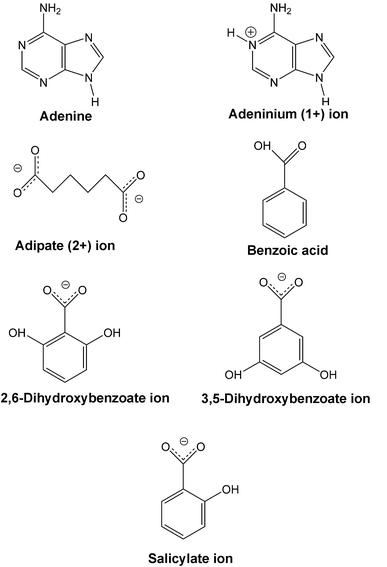
Supramolecular structures of six adenine-carboxylic acid complexes†
Maureen
Byres
,
Philip J.
Cox
*,
Graeme
Kay
and
Elaine
Nixon
School of Pharmacy, The Robert Gordon University, Aberdeen, AB10 1FR, UK. E-mail: p.j.cox@rgu.ac.uk; Fax: +01224 262555; Tel: +01224 262535
First published on 14th October 2008
Abstract
Studies concentrating on hydrogen bonding between the bases of DNA and small molecule proton acceptor/donators have led to an increased understanding of the role such complexes have in DNA binding. Here anhydrous and solvated multicomponent crystals of adenine have been prepared with benzoic acid, adipic acid, salicylic acid, 2,6-dihydroxybenzoic acid and 3,5-dihydroxybenzoic acid. The six crystalline forms reported are an anhydrous cocrystal, a methanolated cocrystal, an anhydrous proton-transfer complex, a hydrated proton-transfer complex, a methanolated proton-transfer complex and a doubly hydrated salt. All products were formed in solution and obtained by the slow evaporation technique. A comparison of hydrogen bonding motifs in the products is presented.
Introduction
The interaction of small molecules with DNA is of major interest in chemotherapy.1,2 The ability of such molecules to bind to DNAvia an intercalative, groove-binding or electrostatic mode has led to the development of many novel anticancer,3,4 antibacterial and antimicrobial agents.5,6 There have been a number of studies investigating the relationship between biological activity and DNA binding,7,8,9 furthermore, studies concentrating on hydrogen bonding between the nucleotide bases (adenine, cytosine, guanine and thymine) and small molecule proton acceptor/donators have led to an increased understanding of the role such complexes have in duplex binding.10,11Adenine is known to form several crystalline complexes with metal ions such as zinc,12cobalt,12manganese13,14 and tellurium.15 Adducts with N-methyl-2-pyrrolidone16 and benzoic acid17 are also known and many crystalline complexes of adenine are hydrated, e.g., adenine-riboflavin trihydrate,18tris(adenine) phthalic hexahydrate19 and deoxycorticosterone-adenine monohydrate.20Adenine itself forms a trihydrate21 in the crystalline state. When protonated, adenine forms the adeninium cation and examples of such salts are adeninium hemisulfate hydrate,22adeninium succinate monohydrate23 and adenium chloroacetate chloroacetic acid solvate.24 Both adenine and adeninium cations can exist in the same crystal as in adeninium perchlorate adenine dihydrate.25
We have determined the molecular and supramolecular structures of complexes formed between adenine and some carboxylic acids. Differences between the various products including molecular disorder, solvent inclusion and hydrogen bonding motifs are discussed.
Experimental
Preparative methods
Starting chemicals, including solvents, were obtained from Sigma. In all preparations equi-millimolar quantities of the two starting materials were weighed, transferred to a 50 ml beaker and 15 ml of solvent added. The mixtures were stirred and warmed on a water bath until a clear solution was achieved. Crystals, formed by slow evaporation of the solvent, were obtained by filtration.(I) [Adenine][benzoic acid]2
Cocrystals were obtained from a dry methanol solution.
(II) [Adenine]2 [adipic acid] [methanol]2
Cocrystals were obtained from a dry methanol solution.
(III) [Adeninium]+ [2,6-dihydroxybenzoate]−
The proton-transfer complex was obtained from a dry methanol solution.
(IV) [Adeninium]+ [2,6-dihydroxybenzoate]−[H2O]
The hydrated proton-transfer complex was obtained from a methanol solution.
(V) [Adeninium]+ [2-hydroxybenzoate]− [methanol]
The methanolated proton-transfer complex was obtained from a dry methanol solution.
(VI) [Adeninium]+ [OH]− [3,5-dihydroxybenzoate]− [H3O]+
The doubly hydrated salt of adenine and 3,5-dihydroxybenzoic acid was obtained from an aqueous ethanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solution.
1) solution.
