A MOF-type magnesium benzene-1,3,5-tribenzoate with two-fold interpenetrated ReO3 nets†
Christophe
Volkringer
,
Thierry
Loiseau
*,
Jérôme
Marrot
and
Gérard
Férey
Institut Lavoisier, UMR CNRS 8180, Université de Versailles Saint Quentin, 45, avenue des Etats-Unis, 78035, Versailles, France. E-mail: loiseau@chimie.uvsq.fr; Fax: +33 1 39 25 43 58; Tel: +33 1 39 25 43 73X
First published on 29th September 2008
Abstract
A novel metal–organic architecture involving mixed chains of magnesium dinuclear units and dioxane species, linked through the benzene-1,3,5-tribenzoate linker is reported; the resulting 3-D framework consists of super-octahedral cavities, with a topology related to a two-fold interpenetrated ReO3 net.
During the last decade, the number of studies related to the synthesis of crystalline porous organic–inorganic hybrid materials has grown rapidly. These compounds, known as metal–organic framework (MOF) or coordination polymers1–3 may exhibit significant porosity properties giving rise to promising applications in the fields of the gas storage, molecule separation, drug delivery, etc. Their atomic structures are based on the association of multinuclear inorganic building motifs connected to each other through rigid organic linkers such as polycarboxylate ligands, in order to generate stable infinite 3-D open networks with tunable pore sizes and functionalities. Most MOF-type compounds reported in the literature involve transition metals (e.g., Zn2+, Cu2+, Cd2+, etc.), however quite a few contributions described the use of light elements like Al3+ or Mg2+. With these lighter cations, the formation of rather low dense and “lightweight” materials would be expected. This feature will improve their molecular storage capacity (wt%) and would be of great interest for hydrogen storage applications, for instance.
The preparation of MOF-type magnesium-based carboxylates has not been so often investigated, despite the pioneering work of Long and Dinca,4 showing hydrogen gas affinity in a magnesium naphthalenedicarboxylate, Mg3(ndc)3(def)4. Following this strategy, other research groups5–14 have reported various phases incorporating magnesium and carboxylate spacers in mixed organic–inorganic networks, obtained from the hydro(solvo)thermal route. These included different rigid aromatic ditopic (terephthalate,13naphthalenedicarboxylate,5,6biphenyldicarboxylate13) or tritopic (trimesate11,13) organic linkers.
Hereafter, this contribution deals with the use of the benzene-1,3,5-tribenzoate (noted btb) linker, which leads to the formation of a large pore 3-D framework (called MIL-123 for Materials Institute Lavoisier) with magnesium, in the presence of 1,4-dioxane as a solvent. This specific tritopic spacer, which is an augmented benzene derivative of the trimesate species, was previously reported with Zn,15,16Cu,17Fe18,19 or Tb20 cations. Interestingly, extra-large pore solids have been isolated, with high symmetry tetrahedral or octahedral cavities for some of them, correlated to the ternary symmetry of the btb molecule.
Crystals of MIL-123 (Mg12(H2O)12(μ2-(H2O)6)(btb)8(dioxane)6)·≈ 11 dioxane have been grown from the solvothermal reaction involving a mixture of the hydrated magnesium nitrate with the tripod-like benzene-1,3,5,-tribenzoic acid in 1,4-dioxane.‡ After heating at 100–110 °C for 24 hours, the title compound crystallizes as small yellowish cube-shaped crystals (Fig. S1, ESI†). The structure was determined in the trigonal system (R-3) by single-crystal X-ray diffraction.§
The structure of MIL-123 consists of four crystallographically inequivalent magnesium centers which can be divided into two identical symmetrically pairs Mg1–Mg2, and Mg3–Mg4 (Fig. 1).
