser. A, 1989, 423 Search PubMed 457-&.
- B. R. Heywood and S. Mann, Chem. Mater., 1994, 6, 311–318 CrossRef CAS.
- D. C. Popescu, M. M. J. Smulders, B. P. Pichon, N. Chebotareva, S. Y. Kwak, O. L. J. van Asselen, R. P. Sijbesma, E. DiMasi and N. Sommerdijkt, J. Am. Chem. Soc., 2007, 129, 14058–14067 CrossRef CAS.
- S. Rajam, B. R. Heywood, J. B. A. Walker, S. Mann, R. J. Davey and J. D. Birchall, J. Chem. Soc., Dalton Trans., 1991, 87, 727–734 Search PubMed.
- L. Addadi, D. Joester, F. Nudelman and S. Weiner, Chem.–Eur. J., 2006, 12, 981–987.
- J. Kmetko, C. J. Yu, G. Evmenenko, S. Kewalramani and P. Dutta, Phys. Rev. Lett., 2002, 89 Search PubMed.
- S. Kewalramani, G. Evmenenko, C. J. Yu, K. Kim, J. Kmetko and P. Dutta, Surf. Sci., 2005, 591, L286–L291 CrossRef CAS.
- J. Kmetko, A. Datta, G. Evmenenko, M. K. Durbin, A. G. Richter and P. Dutta, Langmuir, 2001, 17, 4697–4700 CrossRef CAS.
- J. Kmetko, C. Yu, G. Evmenenko, S. Kewalramani and P. Dutta, Phys. Rev. B: Condens. Matter Mater. Phys., 2003, 68 Search PubMed.
- J. Aizenberg, A. J. Black and G. H. Whitesides, J. Am. Chem. Soc., 1999, 121, 4500–4509 CrossRef CAS.
- J. Aizenberg, A. J. Black and G. M. Whitesides, Nature, 1999, 398, 495–498 CrossRef CAS.
- B. Pokroy and J. Aizenberg, CrystEngComm, 2007, 9, 1219–1225 RSC.
- M. Rinaudo, Prog. Polym. Sci., 2006, 31, 603–632 CrossRef CAS.
- N. Boucard, L. David, C. Rochas, A. Montembault, C. Viton and A. Domard, Biomacromolecules, 2007, 8, 1209–1217 CrossRef CAS.
- G. Falini, S. Albeck, S. Weiner and L. Addadi, Science, 1996, 271, 67–69 CrossRef.
- Y. Levi, S. Albeck, A. Brack, S. Weiner and L. Addadi, Chem.–Eur. J., 1998, 4, 389–396 CrossRef CAS.
- G. Falini, S. Fermani, S. Vanzo, M. Miletic and G. Zaffino, Eur. J. Inorg. Chem., 2005, 162–167 CrossRef CAS.
- S. R. Payne, M. Heppenstall-Butler and M. F. Butler, Cryst. Growth Des., 2007, 7, 1262–1276 CrossRef CAS.
- T. P. Wang, M. Antonietti and H. Colfen, Chem.–Eur. J., 2006, 12, 5722–5730 CrossRef CAS.
- J. Rudloff, M. Antonietti, H. Colfen, J. Pretula, K. Kaluzynski and S. Penczek, Macromol. Chem. Phys., 2002, 203, 627–635 CrossRef CAS.
- L. A. Gower and D. A. Tirrell, J. Cryst. Growth, 1998, 191, 153–160 CrossRef CAS.
- S. Kewalramani, K. Kim, B. Stripe, G. Evmenenko, G. H. B. Dommett and P. Dutta, Langmuir, 2008, 24, 10579–10582 CrossRef CAS.
- B. Pokroy, personal communication.
Footnote |
| † Present address: Brookhaven National Laboratory, Upton, 11973 NY, USA |
| This journal is © The Royal Society of Chemistry 2009 |