Crystal structure determination and refinement
Data were collected using ϕ and ω scans on a Bruker-Nonius KappaCCD diffractometer with graphite-monochromated Mo Kα radiation (λ = 0.7107 Å) at 120 K. The programs DENZO26 and COLLECT27 were used in data collection and cell refinement. Multi-scan adsorption corrections were made with SADABS.28 The structures were solved with SIR9729 and refined with SHELXL97.30Hydrogen atoms were constrained to their parent site except for the hydrogens in water (V) and water ions (VII) that were freely refined. Molecular geometries were obtained with PLATON31 and molecular plots were obtained with WinGX32 and MERCURY.33 Further details of crystal and structure refinement are shown in Table 1. The molecules and ions discussed in this study are presented in Scheme 1.| (I) | (II) | (III) | (IV) | /V | (VI) | |
|---|---|---|---|---|---|---|
| Molecular formula | [C5H5N5].2[C7H6O2] | 2[C5H5N5]. [C6H10O4].2[CH4O] | [C5H6N5]+.[C7H5O4]− | [C5H6N5]+.[C7H5O4]−.[H2O] | [C5H6N5]+.[C7H5O3]−.[CH3OH] | [C5H6N5]+.[OH]−[C7H5O4]−.[H3O]+ |
| M r | 379.38 | 480.49 | 289.25 | 307.27 | 305.30 | 325.28 |
| Crystal system | Triclinic | Triclinic | Monoclinic | Monoclinic | Monoclinic | Monoclinic |
| Space group |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
P21/c | P21 | P21/n | Cc |
| a/Å | 6.9422(2) | 8.9781(5) | 7.0966(3) | 6.9541(2) | 7.1636(2) | 11.6347(5) |
| b/Å | 10.1547(4) | 9.9334(5) | 7.2069(3) | 15.2160(6) | 7.9486(3) | 14.9993(8) |
| c/Å | 14.1896(5) | 14.2815(7) | 23.5630(11) | 7.0195(3) | 24.7336(9) | 7.9794 |
| α/° | 81.003(2) | 74.908(3) | 90 | 90 | 90 | 90 |
| β/° | 76.327(2) | 85.357(3) | 93.978(3) | 118.588(2) | 91.248(2) | 91.496(3) |
| γ/° | 70.344(2) | 66.786(3) | 90 | 90 | 90 | 90 |
| Volume/Å3 | 912.09(6) | 1129.84(11) | 1202.11(9) | 652.21 | 1408.01(8) | 1392.03(12) |
| Z | 2 | 2 | 4 | 2 | 4 | 4 |
| T max , T min | 0.8606, 0.9881 | 0.6436, 0.9935 | 0.7409, 0.9975 | 0.7715, 0.9755 | 0.7372, 0.9784 | 0.8668, 0.9924 |
| D x /Mg m−3 | 1.381 | 1.412 | 1.598 | 1.565 | 1.440 | 1.552 |
| μ/mm−1 | 0.10 | 0.11 | 0.12 | 0.12 | 0.11 | 0.13 |
| Crystal shape | Slab | Slab | Lath | Block | Block | Block |
| Colour | Colourless | Colourless | Colourless | Colourless | Colourless | Pale brown |
| Range of h,k,l | −8 → h → 8 | −11 → h → 11 | −9 → h → 8 | −8 → h → 9 | −9 → h → 9 | −15 → h → 14 |
| −13 → k → 13 | −12 → k → 12 | −9 → k → 9 | −19 → k → 19 | −10 → k → 10 | −193 → k → 18 | |
| −18 → l → 18 | −18 → l → 18 | −30 → l → 29 | −9 → l → 9 | −32 → l → 31 | −10 → l → 10 | |
| Reflections collected | 19221 | 22726 | 13945 | 9017 | 14712 | 7231 |
| Independent reflections | 4145 | 5140 | 2955 | 1553 | 3435 | 1653 |
| Crystal size/mm | 0.34 × 0.28 × 0.12 | 0.42 × 0.20 × 0.06 | 0.36 × 0.08 × 0.02 | 0.28 × 0.24 × 0.20 | 0.34 × 0.20 × 0.20 | 0.14 × 0.14 × 0.06 |
| Data/restraints/parameters | 4139, 26, 288 | 5137, 0, 313 | 2738, 0, 192 | 1538, 4, 205 | 3195, 0, 207 | 1599, 9, 223 |
| Goodness of fit | 1.045 | 1.071 | 1.046 | 1.116 | 1.053 | 1.080 |
| R1 and R2[I > 2σI] | 0.0415, 0.0946 | 0.0462, 0.1075 | 0.0512, 0.1077 | 0.0541, 0.1583 | 0.0464, 0.1126 | 0.0512, 0.1166 |
| R1 and R2 [all data] | 0.0562, 0.1007 | 0.0757, 0.1198 | 0.0877, 0.1245 | 0.0565, 0.1598 | 0.0655, 0.1227 | 0.0690, 0.1245 |
| Weighting scheme P = (Fo2 + 2Fc2)/3 | w = 1/[σ2(Fo2) + (0.0433P)2 + 0.2515P] | w = 1/[σ2(Fo2) + (0.0539P)2 + 0.2864P] | w = 1/[σ2(Fo2) + (0.0488P)2 + 0.5699P] | w = 1/[σ2(Fo2) + (0.0434P)2 + 2.1292P] | w = 1/[σ2(Fo2) + (0.0501P)2 + 0.6703P] | w = 1/[σ2(Fo2) + (0.0328P)2 + 3.9257P] |
| Largest difference peak and hole/e Å−3 | 0.252, −0.224 | 0.250, −0.268 | 0.254, −0.311 | 0.381, −0.375 | 0.239, −0.256 | 0.326, −0.380 |
 | ||
| Scheme 1 Molecules and ions in this study. | ||
Results
Crystal structures
(I) [Adenine][benzoic acid]2The numbering scheme is shown in Fig. 1.