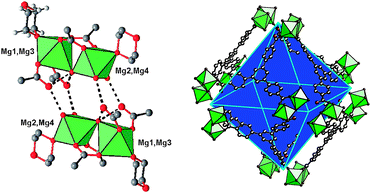 | ||
| Fig. 1 (left) Detailed view of two sets of the magnesium dinuclear units (Mg1–Mg2 or Mg3–Mg4) in MIL-123. (right) View of the super-octahedral cavities showing the position of the six tetrameric units Mg4 at each corner of the octahedron and the six organic tritopic btb linkers located on each face. Green octahedra: MgO5(H2O) or MgO3(H2O)3; red: oxygen; yellow: terminal bonded water: grey: carbon. Only the 1,4-dioxane molecule and fragment of the btb linker are shown for clarity. Hydrogen atoms have been omitted. | ||
The inorganic brick consists of octahedral Mg dimers with a common corner corresponding to a μ2-H2O aquo ligand with the Mg–H2O distances of 2.085(3)–2.208(3) Å. Two types of dinuclear motifs (Mg1–Mg2, and Mg3–Mg4) can be distinguished. The cations Mg1 and Mg3 are linked to four oxygen atoms of the carboxyl groups from the btb linker (Mg–O = 2.019(3)–2.049(2) Å). Two of the carboxylate groups are in a bidentate bridging mode and connect the two magnesium cations (Mg1–Mg2 or Mg3–Mg4) together, whereas the two others are in a monodentate mode. The cations Mg2 and Mg4 are linked to two carboxylate groups from the btb spacer (Mg–O = 2.008(3)–2.028(2) Å) and two other water molecules in terminal position (Mg–H2O = 2.073(3)–2.087(3) Å). The octahedral surrounding is completed with an oxygen atom belonging to the 1,4-dioxane molecules, which is in trans position with the bridging aquo ligand (Mg–Odioxane = 2.097(4)–2.161(2) Å). It results in two types of coordination spheres for magnesium MgO5(H2O) or MgO3(H2O)3. Two crystallographically equivalent dinuclear motifs Mg2 are connected to each other through a double hydrogen bond network composed of the two terminal water species of Mg2 or Mg4 and the non bonded carboxyl oxygen atoms of Mg1 or Mg3 (Mg2–H2O⋯O–C– = 2.631(3) and 2.661(5) Å; Mg4–H2O⋯O–C– = 2.649(3) and 2.696(4) Å). Moreover, the bridging aquo ligand is also hydrogen bonded to the terminal carboxyl oxygen atoms with short μ2-H2O⋯O–C- distances (2.557(3)–2.614(4) Å). Each pair of the dinuclear units Mg1–Mg2 or Mg3–Mg4 (resulting in two independent Mg4 tetranuclear motifs) is positioned around the inversion centers corresponding to the special positions 9e (½ 0 0) and 9d (½ 0 ½), respectively.
The Mg4 tetrameric building blocks are arranged in such a way that they occupy each corner of a super-octahedral polyhedron (Fig. 1). The organic tritopic btb linkers connect three Mg4 units to each other and are located on each triangular face of the octahedron. There are four crystallographically inequivalent btb ligands, which differ because of the distinct connection modes with the Mg4 tetramers. They are linked to five (two bidentate bridging and one monodentate modes; μ5-coordination) or three magnesium cations (three monodentate mode, μ3- coordination) from the (Mg1–Mg2)2 tetrameric block or four (one bidentate bridging and two monodentate modes; μ4- coordination) or six magnesium cations (three bidentate bridging mode, μ6- coordination) from the (Mg3–Mg4)2 tetrameric block. The remaining non-bonded C–O linkages are involved in strong hydrogen bond interactions with the terminal water molecules attached to magnesium Mg2 or Mg4. The ratios of the μ5/μ3 and μ4/μ6 btb linkers are 3/1, respectively. This leads to the formation of two crystallographically independent super-octahedral cages centered on the special positions 3b (0 0 ½) and 3a (0 0 0) coming from the connection of btb with the tetramers (Mg1–Mg2)2 and the tetramers (Mg3–Mg4)2, respectively.
The 3-D cohesion of the structure is ensured by the dioxane species which link two distinct Mg1–Mg2 and Mg3–Mg4 dinuclear units to each other, and therefore act as the connection nodes between the two independent super-octahedral blocks. It results in the formation of rings of the Mg1–Mg2 dimers alternating with the Mg3–Mg4 ones through the dioxane molecules which are developed along the edges of the super-octahedral cavities (Fig. 2). The super-octahedral units are stacked to each other along the [0 0 1], [0 2 1] and [2 0 −1] directions with a rotation of 60° of their triangular faces (Fig. 3), through π–π interactions between the central benzene ring of two distinct btb linkers (C⋯C = ≈ 3.6–3.7 Å).
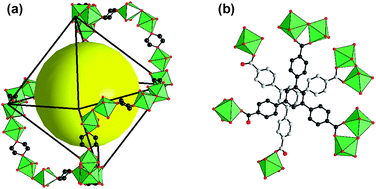 | ||
| Fig. 2 (a) View of the ring of dioxane species linking the dinuclear units Mg1–Mg2 and Mg3–Mg4 to each other along the edge of the super-octahedral cavities. The yellow sphere indicates the center of the super-octahedral cage. (b) Two tritopic btb linkers in π–π interactions between a μ5-btb coordinated to Mg1–Mg2 and a μ4-btb coordinated to Mg3–Mg4 dinuclear units. | ||
 | ||
| Fig. 3 View of the structure of MIL-123 showing the two independent super-octahedral cavities based on the connection of (Mg1–Mg2)2 (blue) and (Mg3–Mg4)2 (yellow) tetramers with the btb linkers. | ||
The latter interaction also contributes to reinforcing the stability of the structure. Similar interactions were previously encountered in a copper benzene-1,3,5-tribenzoate,17 in which the btb linkers also interact between the central benzene rings in an eclipsed manner.
