Two [Au(CN)2]−-bridged heterometallic coordination polymers directed by different 2,2′-bipyridyl-like ligands†
Yang
Guo
a,
Zhan-Quan
Liu
b,
Bin
Zhao
a,
Yu-Hua
Feng
a,
Gong-Feng
Xu
a,
Shi-Ping
Yan
a,
Peng
Cheng
a,
Qing-Lun
Wang
a and
Dai-Zheng
Liao
*a
aDepartment of Chemistry, Nankai University, Tianjin, 300071, P. R. China. E-mail: liaodz@nankai.edu.cn; Fax: +86-22-23504855
bDepartment of Material Chemistry, Nankai University, Tianjin, 300071, P. R. China
First published on 26th September 2008
Abstract
Two new cyanide-bridged heterobimetallic coordination polymers constructed by using the [Au(CN)2]− building block, {Cd(bipy)[Au(CN)2]2}n (1) and {{Cd(phen)[Au(CN)2]2(H2O)}·iPrOH}n (2) (bipy = 2,2′-bipyridine, phen = 1,10-phenanthroline), have been synthesized and characterized. Complex 1 with chiral P3112 space group exhibits a novel three-fold interpenetrating quartz-like 3-D network, which is completely unsupported by aurophilic interactions, while complex 2 with the replacement of bipy by phen shows C2/c space group and features the 1-D zigzag chain structure and an extended supramolecular 2-D array in which aurophilic interactions may play an important role in sustaining the supramolecular solid-state architectures. 1 and 2 feature various networks most likely due to the different steric hindrances of the bipy and phen ligands. In addition, the solid state photoluminescence spectrum of complex 2 exhibits a strong green emission band (λmax = 524 nm) at room temperature.
Introduction
The construction of coordination polymers built upon molecular building blocks has become an area of intense interest in recent years.1 This is due not only to their useful optical, conducting or magnetic properties, but also to their porous functions such as catalysis, separation, molecular recognition, ion-exchange, sorption–desorption, etc.2–5 Among these materials, cyanide-bridged coordination polymers which are prepared from assembling cyanometallate building blocks and transition metal centers, have been shown to exhibit a remarkable diversity of structural types with interesting magnetic, magneto-optical, electrochemical and zeolitic materials properties.6The linear dicyanoaurate anion [Au(CN)2]− is an ideal building block for the construction of cyanide-bridged coordination polymers. From a material point of view, it can be utilized to construct heterometallic complexes showing interesting magnetic, spin-crossover, vapochromic, birefringent and zeolite-like properties.7 On the other hand, the bidentate [Au(CN)2]−group can be served as a 2-connector to establish strong coordinative bonds between the cyanide groups and a second metal cations. Using this 2-connecting unit, some cyanide-bridged extended systems with 1-D,8 2-D7h,9 and 3-D9 polymeric structures have been obtained. Apart from being a μ2- bridging ligand, [Au(CN)2]− can also produce supramolecular solid-state architectures through weakly bonding aurophilic interactions which have order-of-magnitude strengths comparable to hydrogen-bonding interactions,10 and this attractive Au⋯Au interactions can be used as a useful tool to control supramolecular dimensionality.7h,8b–8d,11 As a supramolecular element, this aurophilicity can play an important role in producing some fascinating networks with intriguing topologies such as interpenetrating,7a–7c,8c,12 microporous,7a,12f Hoffman-like13a and interwoven13b frameworks. In addition, [Au(CN)2]− salts are known to have luminescent properties14 and some complexes assembled using these building blocks may retain this useful material property as a result of the aggregates of [Au(CN)2]− ions.11
In view of the important role of the aromatic chelate 2,2′-bipyridyl-like ligands such as 2,2′-bipyridine (bipy) and 1,10-phenanthroline (phen) in the development of coordination supramolecular networks,15 we present here the syntheses and characterizations of two novel cyanide-bridged heterometallic coordination polymers incorporating [Au(CN)2]− building block and 2,2′-bipyridyl-like ligands, {Cd(bipy)[Au(CN)2]2}n (bipy = 2,2′-bipyridine) (1) and {{Cd(phen)[Au(CN)2]2(H2O)}·iPrOH}n (phen = 1,10-phenanthroline, iPrOH = isopropanol) (2). In the two complexes, using different bipy and phen chelating ligands, [Au(CN)2]− tecton links cadmium ions in various coordination modes, giving rise to one- or three-dimensional heterobimetallic Cd(II)–Au(I) coordination polymers, respectively.