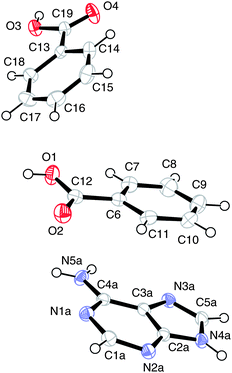 | ||
| Fig. 1 The atomic arrangement in (I), major site for adenine, displacement ellipsoids are shown at the 50% probability level. | ||
The (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) cocrystal is held together by hydrogen bonding. The cell dimensions are similar to those previously published17 but unlike the known structure the present study has revealed a second position for the adenine molecule such that two sites are occupied in a ratio of 0.90
2) cocrystal is held together by hydrogen bonding. The cell dimensions are similar to those previously published17 but unlike the known structure the present study has revealed a second position for the adenine molecule such that two sites are occupied in a ratio of 0.90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.10. Prior to location of the minor site the Fourier difference synthesis revealed a difference peak maximum of only 0.8 e Å−3. The electron density associated with the minor site of adenine was very low and hence common isotropic displacement parameters were used for the atoms and its geometry was constrained to be the same as that of the major site. The hydrogen bonding motif for the major site for adenine is shown in Fig.2 and the motif for the minor site is shown in Fig 3. Hydrogen bonding geometry is shown in Table 2.
0.10. Prior to location of the minor site the Fourier difference synthesis revealed a difference peak maximum of only 0.8 e Å−3. The electron density associated with the minor site of adenine was very low and hence common isotropic displacement parameters were used for the atoms and its geometry was constrained to be the same as that of the major site. The hydrogen bonding motif for the major site for adenine is shown in Fig.2 and the motif for the minor site is shown in Fig 3. Hydrogen bonding geometry is shown in Table 2.
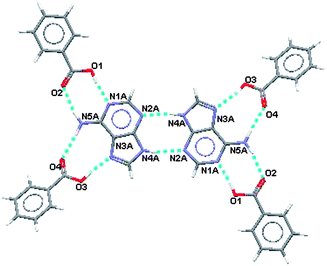 | ||
| Fig. 2 Hydrogen bonding motif for the major site for adenine with benzoic acid. | ||
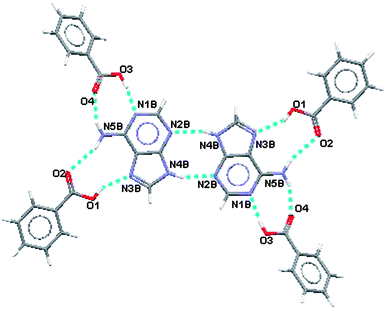 | ||
| Fig. 3 Hydrogen bonding motif for the minor site for adenine with benzoic acid. | ||
From Fig. 2 and Fig. 3 it can be seen that the hydrogen bonding motif is identical for cells containing either the minor or major sites of adenine. However, the hydrogen bonds between adenine and benzoic acid, for the two possible adenine sites, involve different oxygen atoms, as indicated by the oxygen labels.
The homosynthon between adenine molecules is a planar R22(8) ring and the two heterosynthons are planar R22(8) and non-planar R22(9) formations.
The closest approach of ring centres in the crystal packing is 3.337(1) Å between the pyrimidine and imidazole rings, the latter translated by 1 − x, 2 − y, −z. Weak C–H⋯π bonding is also present (Table 2).
| D–H⋯A | d(D–H) | d(H⋯A) | d(D⋯A) | ∠(DHA) |
|---|---|---|---|---|
| a Symmetry transformations used to generate equivalent atoms: #1 −x + 1, −y + 1, −z #2 −x, −y + 1, −z + 1 #3 x − 1, y, z + 1 #4 −x + 2, −y + 2, −z #5 x + 1, y, z − 1 #6 −x, −y, −z. Cg(I) = centre of benzoid ring [C(6)—C(11)] transformed by: −x + 1, y + 1/2, −z + 1/2. | ||||
| O1–H1⋯N1A#1 | 0.84 | 1.80 | 2.6357(15) | 170 |
| O1–H1⋯N3B | 0.84 | 1.92 | 2.694(14) | 152 |
| O3–H3⋯N3A#2 | 0.84 | 1.84 | 2.6721(15) | 170 |
| O3–H3⋯N1B#3 | 0.84 | 1.77 | 2.596(12) | 167 |
| N5A–H5B⋯O4#2 | 0.88 | 2.15 | 3.017(2) | 166 |
| N5A–H5A⋯O2#1 | 0.88 | 2.06 | 2.927(2) | 167 |
| N4A–H4A⋯N2A#4 | 0.88 | 1.98 | 2.8506(17) | 168 |
| N5B–H5X⋯O4#5 | 0.88 | 1.97 | 2.80(3) | 157 |
| N5B–H5Y⋯O2 | 0.88 | 2.33 | 3.17(2) | 159 |
| N4B–H4B⋯N2B#6 | 0.88 | 1.95 | 2.796(19) | 160 |
| C16–H16⋯O1 | 0.95 | 2.51 | 3.4502(17) | 169 |
| C17–H17⋯Cg(I) | 0.95 | 2.74 | 3.4254(16) | 130 |
(II) [Adenine]2 [adipic acid] [methanol]2
The numbering scheme of (II) is shown in Fig. 4.