Topologically, both independent super-octahedral blocks are connected to each other by sharing all their corners, giving rise to a 3-D network corresponding to the primitive cell of the ReO3 type. The structure can be viewed as two interpenetrating but nonintersecting ReO3 nets, which are shifted to each other by a ½ ½ ½ translation (Fig. 4). This specific arrangement is a new example of interpenetrating ReO3 nets, which were previously classified by Batten and Robson21 and, for instance, described in Cu3(tpt)4(ClO4)3.
 | ||
| Fig. 4 Polyhedral representation of the two-fold interpenetrating ReO3 nets in MIL-123. The yellow ReO3 net is shifted to the blue one by the ½ ½ ½ translation. The blue octahedron (centered on 0 0 ½) consists of 12 Mg1, 12 Mg2 and six btb linkers whereas the yellow one (centered on 0 0 0) consists of 12 Mg3, 12 Mg4 and six btb linkers. | ||
The estimated pore sizes are 16 Å from face to face and 21 Å between two opposite corners of the super-octahedral cavities. Although it was not possible to locate species by XRD, it was assumed that a large amount of 1,4-dioxane species reside within the cage. Thermogravimetric analysis (Fig. S2, ESI†) indicates that about free 11 1,4-dioxane molecules per Mg12 unit are encapsulated within the cavities. IR spectroscopy (Fig. S3 ESI†) also indicates a specific intense vibration band corresponding to a νC–O frequency at 1634 cm−1 from the 1,4-dioxane species. The apparent solvent accessible space is estimated to be 54% from the PLATON22 calculations. Despite this volume ratio, the adsorption of N2 was not so high and after many attempts to remove the trapped solvent molecules (1,4-dioxane) under primary vacuum at different temperatures (≤ 80 °C), a BET surface area of 216(6) m2 g−1 (Langmuir: 313(1) m2 g−1) was measured from the N2 isotherm at 77 K. This low value indicates that the trapped organic species still protrude the cavities and it was not possible to remove all of them, otherwise the structure collapsed. Thermal XRD analysis showed that the Bragg peaks disappeared after 80–90 °C, indicating the decomposition of the phase.
In conclusion, MIL-123 is a new example of MOF-type compound incorporating magnesium, which is rarely described in the literature. Its structure is built up from two types of large super-octahedral cavities, independently connected to each other to generate two-fold interpenetrated ReO3 nets.
We would like to thank Mrs P. Dreux and S. de Bigault de Cazanove for their help in the synthesis work, Dr E. Leroy and Dr M. Latroche for their assistance with the SEM image and Dr C. Kiener (BASF - Ludwigshafen, Germany) for kindly providing the benzene-1,3,5-tribenzoic acid reactant.
References
- G. Férey, Chem. Soc. Rev., 2008, 37, 191 RSC.
- O. M. Yaghi, M. O'Keeffe, N. W. Ockwig, H. K. Chae, M. Eddaoudi and J. Kim, Nature, 2003, 423, 705 CrossRef CAS.
- S. Kitagawa, R. Kitaura and S.-I. Noro, Angew. Chem., Int. Ed., 2004, 43, 2334 CrossRef CAS.
- M. Dinca and J. R. Long, J. Am. Chem. Soc., 2005, 127, 9376 CrossRef CAS.
- I. Senkovska and S. Kaskel, Eur. J. Inorg. Chem., 2006, 4564 CrossRef CAS.
- I. Senkovska, J. Fritsch and S. Kaskel, Eur. J. Inorg. Chem., 2007, 5475 CrossRef CAS.
- J. A. Rood, B. C. Noll and K. W. Henderson, Inorg. Chem., 2006, 45, 5521 CrossRef CAS.
- K. C. Kam, L. M. Young and A. K. Cheetham, Cryst. Growth Des., 2007, 7, 1522 CrossRef CAS.
- A. Mesbah, C. Juers, F. Lacouture, S. Mathieu, E. Rocca, M. François and J. Steinmetz, Solid State Sci., 2007, 9, 322 CrossRef CAS.
- Z. Hulvey and A. K. Cheetham, Solid State Sci., 2007, 9, 137 CrossRef CAS.
- S. Ma, J. A. Fillinger, M. W. Ambrogio, J.-L. Zuo and H.-C. Zhou, Inorg. Chem. Commun., 2007, 10, 220 CrossRef CAS.