Experimental
Materials and general methods
The solvents and reagents for synthesis were commercially available and used as received. Elemental analyses (C, H and N) were performed on a Perkin-Elmer 240 analyzer. The FT-IR spectra were measured with a Bruker Tensor 27 spectrometer on KBr pellets in the region of 4000–400 cm−1. Emission spectra in solid state at room temperature were taken on a Cary Eclips fluorescence spectrophotometer.Caution! The cyanides are very toxic and the perchlorate salts of metal compounds with organic ligands are potentially explosive. Only small amounts of material should be cautiously handled.
Synthesis of complexes 1 and 2
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 6 mL), the other arm contains a water solution (6 mL) of K[Au(CN)2] (0.4 mmol). The remaining of the vessel was full of isopropanol. Diamond-shaped colorless crystals were obtained after 5 months. Yield: 39% (based on Cd(II) salts). IR (KBr disk): νCN 2144, 2155, and 2176 cm−1. Elemental analysis (%) calcd for C14H8Au2CdN6 (1): C 21.93, H 1.05, N 10.96; Found: C 21.82, H 1.10, N 10.88.
2, 6 mL), the other arm contains a water solution (6 mL) of K[Au(CN)2] (0.4 mmol). The remaining of the vessel was full of isopropanol. Diamond-shaped colorless crystals were obtained after 5 months. Yield: 39% (based on Cd(II) salts). IR (KBr disk): νCN 2144, 2155, and 2176 cm−1. Elemental analysis (%) calcd for C14H8Au2CdN6 (1): C 21.93, H 1.05, N 10.96; Found: C 21.82, H 1.10, N 10.88.
X-Ray data collection and structure determinations
X-Ray single-crystal diffraction data for complexes 1 and 2 were collected on Bruker Smart 1000 CCD diffractometer with Mo Kα radiation (λ = 0.71073 Å). All the structures were solved by direct methods using the SHELXS program of the SHELXTL package and refined by full-matrix least-squares methods with SHELXL.16 The metal atoms in each complex were located from the E-maps and other non-hydrogen atoms were located in successive difference Fourier syntheses and refined with anisotropic thermal parameters on F2. The hydrogen atoms of the ligand were generated theoretically onto the specific atoms and refined isotropically with fixed thermal factors. Crystallographic data and experimental details for structural analyses are summarized in Table 1. Selected bond lengths and angles are listed in Tables 2 and 3, respectively.| 1 | 2 | |
|---|---|---|
| Empirical formula | C14H8Au2CdN6 | C19H18Au2CdN6O2 |
| Formula weight | 766.60 | 868.73 |
| Temperature/K | 294(2) | 294(2) |
| Crystal system | Hexagonal | Monoclinic |
| Space group | P3112 | C2/c |
| a/Å | 8.9349(19) | 18.991(4) |
| b/Å | 8.9349(19) | 9.987(2) |
| c/Å | 19.992(4) | 24.707(5) |
| α/° | 90 | 90 |
| β/° | 90 | 100.697(3) |
| γ/° | 120 | 90 |
| V/Å3 | 1382.2(5) | 4604.8(16) |
| Z | 3 | 8 |
| D/g cm−3 | 2.763 | 2.500 |
| μ/mm−1 | 17.030 | 13.654 |
| F(000) | 1020 | 3152 |
| Reflections collected | 7751 | 10815 |
| Unique reflections | 1901 | 4002 |
| Data/restraints/parameters | 1901/0/106 | 4002/32/274 |
| R(int) | 0.0709 | 0.0742 |
| GOF | 0.968 | 1.030 |
| R 1 [I >2σ(I)] | 0.0346 | 0.0636 |
| wR2 [I >2σ(I)] | 0.0589 | 0.1530 |
| a Symmetry codes: #2 −y + 1, −x + 1, −z + 5/3. | |
|---|---|
| Cd(1)–N(1) | 2.346(9) |
| Cd(1)–N(2) | 2.303(9) |
| Cd(1)–N(3) | 2.327(7) |
| N(1)–Cd(1)–N(1)#2 | 175.2(5) |
| N(2)–Cd(1)–N(1) | 87.6(3) |
| N(2)–Cd(1)–N(1)#2 | 89.4(3) |
| N(2)–Cd(1)–N(2)#2 | 103.2(5) |
| N(2)–Cd(1)–N(3) | 94.3(3) |
| N(2)–Cd(1)–N(3)#2 | 160.5(3) |
| N(2)#2–Cd(1)–N(1) | 89.4(3) |
| N(2)#2–Cd(1)–N(1)#2 | 87.6(3) |
| N(2)#2–Cd(1)–N(3) | 160.4(3) |
| N(2)#2–Cd(1)–N(3)#2 | 94.3(3) |
| N(3)–Cd(1)–N(1) | 100.0(3) |
| N(3)–Cd(1)–N(1)#2 | 84.0(3) |
| N(3)–Cd(1)–N(3)#2 | 69.9(4) |
| N(3)#2–Cd(1)–N(1) | 84.0(3) |
| N(3)#2–Cd(1)–N(1)#2 | 100.0(3) |
| Cd(1)–N(1) | 2.344(15) |
| Cd(1)–N(3) | 2.245(18) |
| Cd(1)–N(5) | 2.366(14) |
| Cd(1)–N(2) | 2.351(15) |
| Cd(1)–O(1) | 2.283(14) |
| Cd(1)–N(6) | 2.304(14) |