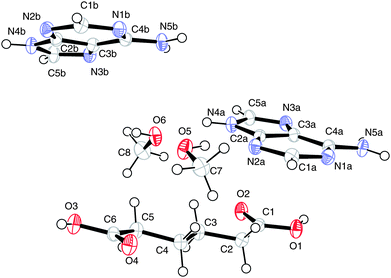 | ||
| Fig. 4 The atomic arrangement in (II). Displacement ellipsoids are shown at the 50% probability level. | ||
This is a methanolated cocrystal and the hydrogen bonding motif is shown in Fig. 5. Hydrogen bonding geometry is shown in Table 3.
| D–H⋯A | d(D–H) | d(H⋯A) | d(D⋯A) | ∠(DHA) |
|---|---|---|---|---|
| a Symmetry transformations used to generate equivalent atoms: #1 −x + 1, −y, −z #2 x + 1, y, z #3 −x + 1, −y + 1, −z #4 −x + 1, −y + 1, −z + 1 #5 x − 1, y, z #6 −x + 2, −y, −z. | ||||
| O1–H1⋯N3A#1 | 0.84 | 1.85 | 2.6779(18) | 166 |
| O3–H3⋯N3B#2 | 0.84 | 1.84 | 2.6668(18) | 166 |
| N4A–H4A⋯O6 | 0.88 | 1.86 | 2.7001(18) | 158 |
| N5A–H5A⋯N1B#3 | 0.88 | 2.16 | 3.029(2) | 171 |
| N5A–H5B⋯O2#1 | 0.88 | 2.09 | 2.9481(19) | 166 |
| N4B–H4B⋯O5#4 | 0.88 | 1.91 | 2.7366(18) | 156 |
| N5B–H5X⋯N1A#3 | 0.88 | 2.15 | 3.018(2) | 168 |
| N5B–H5Y⋯O4#5 | 0.88 | 2.12 | 2.9731(18) | 162 |
| O5–H5⋯N2A | 0.84 | 1.97 | 2.7988(18) | 167 |
| O6–H6⋯N2B#4 | 0.84 | 1.95 | 2.7680(19) | 166 |
| C2–H2B⋯N1A#6 | 0.99 | 2.58 | 3.557(2) | 171 |
| C5B–H5E⋯O2#3 | 0.95 | 2.49 | 3.252(2) | 136 |
| C5A–H5Z⋯O4#5 | 0.95 | 2.45 | 3.176(2) | 133 |
 | ||
| Fig. 5 Hydrogen bonding motif for (II). | ||
Unlike (I) a R22(8) homosynthon involving the NH2groups directly links adenine molecules. A further R44(12) hydrogen bonded ring is formed when methanol molecules are interdispersed between two adenine molecules. Adenine molecules also link to the −COOH groups of adipic acid in R22(9) formations. Extending the supramolecular structure over several molecules shows the formation of a R1010(42) ring.
The closest approach of ring centres in the crystal packing is 3.625(1) Å between two pyrimidine rings, one being translated by 1 − x, 1 − y, 1 − z.
(III) [Adeninium]+ [2,6-dihydroxybenzoate]−
The numbering scheme of (III) is shown in Fig. 6.
 | ||
| Fig. 6 The atomic arrangement in (III). Displacement ellipsoids are shown at the 50% probability level. | ||
This is a salt where the hydrogen atom from the carboxylic group of 2,6-dihydroxybenzoic acid has transferred to the N1 nitrogen of the pyrimidine ring. The carboxylic group now possesses a negative charge with similar C–O bond lengths and the acceptor is positively charged. This enables formation of a R22(8) hydrogen bonded ring between the acid and the base where the charged nitrogen atoms are the donors and the charged oxygen atoms are the acceptors. The anion and cation are also linked by a single hydrogen bond.
Adeninium–adeninium self-association base pairs are present as R22(10) formations. Intramolecular hydrogen bonds form within the 2,6-dihydroxybenzoate ion between the orthohydroxy groups and the partially charged carboxylate oxygens. Further extension of the supramolecular structure reveals a R1414(40) ring formation as shown in Fig. 7. Hydrogen bonding geometry is shown in Table 4.
| D–H⋯A | d(D–H) | d(H⋯A) | d(D⋯A) | ∠(DHA) |
|---|---|---|---|---|
| a Symmetry transformations used to generate equivalent atoms: #1 −x + 2, y + 1/2, −z + 1/2 #2 −x + 2, −y + 1, −z #3 −x + 1, −y + 2, −z #4 x + 1, y, z #5 x − 1, y, zCg(I) = centre of benzoid ring [C(7)—C(12)] transformed by: −x + 1, y + 1/2, −z + 1/2. | ||||
| N4–H4A⋯O4#1 | 0.88 | 2.04 | 2.901(2) | 166 |
| N5–H5A⋯O2#2 | 0.88 | 2.09 | 2.971(2) | 175 |
| N5–H5B⋯N3#3 | 0.88 | 2.14 | 2.974(2) | 158 |
| N1–H1A⋯O1#2 | 0.88 | 1.76 | 2.635(2) | 175 |
| O3–H3⋯O1 | 0.84 | 1.77 | 2.523(2) | 147 |
| O4–H4⋯O2 | 0.84 | 1.81 | 2.567(2) | 148 |
| C1–H1⋯O3#4 | 0.95 | 2.33 | 3.226(3) | 157 |
| C9–H9⋯N2#5 | 0.95 | 2.59 | 3.471(3) | 155 |
| C10–H10⋯Cg(I) | 0.95 | 2.86 | 3.667(2) | 143 |
 | ||
| Fig. 7 Partial hydrogen bonding pattern in (III). | ||
The closest approach of ring centres in the crystal packing is 3.582(1) Å between the aryl ring of 2,6-dihydroxybenzoate and the imidazole ring translated by 1 − x, 2 − y, −z. Weak C–H⋯π bonding is also present (Table 4).