- J. Zhang, S. Chen, H. Valle, M. Wong, C. Austria, M. Cruz and X. Bu, J. Am. Chem. Soc., 2007, 129, 14168 CrossRef CAS.
- R. P. Davies, R. J. Less, P. D. Lickiss and A. J. P. White, Dalton Trans., 2007, 2528 RSC.
- P. D. C. Dietzel, R. Blom and H. Fjellvag, Eur. J. Inorg. Chem., 2008, 3624 CrossRef CAS.
- J. Kim, B. Chen, T. M. Reineke, H. Li, M. Eddaoudi, D. B. Moler, M. O'Keeffe and O. M. Yaghi, J. Am. Chem. Soc., 2001, 123, 8239 CrossRef CAS.
- H. K. Chae, D. Y. Siberio-Perez, J. Kim, Y. Go, M. Eddaoudi, A. J. Matzger, M. O'Keeffe and O. M. Yaghi, Nature, 2004, 427, 523 CrossRef CAS.
- B. Chen, M. Eddaoudi, S. T. Hyde, M. O'Keeffe and O. M. Yaghi, Science, 2001, 291, 1021 CrossRef CAS.
- A. C. Sudik, A. R. Millward, N. W. Ockwig, A. P. Côté, J. Kim and O. M. Yaghi, J. Am. Chem. Soc., 2005, 127, 7110 CrossRef CAS.
- S. B. Choi, M. J. Seo, M. Cho, Y. Kim, M. K. Jin, D.-Y. Jung, J.-S. Choi, W.-S. Ahn, J. L. C. Rowsell and J. Kim, Cryst. Growth Des., 2007, 7, 2290 CrossRef CAS.
- T. Devic, C. Serre, N. Audebrand, J. Marrot and G. Férey, J. Am. Chem. Soc., 2005, 127, 12788 CrossRef CAS.
- S. R. Batten and R. Robson, Angew. Chem., Int. Ed., 1998, 37, 1460 CrossRef.
- A. L. Spek, PLATON, A Multipurpose Crystallographic Tool, Ultrecht University, Utrecht, The Netherlands, 2002 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: SEM photograph, TG analysis. CCDC reference numbers 693012. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/b814943g |
| ‡ The magnesium benzene-1,3,5-tribenzoate (MIL-123) was solvothermally synthesized from a mixture of magnesium nitrate (Mg(NO3)2, 6H2O, Aldrich, 99%), benzene-1,3,5–tribenzoic acid (BASF, noted H3btb) and 1,4-dioxane (Fisher Scientific, Reagent Grade). The starting reactants are mixed together with the following molar compositions 2 Mg(NO3)2, 6 H2O (0.2033 g, 0.79 mmol), 1 btb (0.1703 g, 0.387 mmol) and 208 1,4-dioxane (5 ml, 80.5 mmol) and placed in a Teflon-lined stainless steel Parr autoclave (23 ml). The vessel was heated at 100–110 °C in an oven for 24 h. The reaction mixture was then cooled down to room temperature, leading to the formation of yellowish cubic crystals. The resulting product was filtered off, washed with 1,4-dioxane and left in ambient atmosphere (yield ≈ 80% based on magnesium). Elemental chemical analysis: Mg (obs: 5.4%; calc: 5.2%), C (obs: 58.7%; calc: 60.9%) and H (obs: 5.2%; calc: 5.5%). |
| § A suitable cube-shaped crystal (0.08 × 0.08 × 0.08 mm) of the MIL-123 compound was mounted on a glass fiber and X-ray diffraction intensities were collected with a Bruker X8-APEX2 CCD area-detector diffractometer using graphite-monochromatized Mo Kα radiation (λ = 0.71073 Å) at room temperature. The substantial redundancy in the data allowed a semi-empirical absorption correction (SADABS) to be applied, on the basis of multiple measurements of equivalent reflections. The structures were solved by direct methods, developed by successive difference Fourier syntheses, and refined by full-matrix least-squares on all F2 data using SHELXTL. The hydrogen atoms belonging to the btb and 1,4-dioxane species were included in calculated positions and allowed to ride on their parent atoms. The final R = 0.0647, wR2 = 0.1555 for 11445 observed reflections with I > 2σ(I). Crystal data for C40H28Mg2O13, FW = 765.24 g mol−1, trigonal system, R-3 (n° 148); a = b = 38.3092(6), c = 47.3977(13) Å, γ = 120°, V = 60241(2) Å3, Z = 36, Dc = 0.759 g cm−3, F(000) = 14256. |
| This journal is © The Royal Society of Chemistry 2009 |