| N(1)–Cd(1)–N(2) | 172.7(5) |
| N(1)–Cd(1)–N(5) | 84.7(5) |
| N(2)–Cd(1)–N(5) | 92.7(5) |
| N(3)–Cd(1)–N(1) | 90.5(6) |
| N(3)–Cd(1)–N(2) | 93.6(6) |
| N(3)–Cd(1)–N(5) | 164.9(6) |
| N(3)–Cd(1)–N(6) | 95.0(6) |
| N(6)–Cd(1)–N(1) | 94.2(5) |
| N(6)–Cd(1)–N(2) | 91.4(5) |
| N(6)–Cd(1)–N(5) | 71.1(6) |
| N(3)–Cd(1)–O(1) | 94.6(7) |
| O(1)–Cd(1)–N(1) | 87.3(6) |
| O(1)–Cd(1)–N(2) | 86.4(5) |
| O(1)–Cd(1)–N(5) | 99.5(7) |
| O(1)–Cd(1)–N(6) | 170.2(7) |
Full crystallographic data for 1 and 2 have been deposited with the CCDC (291090 and 290064, respectively.)
Results and discussion
Description of crystal structures
 | ||
| Fig. 1 (a) View of the coordination environment of Cd atom in 1 (some equivalent atoms have been generated to complete the Ni coordination, H atom omitted). (b) Perspective view of the quartz-like network of 1 along the c axis. (c) View of the three-fold interpenetrating networks of 1 along the c axis. | ||
 | ||
| Fig. 2 (a) Perspective views of the coordination environments of Cd atoms in 2 (some equivalent atoms have been generated to complete the Zn coordination, H atoms and iPrOH molecule omitted). (b) Perspective view of the aurophilicity-induced 1-D ladder chain of 2. (c) View of 2-D layer structure constructed from aurophilic interactions with a “tetramer” type of 2 with Au atoms represented by gold spheres and aurophilic interactions represented by gold dotted lines. | ||
Discussion of crystal structures
The quartz-like network occupies a special position within crystal engineering. Few examples of coordination polymers with quartz topology have been reported.12c,21,22 The present net is a second member of rare three-fold interpenetrating coordination compounds with a quartz-like topology.21c Interestingly, one of the best known examples is {Zn[Au(CN)2]2}n reported by Robson and co-workers which is six interpenetrating quartz-like and contains exactly the same tecton ([Au(CN)2]−) as 1.12c The two structures are both interpenetrating quartz-like networks. Besides the different metal ions, there is one significant alteration with the interpenetrating type. The reason why the two structures are much different is not fully understood. Significant Au⋯Au interactions between nets may play a role in the quartz topology of {Zn[Au(CN)2]2}n.22a The auxiliary bipy ligand may act as part of the frameworks in addition to the effect of the Cd(II) ions in 1 considering that there are no Au⋯Au interactions. For the interpenetrated networks in 1, each independent net is constructed of metal–ligand coordinate bonds, and there is no other intermolecular (aurophilic, hydrogen-bonding, π–π stacking, etc.) interaction between the interpenetrating nets. So the interpenetrating networks are completely unsupported and this system can be regarded as interaction-unsupported interpenetration. Leznoff et al. have pointed out in previous reports that the coordination polymers involving the [Au(CN)2]− unit have a propensity to display aurophilicity-supported interpenetration.8c,12a–12e The interaction-unsupported interpenetration type of 1 represents a novel example in the family of [Au(CN)2]−-based interpenetrating networks. Some [N(CN)2]−-based interpenetrating networks have been reported and they are not supported by metal–metal (metallophilic) interactions.23 For the related [Ag(CN)2]−-bridged polymeric systems, some interpenetrating networks are supported by Ag⋯Ag (argentophilic) interactions,7a,24 and some are not.25Although both complexes were prepared from Cd2+ ions, [Au(CN)2]− ligand, H2O/iPrOH solvents and the same synthesis procedure, the crystal structures are quite different. The size of the terminal ligands can play an important role in coordination environments though 1 and 2 have the same metal-to-ligand ratio.26 The reason why 2 does not show a similar structure to 1 may be the larger steric hindrance of the phen ligand than that of the bipy.