(IV) [Adeninium]+ [2,6-dihydroxybenzoate]−[H2O]
The numbering scheme of (IV) is shown in Fig. 8.
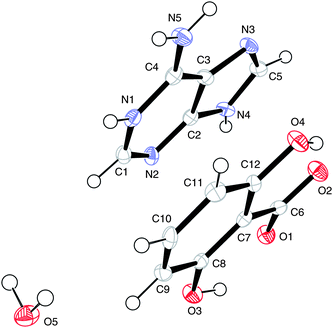 | ||
| Fig. 8 The numbering scheme in (III). Displacement ellipsoids are shown at the 50% probability level. | ||
The numbering scheme here is identical to that used in (III) and the water oxygen is labelled O(5). This is the hydrated form of (III) and the hydrogen bonding pattern is shown in Fig. 9. Hydrogen bonding geometry is shown in Table 5.
| D–H⋯A | d(D–H) | d(H⋯A) | d(D⋯A) | ∠(DHA) |
|---|---|---|---|---|
| a Symmetry transformations used to generate equivalent atoms: #1 x − 1, y, z − 1 #2 −x, y − 1/2, −z + 1 #3 x + 1, y, z #4 x + 1, y, z + 1 #5 −x + 1, y + 1/2, −z + 2 Cg(I) is the cenrte of benzoid ring [C(7)—C(12)] transformed by: x, y − 1/2, −z + 2. | ||||
| O3–H3⋯O1 | 0.84 | 1.85 | 2.546(5) | 139 |
| O3–H3⋯O4#1 | 0.84 | 2.44 | 2.854(5) | 111 |
| O4–H4⋯O2 | 0.84 | 1.78 | 2.533(6) | 148 |
| N4–H4A⋯O5#2 | 0.88 | 1.83 | 2.708(6) | 178 |
| N5–H5A⋯O2#3 | 0.88 | 1.90 | 2.776(6) | 176 |
| N5–H5B⋯N2#4 | 0.88 | 2.42 | 3.086(6) | 133 |
| N1–H1A⋯O1#3 | 0.88 | 1.83 | 2.714(6) | 177 |
| O5–H5X⋯N3#5 | 0.85 | 2.00 | 2.827(6) | 164 |
| O5–H5Y⋯O3#3 | 0.86 | 2.13 | 2.942(6) | 158 |
| C11–H11⋯O3#4 | 0.95 | 2.52 | 3.261(7) | 135 |
| C5–H5C⋯Cg(I) | 0.95 | 2.49 | 3.256(6) | 137 |
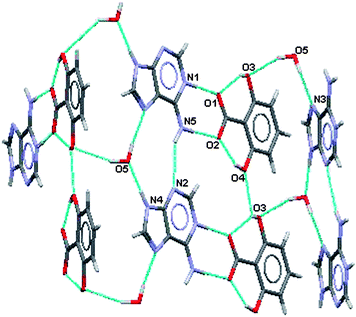 | ||
| Fig. 9 Partial hydrogen bonding in (IV). | ||
Various ring formations result from the hydrogen bonding in this salt. As in (III) there are R22(8)hydrogen bonded rings between the acid and the base where the charged nitrogen atoms are the donors and the charged oxygen atoms are the acceptors. Pairs of adeninium ions are linked via a water molecule in R33(11) formations. Two adeninium ions and two 2,6-dihydroxybenzoate ions link together through intra- and intermolecular hydrogen bonding in a R55(13) formation. Further, two 2,6-dihydroxybenzoate ions, two water molecules and an adeninium ion link in a R77(18) formation. Other, larger ring formations may also be described.
The closest approach of ring centres in the crystal packing is 3.549(3) Å between the aryl ring of 2,6-dihydroxybenzoate ion and the pyrimidinium ring in the same asymmetric unit. Weak C–H⋯π bonding is also present (Table 5).
(V) [Adeninium]+ [2-hydroxybenzoate]− [methanol]
The numbering scheme is shown in Fig. 10.
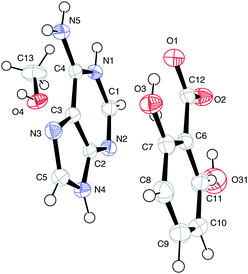 | ||
| Fig. 10 The atomic arrangement in (V) — major and minor sites superimposed. Displacement ellipsoids are shown at the 50% probability level. | ||
Here a methanolated salt is formed with hydrogen transfer from the carboxylic acid group to the nitrogen of the pyrimidine ring.
The hydroxy group of the salicylate ion is disordered over both ortho sites in a ratio of 0.77![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.23. Here the major and minor sites are occupied by O3 and O31, respectively.