Luminescent property of complex 2
The photoluminescent (PL) property of complex 2 has been investigated in the solid state. Complex 2 exhibits intensive green emission with peak maximum around 524 nm when irradiated with UV radiation (403 nm) at room temperature (Fig. 3). Solid K[Au(CN)2] had one emission band at 390 nm.14,27 The free phen·H2O also fluoresces in the solid state and the emission bands for it are at 365 and 388 nm (λex = 310 nm).28a Compound [Cd(phen)(NO3)2] is usually located at about 400 nm which is assigned to the intraligand fluorescence of coordinated phen ligands.28b The significant red-shift in 2 may be due to the aurophilic bonding interactions (originated from the close proximity of the [Au(CN)2]− ions),14,27 considering the chelation effect of the phen ligand to the metal ion28c and modification effect of the metal–metal interactions of d10 system to photoluminescent behavior.17a,27 The luminescence of aggregates of [Au(CN)2]− ions has attracted considerable research and, up to date, there are many studies on MI[Au(CN)2] (M = K, Cs, Tl),14Au(I)-Au(I) and Ln(III)–Au(I) (Ln are lanthanides) emission systems,29,30 but there are very limited reports on M(II)–Au(I) (M2+ are close-shell d10 metal ions or pseudo-closed-shell d8 metal ions) emission systems.17a,27 The incorporation of a preformed luminescent [Au(CN)2]− precursor into the desired structures may be an effective method for luminescent systems and complex 2 has provided a new material for them.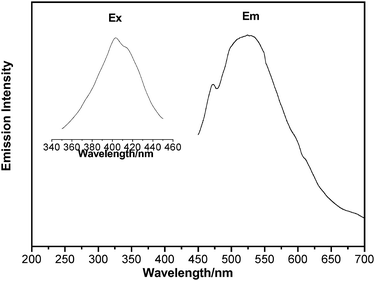 | ||
| Fig. 3 Emission spectra of 2 in the solid state at room temperature excited at 403 nm. (Ex = excitation, Em = emission). | ||
Conclusion
In this paper, two novel [Au(CN)2]−-bridged heterometallic coordination polymers with unusual network topologies have been obtained by combining the [Au(CN)2]− building block with 2,2′-bipyridyl-like ligands. The luminescent property of complex 2 has also been studied. Complex 1 possesses a novel three-fold interpenetrating quartz-like network in which the triple interpenetrating networks are completely unsupported. To the best of our knowledge, 1 represents the second example of three-fold interpenetrating coordination polymer with quartz topology. Complex 2 features the 1-D zigzag chain structure and a further extended 2-D supramolecular array in which aurophilic interactions may play an important role. It is possible that the larger steric hindrance of the phen ligand than that of the bipy plays a key role in the construction of 1 and 2. The solid state sample of 2 exhibits a strong green emission at room temperature which may be the result of aurophilic interactions. The unusual structures of the two compounds incorporating [Au(CN)2]− building block imply that this building block has a great potential for constructing novel coordination polymers and heterometallic complexes. Meanwhile, different 2,2′-bipyridyl-like ligands such as bipy and phen can modulate the frameworks of the coordination polymers. Incorporating [Au(CN)2]− into the structure is a useful route to luminescent material and the [Au(CN)2]−-based complexes can provide an impetus for further exploration of various applications of this useful system.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 20631030, 20601014) and National Basic Research Program of China (973 Program, 2007CB815305).References
- (a) A. J. Blake, N. R. Champness, P. Hubberstey, W. S. Li, M. A. Withersby and M. Schröder, Coord. Chem. Rev., 1999, 183, 117 CrossRef CAS; (b) R. Robson, J. Chem. Soc., Dalton Trans., 2000, 3735 RSC; (c) M. Eddaoudi, D. B. Moler, H. Li, B. Chen, T. M. Reineke, M. O'Keeffe and O. M. Yaghi, Acc. Chem. Res., 2001, 34, 319 CrossRef CAS; (d) M. J. Zaworotko, Chem. Commun., 2001, 1 RSC; (e) O. M. Yaghi, M. O'Keeffe, N. W. Ockwig, H. K. Chae, M. Eddaoudi and J. Kim, Nature, 2003, 423, 705 CrossRef CAS; (f) L. Brammer, Chem. Soc. Rev., 2004, 33, 476 RSC.
- (a) S. R. Batten, Curr. Opin. Solid State Mater. Sci., 2001, 5, 107 CrossRef CAS; (b) S. L. James, Chem. Soc. Rev., 2003, 32, 276 RSC; (c) C. Janiak, Dalton Trans., 2003, 2781 RSC.
- (a) E. Coronado, J. R. Galán-Mascarós, C. J. Gómez-García and V. Laukhin, Nature, 2000, 408, 447 CrossRef CAS; (b) O. R. Evans and W. B. Lin, Acc. Chem. Res., 2002, 35, 511 CrossRef CAS; (c) T. P. Radhakrishnan, Acc. Chem. Res., 2008, 41, 367 CrossRef CAS.
- (a) D. Maspoch, D. Ruiz-Molina and J. Veciana, J. Mater. Chem., 2004, 14, 2713 RSC; (b) C. J. Kepert, Chem. Commun., 2006, 695 RSC.
- (a) O. M. Yaghi, H. Li, C. Davis, D. Richardson and T. L. Groy, Acc. Chem. Res., 1998, 31, 474 CrossRef CAS; (b) P. H. Dinolfo and J. T. Hupp, Chem. Mater., 2001, 13, 3113 CrossRef CAS; (c) C. N. R. Rao, S. Natarajan and R. Vaidhyanathan, Angew. Chem., Int. Ed., 2004, 43, 1466 CrossRef CAS; (d) S. Kitagawa, R. Kitaura and S.-I. Noro, Angew. Chem., Int. Ed., 2004, 43, 2334 CrossRef CAS; (e) J. L. C. Rowsell and O. M. Yaghi, Angew. Chem., Int. Ed., 2005, 44, 4670 CrossRef CAS.
- (a) K. R. Dunbar and R. A. Heintz, Prog. Inorg. Chem., 1997, 45, 283 CAS; (b) M. Verdaguer, A. Bleuzen, V. Marvaud, J. Vaissermann, M. Seuleiman, C. Desplanches, A. Scuiller, C. Train, R. Garde, G. Gelly, C. Lomenech, I. Rosenman, P. Veillet, C. Cartier and F. Villain, Coord. Chem. Rev., 1999, 190, 1023 CrossRef; (c) M. Ohba and H. Okawa, Coord. Chem. Rev., 2000, 198, 313 CrossRef CAS; (d) J. S. Miller and J. L. Manson, Acc. Chem. Res., 2001, 34, 563 CrossRef CAS; (e) J. Černák, M. Orendáč, I. Potočňák, J. Chomič, A. Orendáčová, J. Skoršepa and A. Feher, Coord. Chem. Rev., 2002, 224, 51 CrossRef CAS; (f) O. Sato, T. Iyoda, A. Fujishima and K. Hashimoto, Science, 1996, 272, 704 CrossRef CAS.
- (a) V. Niel, A. L. Thompson, M. C. Muñoz, A. Galet, A. E. Goeta and J. A. Real, Angew. Chem., Int. Ed., 2003, 42, 3760 CrossRef CAS; (b) A. Galet, M. C. Muñoz, V. Martínez and J. A. Real, Chem. Commun., 2004, 2268 RSC; (c) A. Galet, M. C. Muñoz and J. A. Real, Chem. Commun., 2006, 4321 RSC; (d) J. Lefebvre, R. J. Batchelor and D. B. Leznoff, J. Am. Chem. Soc., 2004, 126, 16117 CrossRef CAS; (e) J. Lefebvre, F. Callaghan, M. J. Katz, J. E. Sonier and abd D. B. Leznoff, Chem.–Eur. J., 2006, 12, 6748 CrossRef CAS; (f) M. J. Katz, P. M. Aguiar, R. J. Batchelor, A. A. Bokov, Z.-G. Ye, S. Kroeker and D. B. Leznoff, J. Am. Chem. Soc., 2006, 128, 3669 CrossRef CAS; (g) M. J. Katz, H. Kaluarachchi, R. J. Batchelor, A. A. Bokov, Z.-G. Ye and D. B. Leznoff, Angew. Chem., Int. Ed., 2007, 46, 8804 CrossRef CAS; (h) E. Colacio, F. Loret, R. Kivekäs, J. Ruiz, J. Suárez-Varela and M. R. Sundberg, Chem. Commun., 2002, 592 RSC.