0.23. Here the major and minor sites are occupied by O3 and O31, respectively.
As in (III) this results in formation of a R22(8) hydrogen bonded ring between the acid and the base where the charged nitrogen atoms are the donors and the charged oxygen atoms are the acceptors. The methanol molecule is involved in a R33(11) formation that links two adeninium ions together. These two features are shown in Fig. 11 and 12 along with the difference in intramolecular hydrogen binding at the ortho hydroxy in the acid. Hydrogen bonding geometry is shown in Table 6.
| D–H⋯A | d(D–H) | d(H⋯A) | d(D⋯A) | ∠(DHA) |
|---|---|---|---|---|
| a Symmetry transformations used to generate equivalent atoms: #1 −x + 1, −y, −z + 1 #2 −x + 1, −y + 1, −z + 1 #3 x + 1, y, z #4 −x, −y, −z + 1 #5 x − 1, y, z. | ||||
| O3–H3⋯O1 | 0.84 | 1.80 | 2.547(2) | 148 |
| O31–H31⋯O2 | 0.84 | 1.75 | 2.516(7) | 152 |
| O4–H4⋯N3#1 | 0.84 | 1.93 | 2.7638(17) | 172 |
| N1–H1⋯O1#2 | 0.88 | 1.82 | 2.6927(18) | 170 |
| N5–H5A⋯O2#2 | 0.88 | 1.85 | 2.7174(18) | 170 |
| N5–H5B⋯N2#3 | 0.88 | 2.43 | 3.0575(19) | 128 |
| N4–H4A⋯O4#4 | 0.88 | 1.81 | 2.6855(17) | 178 |
| C1–H1A⋯O2#4 | 0.95 | 2.44 | 3.3079(19) | 152 |
| C10–H10A⋯O3#5 | 0.95 | 2.34 | 3.185(3) | 152 |
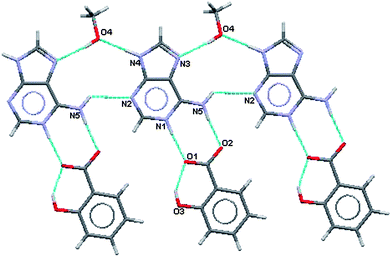 | ||
| Fig. 11 Partial hydrogen bonding, major site of salicylate ion. | ||
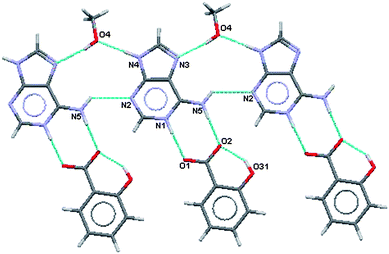 | ||
| Fig. 12 Partial hydrogen bonding, minor site of salicylate ion. | ||
The closest approach of ring centres in the crystal packing is 3.543(1) Å between the aryl ring of the salicylate ion and the imidazole ring in the same asymmetric unit.
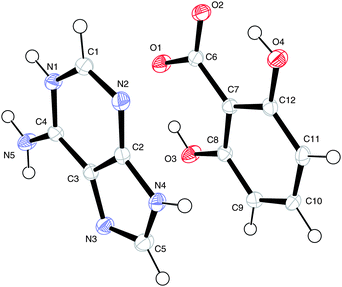
(VI) [Adeninium]+ [OH]− [3,5-dihydroxybenzoate]− [H3O]+
The numbering scheme is shown in Fig. 13.
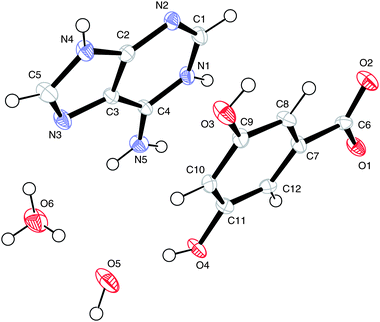 | ||
| Fig. 13 The atomic arrangement in (VI). Displacement ellipsoids are shown at the 50% probability level. | ||
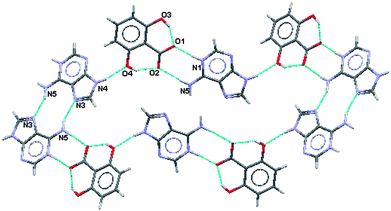
For this salt the two water molecules exist in ionised forms as hydroxide ions (OH−) and as hydroxonium ions (H3O+). As in (III), (IV) and (V) there is formation of a R22(8) hydrogen bonded ring between the acid and the base where the charged nitrogen atoms are the donors and the charged oxygen atoms are the acceptors. The planar sheets of ions (Fig. 14) are also hydrogen bonded together in R33(8), R22(11), R55(15) and R55(21) formations. Hydrogen bonding geometry is shown in Table 7.
| D–H⋯A | d(D–H) | d(H⋯A) | d(D⋯A) | ∠(DHA) |
|---|---|---|---|---|
| a Symmetry transformations used to generate equivalent atoms: #1 x − 1/2, −y + 1/2, z + 1/2 #2 x, −y + 1, z − 1/2 #3 x − 1/2, y − 1/2, z #4 x + 1/2, −y + 1/2, z − 1/2 #5 x, −y, z − 1/2. | ||||
| O3–H3⋯O4#1 | 0.84 | 1.95 | 2.776(5) | 168 |
| O4–H4⋯O5 | 0.84 | 1.84 | 2.675(5) | 173 |
| N1–H1⋯O2#2 | 0.88 | 1.76 | 2.634(5) | 172 |
| N4–H4A⋯O1#3 | 0.88 | 1.89 | 2.744(5) | 163 |
| N5–H5A⋯O1#2 | 0.88 | 2.08 | 2.952(5) | 172 |
| N5–H5B⋯N2#4 | 0.88 | 2.52 | 2.996(5) | 115 |
| O6–H6A⋯O5 | 0.87 | 1.89 | 2.754(6) | 172 |
| O6–H6B⋯N3 | 0.86 | 2.04 | 2.789(5) | 145 |
| O6–H6C⋯O5#5 | 0.84 | 1.85 | 2.673(5) | 167 |
| O5–H5X⋯O2#4 | 0.86 | 1.92 | 2.745(5) | 162 |
| C1–H1A⋯O6#1 | 0.95 | 2.29 | 3.191(6) | 157 |
| C5–H5C⋯O3#5 | 0.95 | 2.34 | 3.243(6) | 159 |
| C8–H8⋯O5#1 | 0.95 | 2.49 | 3.410(6) | 162 |
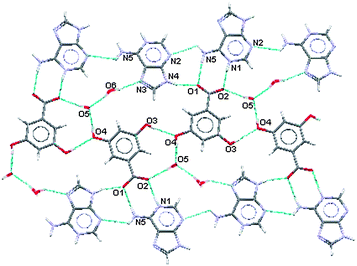 | ||
| Fig. 14 Planar portion of the hydrogen bonding in (VI). | ||
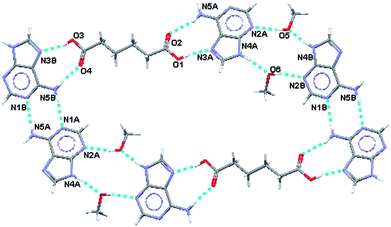
The water ions hydrogen bond to each other in the planar portions of the supramolecular structure and also link these planes together as shown in Fig. 15.
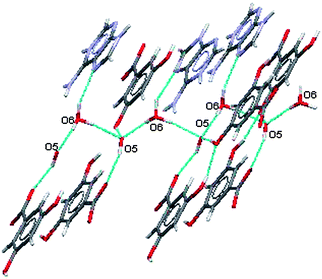 | ||
| Fig. 15 Participation of water ions with in-plane and out-of-plane hydrogen bonding in (VI). | ||
The closest approach of ring centres in the crystal packing is 3.486(3) Å between the aryl ring of the 3,5-dihydroxybenzoate ion and the pyrimidinium ring in the same asymmetric unit.
Discussion
From this study it can been seen that adenine will form cocrystals or salts with carboxylic acids if complementary hydrogen bonding groups are present. When the thermodynamics and kinetics are favourable the product will be a cocrystal or a salt. If water is present the tendency is for it to be incorporated into the crystal lattice so the use of dry solvents may be required. Methanol is an obvious choice but this can also be incorporated into the product as in (II) and (V).When pKa's of starting materials are similar it may be considered favourable for cocrystal formation. Aqueous pKa's for adenine (4.15), benzoic acid (4.20) and adipic acid (4.43) are similar and indeed cocrystals do form with benzoic acid (I) and adipic acid (II). However, with 3,5-dihydroxybenzoic acid (pKa = 4.04) and 2-hydroxybenzoic acid (pKa = 2.97), where pKa values are similar to that for adenine, salts [(VI) and (V)] are formed. As expected salts [anhydrous (III) and hydrated (IV)] are formed with the strong acid 2,6-dihydroxybenzoic acid (pKa = 1.30). All the salts are formed by the proton transfer process resulting in an adeninium ion with a single positive charge. For each salt the nitrogen in the pyrimidine ring adjacent to the amine group is protonated as this is the most basic nitrogen in adenine. For the acids the pKa relates to the deprotonation of the carboxylic acid group.
The two co-crystals [(I) and (II)] have densities of 1.381 and 1.412 g cm−3, respectively, whereas the proton transfer complexes that possess ionic interactions all have higher densities. The anhydrous 2,6-dihydroxybenzoate salt (III) has the highest density at 1.598 g cm−3.
All nitrogen atoms in the adenine moieties are involved in hydrogen bonding as are all the oxygens in the associated molecules and ions. All these bonds involve nitrogen and oxygen atoms. With the exception of N2 from (III), all these bonds are classical hydrogen bonds. In addition all products posess weak C–H⋯O bonds but only (II) and (III) possess C–H⋯N hydrogen bonds. Evidence for C–H⋯π interaction is present in (I), (III) and (IV).
All products contain the R22(8) synthon; in (I) this heterosynthon is in the Watson–Crick mode, in (II) the homosynthon links adenine molecules together and in the remaining products the heterosynthon links acid and base in anion-cation hydrogen bonding. The R22(9) heterosynthons in (I) and (II) are evidence of hydrogen bonding in the Hoogsteen mode.
The presence of solvent extends the hydrogen bonding ring formations such that in (II) and (V) the methanol solvent forms part of R44(12) and R33(11) formations, respectively.
Large hydrogen bonded ring formations are also evident in the presence of molecular water (IV) and water ions (VI).
Evidence of π⋯π interactions is shown by the closest separations between centres of aromatic rings in adjacent molecules. In this respect the shortest separation, 3.337(1) Å is found in (I) and the greatest separation, 3.625(1) Å is found in (II).
Conclusions
Adenine can form a number of different products with suitable coformers. In the selected range of carboxylic acids studied here products include an anhydrous cocrystal, a methanolated cocrystal, an anhydrous proton-transfer complex, a hydrated proton-transfer complex, a methanolated proton-transfer complex and a doubly hydrated salt. Several ionic and hydrogen bonding interactions of the nucleotide base with the carboxylic acid group have been demonstrated.Acknowledgements
We thank the EPSRC X-ray data collection service at Southampton University, UK for the collection of X-ray data sets.References
- L. H. Hurley, Nat. Rev. Cancer, 2002, 2, 188 CrossRef CAS.