- (a) I. K. Chu, I. P. Y. Shek, K. W. M. Siu, W.-T. Wong, J.-L. Zuo and T.-C. Lau, New J. Chem., 2000, 24, 765 RSC; (b) D. B. Leznoff, B.-Y. Xue, B. O. Patrick, V. Sanchez and R. C. Thompson, Chem. Commun., 2001, 259 RSC; (c) D. B. Leznoff, B.-Y. Xue, R. J. Batchelor, F. W. B. Einstein and B. O. Patrick, Inorg. Chem., 2001, 40, 6026 CrossRef CAS; (d) E. Colacio, F. Loret, R. Kivekäs, J. Ruiz, J. Suárez-Varela, M. R. Sundberg and R. Uggla, Inorg. Chem., 2003, 42, 560 CrossRef CAS; (e) G.-F. Xu, Z.-Q. Liu, H.-B. Zhou, Y. Guo and D.-Z. Liao, Aust. J. Chem., 2006, 59, 640 CrossRef CAS; (f) A. R. Geisheimer, M. J. Katz, R. J. Batchelor and D. B. Leznoff, CrystEngComm, 2007, 9, 1078 RSC.
- J. Lefebvre, D. Chartrand and D. B. Leznoff, Polyhedron, 2007, 26, 2189 CrossRef CAS.
- (a) H. Schmidbaur, Chem. Soc. Rev., 1995, 24, 391 RSC; (b) P. Pyykkö, Chem. Rev., 1997, 97, 597 CrossRef; (c) H. Schmidbaur, Gold Bull., 2000, 33, 3 CAS; (d) R. J. Puddephatt, Coord. Chem. Rev., 2001, 216, 313 CrossRef; (e) P. Pyykkö, Angew. Chem., Int. Ed., 2004, 43, 4412 CrossRef.
- D. B. Leznoff and J. Lefebvre, Gold Bull., 2005, 38, 47 CAS.
- (a) S. C. Abrahams, J. L. Bernstein, R. Liminga and E. T. Eisenmann, J. Chem. Phys., 1980, 73, 4585 CrossRef CAS; (b) S. C. Abrahams, L. E. Zyontz and J. L. Bernstein, J. Chem. Phys., 1982, 76, 5458 CrossRef CAS; (c) B. F. Hoskins, R. Robson and N. V. Y. Scarlett, Angew. Chem., Int. Ed. Engl., 1995, 34, 1203 CrossRef CAS; (d) W.-F. Yeung, W.-T. Wong, J.-L. Zuo and T.-C. Lau, J. Chem. Soc., Dalton Trans., 2000, 629 RSC; (e) D. B. Leznoff, B.-Y. Xue, C. L. Stevens, A. Storr, R. C. Thompson and B. O. Patrick, Polyhedron, 2001, 20, 1247 CrossRef CAS; (f) W. Dong, L.-N. Zhu, Y.-Q. Sun, M. Liang, Z.-Q. Liu, D.-Z. Liao, Z.-H. Jiang, S.-P. Yan and P. Cheng, Chem. Commun., 2003, 2544 RSC; (g) C. Paraschiv, M. Andruh, S. Ferlay, M. Wais Hosseini, N. Kyritsakas, J.-M. Planeix and N. Stanica, Dalton Trans., 2005, 1195 RSC.
- (a) J. Suárez-Varela, H. Sakiyama, J. Cano and E. Colacio, Dalton Trans., 2007, 249 RSC; (b) Y.-H. Feng, Y. Guo, Y. OuYang, Z.-Q. Liu, D.-Z. Liao, P. Cheng, S.-P. Yan and Z.-H. Jiang, Chem. Commun., 2007, 3643 RSC.
- (a) N. Nagasundaram, G. Roper, J. Biscoe, J. W. Chai, H. H. Patterson, N. Blom and A. Ludi, Inorg. Chem., 1986, 25, 2947 CrossRef CAS; (b) Z. Assefa, F. DeStefano, M. A. Garepapaghi, J. H. LaCasce, Jr., S. Ouellete, M. R. Corson, J. K. Nagle and H. H. Patterson, Inorg. Chem., 1991, 30, 2868 CrossRef CAS; (c) M. A. Rawashdeh-Omary, M. A. Omary and H. H. Patterson, J. Am. Chem. Soc., 2000, 122, 10371 CrossRef CAS; (d) M. A. Rawashdeh-Omary, M. A. Omary, H. H. Patterson and J. P. Jr. Fackler, J. Am. Chem. Soc., 2001, 123, 11237 CrossRef CAS.
- B.-H. Ye, M.-L. Tong and X.-M. Chen, Coord. Chem. Rev., 2005, 249, 545 CrossRef CAS.
- G. M. Sheldrick, SHELXTL NT Version 5.1. Program for Solution and Refinement of Crystal Structures, University of Göttingen, Germany, 1997 Search PubMed.
- (a) H. Zhang, J.-W. Cai, X.-L. Feng, T. Li, X.-Y. Li and L.-N. Ji, Inorg. Chem. Commun., 2002, 5, 637 CrossRef CAS; (b) S.-P. Wang, D.-Z. Gao, Z.-Q. Liu, Z.-H. Jiang, S.-P. Yan and D.-Z. Liao, J. Coord. Chem., 2007, 60, 1599 CrossRef CAS.
- (a) S. S. Pathaneni and G. R. Desiraju, J. Chem. Soc., Dalton Trans., 1993, 319 RSC; (b) P. Pyykkö and N. Runeberg, Chem. Phys. Lett., 1994, 218, 133 CrossRef; (c) M. Bardají and A. Laguna, J. Chem. Educ., 1999, 76, 201 CrossRef CAS.
- (a) A. F. Wells, Three Dimensional Nets and Polyhedra, Wiley-Interscience, New York, 1977 Search PubMed; (b) Further Studies of Three-dimensional Nets, ACA Monograph No. 8, American Crystallographic Association, 1979; (c) O. V. Dolomanov, A. J. Blake, N. R. Champness and M. Schröder, J. Appl. Crystallogr., 2003, 36, 1283 CrossRef CAS.
- C. Janiak, J. Chem. Soc., Dalton Trans., 2000, 3885 RSC.
- (a) J. Sun, L. Weng, Y. Zhou, J. Chen, Z. Chen, Z. Liu and D. Zhao, Angew. Chem., Int. Ed., 2002, 41, 4471 CrossRef CAS; (b) E. Tynan, P. Jensen, N. R. Kelly, P. E. Kruger, A. C. Lees, B. Moubaraki and K. S. Murray, Dalton Trans., 2004, 3440 RSC; (c) S. Hu and M.-L. Tong, Dalton Trans., 2005, 1165 RSC; (d) Z.-F. Chen, S.-F. Zhang, H.-S. Luo, B. F. Abrahams and H. Liang, CrystEngComm, 2007, 9, 27 RSC; (e) T.-T. Luo, Y.-H. Liu, C.-C. Chan, S.-M. Huang, B.-C. Chang, Y.-L. Lu, G.-H. Lee, S.-M. Peng, J.-C. Wang and K.-L. Lu, Inorg. Chem., 2007, 46, 10044 CrossRef CAS; (f) Q. Zhang, Z. Lin, X. Bu, T. Wu and P. Feng, Chem. Mater., 2008, 20, 3239 CrossRef CAS.
- (a) Reviews, S. R. Batten and R. Robson, Angew. Chem., Int. Ed., 1998, 37, 1460 Search PubMed; (b) S. R. Batten, CrystEngComm, 2001, 3, 67 RSC; (c) L. Carlucci, G. Ciani and D. M. Proserpio, Coord. Chem. Rev., 2003, 246, 247 CrossRef CAS.
- (a) For example, J. L. Manson, C. D. Incarvito, A. L. Rheingold and J. S. Miller, J. Chem. Soc., Dalton Trans., 1998, 3705 Search PubMed; (b) S. R. Batten, A. R. Harris, P. Jensen, K. S. Murray and A. Ziebell, J. Chem. Soc., Dalton Trans., 2000, 3829 RSC; (c) B.-W. Sun, S. Gao, B.-Q. Ma and Z.-M. Wang, New J. Chem., 2000, 24, 953 RSC; (d) E.-Q. Gao, S.-Q. Bai, Z.-M. Wang and C.-H. Yan, Dalton Trans., 2003, 1759 RSC; (e) A. M. Kutasi, A. R. Harris, S. R. Batten, B. Moubaraki and K. S. Murray, Cryst. Growth Des., 2004, 4, 605 CrossRef CAS; (f) T. K. Maji, R. Matsuda and S. Kitagawa, Nat. Mater., 2007, 6, 142 CrossRef CAS.