- R. P. Verma and C. Hansch, J. Pharm. Sci., 2008, 97, 88 CrossRef CAS.
- W. A. Denny, B. C. Baguley, Molecular Aspect of Anticancer Drug-DNA interaction. 1994, UK, 1st edn, MacMillan, p. 270 Search PubMed.
- K. Ohara, M. Smietana, A. Restouin, S. Mollard, J. Borg, Y. Collette and J. Vasseur, J. Med. Chem., 2007, 50, 6465 CrossRef CAS.
- B. S. Praveen Reddy, S. Murari Sondhi and J. W. Lown, Pharmacol. Ther., 1999, 84, 1 CrossRef.
- Y.-L. Song, Y.-T. Li and Z.-Y. Wu, J. Inorg. Biochem., 2008 DOI:10.1016/ j.jinorgbio.2008.04.005.
- J. M. Woynarowski, Biochem. Biophys. Acta, 2002, 1587, 300 CAS.
- A. Sukhanova, S. Grovsky, M. Ermisov, Z. Mocholov and A. Zhuze, Biochem. Pharmacol., 2002, 64, 79 CrossRef CAS.
- I. Ishida and T. Asao, Biochem. Biopys. Acta, 2002, 1587, 155 Search PubMed.
- K.T. Ghosh Sen and R. Frohlich, Tetrahedron Lett., 2007, 48, 7022 CrossRef.
- M. L. Głowka, D. Martynowski, A. Olczak, J. Bojarska, M. Szczesio and K. Kozłowska, J. Mol. Struct., 2003, 658, 43 CrossRef CAS.
- J. P. Garcia-Teran, O. Castillo, A. Lique, U. Garcia-Couceiro, P. Roman and F. Loret, Inorg. Chem., 2004, 43, 5761 CrossRef CAS.
- J. P. Garcia-Teran, O. Castillo, A. Lique, U. Garcia-Couceiro, G. Beobide and P. Roman, Dalton Trans., 2006, 902 RSC.
- D. Dobrzynska and L. B. Jerzykiewicz, J. Am. Chem. Soc., 2004, 126, 1118.
- M. Driess, K. Merz and R. B. Rowlings, Z. Anorg. Allg. Chem., 2001, 627, 213 CrossRef CAS.
- V. Langer, K. Huml and J. Zachova, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1984, 40, 2080 CrossRef.
- S. R. Perumalla, E. Suresh and V. R. Pedireddi, Angew. Chem., Int. Ed., 2005, 44, 7752 CrossRef CAS.
- S. Fujii, K. Kawasaki, A. Sato, T. Fujiwara and K.-I. Tomlta, Arch. Biochem. Biophys., 1977, 181, 363 CrossRef CAS.
- A. Takenaka and Y. Sasada, Bull. Chem. Soc. Jpn., 1982, 55, 680 CAS.
- C. M. Weeks, D. C. Rohrer and W. L. Duax, Science, 1975, 190, 1096 CrossRef CAS.
- S. M. Tret'yak, V. V. Mitkevich and L. F. Sukhodub, Kristallografiya , 1987, 32, 1268 CAS.
- V. Langer, K. Huml and L. Lessinger, Acta Crystallogr., Sect. B: Structural Science., 1978, 34, 2229 Search PubMed.
- S. Sridhar and K. Ravikumar, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 2007, 63 Search PubMed o415 .
- J. Maixner, J. Zachova and K. Huml, Collect. Czech. Chem. Commun., 1993, 58, 861 CrossRef CAS.
- V. Zelenak, Z. Vargova and I. Cisarova, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2004 Search PubMed 60, o742.
- Z. Otwinowski and W. , Minor. Methods in Enzymology, Vol. 276, Macromolecular Crystallography, Part A, ed. C. W. Carter Jr and R. M. Sweet, Academic Press, New York, 1997, pp 307–326 Search PubMed.
- R. W. W. Hooft. COLLECT. Nonius BV, Delft, Holland, 1999 Search PubMed.
- G. M. Sheldrick. SADABS. Version 2.10. University of Göttingen, Germany, 2003 Search PubMed.
- A. Altomare, M. C. Burla, M. Camalli, G. L. Cascarano, C. Giacovazzo, A. Guagliardi, A. G. G. Moliterni, G. Polidori and R. , Spagna, J. Appl. Crystallogr., 1999, 32, 115 CrossRef CAS.
- G. M. Sheldrick. SHELXL97. University of Göttingen, Germany, 1997 Search PubMed.
- A. L. Spek, J. Appl. Crystallogr., 2003, 36, 7 CrossRef CAS.
- L. J. Farrugia, J. Appl. Crystallogr., 1999, 32, 837 CrossRef.
- C. F. Macrae, P. R. Edgington, P. McCabe, E. Pidcock, G. P. Shields, R. Taylor, M. Towler and J. van de Streek, J. Appl. Crystallogr., 2006, 39, 453 CrossRef CAS.
Footnote |
| † CCDC reference numbers 686683–686688. For crystallographic data in CIF or other electronic format see DOI: 10.1039/b811243f |
| This journal is © The Royal Society of Chemistry 2009 |