- (a) For example, C. J. Shorrock, B.-Y. Xue, P. B. Kim, R. J. Batchelor, B. O. Patrick and D. B. Leznoff, Inorg. Chem., 2002, 41, 6743 Search PubMed; (b) A. Galet, V. Niel, M. C. Muñoz and J. A. Real, J. Am. Chem. Soc., 2003, 125, 14224 CrossRef CAS; (c) A. Galet, M. C. Muñoz, A. B. Gaspar and J. A. Real, Inorg. Chem., 2005, 44, 8749 CrossRef CAS.
- (a) For example, T. Soma, H. Yuge and T. Iwamoto, Angew. Chem., Int. Ed. Engl., 1994, 33, 1665 Search PubMed; (b) W. Dong, Q.-L. Wang, S.-F. Si, D.-Z. Liao, Z.-H. Jiang, S.-P. Yan and P. Cheng, Inorg. Chem. Commun., 2003, 6, 873 CrossRef CAS.
- A.-L. Cheng, Y. Ma, J.-Y. Zhang and E.-Q. Gao, Dalton Trans., 2008, 1993 RSC.
- (a) J.-X. Chen, W.-H. Zhang, X.-Y. Tang, Z.-G. Ren, H.-X. Li, Y. Zhang and J.-P. Lang, Inorg. Chem., 2006, 45, 7671 CrossRef CAS; (b) M. Stender, R. L. White-Morris, M. M. Olmstead and A. L. Balch, Inorg. Chem., 2003, 42, 4504 CrossRef CAS; (c) C. N. Pettijohn, E. B. Jochnowitz, B. Chuong, J. K. Nagle and A. Vogler, Coord. Chem. Rev., 1998, 171, 85 CrossRef CAS.
- (a) X. Shi, G. S. Zhu, Q. R. Fang, G. Wu, G. Tian, R. W. Wang, D. L. Zhang, M. Xue and S. L. Qiu, Eur. J. Inorg. Chem., 2004, 185 CrossRef; (b) J.-Y. Wu, C.-H. Chang, T.-W. Tseng and K.-L. Lu, J. Mol. Struct., 2006, 796, 69 CrossRef CAS; (c) A. Thirumurugan, M. B. Avinash and C. N. R. Rao, Dalton Trans., 2006, 221 RSC.
- (a) For example, W.-F. Fu, K.-C. Chan, V. M. Miskowski and C.-M. Che, Angew. Chem., Int. Ed., 1999, 38, 2783 Search PubMed; (b) M.-C. Brandys and R. J. Puddephatt, J. Am. Chem. Soc., 2001, 123, 4839 CrossRef CAS; (c) Z. Assefa, M. A. Omary, B. G. McBurnett, A. A. Mohamed, H. H. Patterson, R. J. Staples and J. P. Fackler, Jr., Inorg. Chem., 2002, 41, 6274 CrossRef CAS.
- (a) For example, Z. Assefa, G. Shankle, H. H. Patterson and R. Reynolds, Inorg. Chem., 1994, 33, 2187 Search PubMed; (b) Z. Assefa and H. H. Patterson, Inorg. Chem., 1994, 33, 6194 CrossRef CAS; (c) M. A. Rawashdeh-Omary, C. L. Larochelle and H. H. Patterson, Inorg. Chem., 2000, 39, 4527 CrossRef CAS; (d) J. C. F. Colis, C. Larochelle, R. Staples, R. Herbst-Irmerd and H. H. Patterson, Dalton Trans., 2005, 675 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Perspective view of the pseudo-hexagonal channel in 1 (a) and of the phen ligands in the network of 1 (b) along the c axis (Fig. S1); view of the supramolecular structure of 2 showing Au⋯Au and two kinds of hydrogen bonds (Fig. S2); distances and angles of hydrogen bonds for complex 2 (Table S1); . CCDC reference numbers 290064 and 291090. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/b809381d |
| This journal is © The Royal Society of Chemistry 2009 |
