A natural carbohydrate substrate for Mycobacterium tuberculosismethionine sulfoxide reductase A†
Susanne A.
Stalford
a,
Martin A.
Fascione
a,
Smitha J.
Sasindran
b,
Delphi
Chatterjee
c,
Subramanian
Dhandayuthapani
b and
W. Bruce
Turnbull
*a
aSchool of Chemistry and Astbury Centre for Structural Molecular Biology, University of Leeds, Leeds, UK LS2 9JT. E-mail: w.b.turnbull@leeds.ac.uk; Fax: +44 (0)113 343 6565; Tel: +44 (0)113 343 7438
bUniversity of Texas Health Science Center at San Antonio, Department of Microbiology and Immunology, and Regional Academic Health Center, 1214 West Schunior Street, Edinburg, TX 78541, USA
cDepartment of Microbiology, Immunology, and Pathology, Colorado State University, Fort Collins, CO 80523-1682, USA
First published on 11th November 2008
Abstract
Enzymatic reduction of the methylsulfinylxylofuranosyl (MSX) groups in lipoarabinomannan provides proof of the absolute configuration of MSX and a possible biochemical mechanism for oxidative protection in Mycobacterium tuberculosis.
Tuberculosis (TB) remains a major threat to world health,1 with 9.2 million new cases and 1.7 million deaths from TB in 2006.2 The disease is caused by Mycobacterium tuberculosis (Mtb) which invades and colonises macrophage cells. The ability of Mtb to survive within this inhospitable environment has been attributed to its robust cell wall,3 that comprises complex glycolipids including mycolyl-arabinogalactan-peptidoglycan (mAGP)4 and lipoarabinomannan (LAM).5LAM facilitates the entry of the bacterium into macrophages,5 prevents macrophage activation, and protects Mtb from damage by superoxide and hydroxyl radicals.6 Although macrophages also produce H2O2 during a respiratory burst,7 the effect of H2O2 on LAM was not reported alongside the studies on superoxide and hydroxyl radicals.6
Recent structural studies have revealed two novel sugar substituents on the terminal mannose caps of LAM,8,9 which were identified as an α-5-methylthioxylofuranosyl (MTX) group and its corresponding sulfoxide derivative (MSX) (Scheme 1b).10,11 The cell surface location of MTX8 will make it particularly exposed to damage by reactive oxygen species (ROS) produced by macrophages. Oxidation of the sulfide group would almost certainly modulate any functional interactions between the sugar and other biomolecules. For example, an MTX-α(1,4)-mannosyl disaccharide has been shown to inhibit the production of cytokine TNF-α in the human monocyte cell line THP-1,11 but the effect is reduced upon oxidation of the sugar.
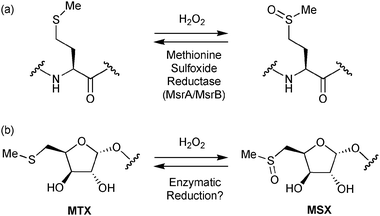 | ||
| Scheme 1 Oxidation and reduction of (a) methionine and (b) MTX. | ||
Conversely, sacrificial oxidation of MTX would sequester ROS from solution and thus prevent other sensitive molecules from oxidative damage. Levine has proposed12 that methionine residues in proteins could provide anti-oxidative protection by sequestering peroxides through a redox cycle (Scheme 1a) comprising oxidation by H2O2 and subsequent enzymatic reduction by methionine sulfoxide reductase (Msr) enzymes that are found in all organisms.13,14 Indeed, over-expression of the methionine sulfoxide reductase A enzyme (MsrA) in human fibroblasts can reduce the level of protein carbonyl formation when challenged with H2O2.15 The MsrA enzyme from Mycobacterium smegmatis has also been shown to be important for survival of mycobacteria inside macrophage cells.16 MsrA is known to accommodate alternative substrates bearing a methylthio group.17 Therefore, the structural similarities between methionine and MTX led us to consider if a similar redox cycle could be possible in which methionine is replaced by MTX (Scheme 1b). We also use this oxidation/reduction mechanism to provide a stereochemical proof for the absolute configuration of MTX.
LAM was purified from the Mtb clinical isolate CSU20 as described previously.81H NMR spectroscopy of CSU20-LAM (Fig. 1a) indicated that there was approximately one MTX or MSX residue for every 45 monosaccharide units. This ratio corresponds to one xylofuranosyl residue per LAM molecule, or one MTX/MSX for every 5–6 mannose caps.5,8 When one considers the relative concentrations of proteins and LAM in mycobacterial cell walls,18 MTX could be as prevalent as methionine residues in this part of the cell. The presence of two overlapping doublets (corresponding to H-1) at ca. 5.45 ppm indicated that the sample was already partially oxidised to a 1 : 1 mixture of MSX diastereoisomers. The occurrence of MSX in previous studies has been attributed to oxidation during purification of the LAM.8 Upon addition of excess H2O2 to the sample, the MTX anomeric signal decreased in size to be replaced by the overlapping MSX anomeric signals (Fig. 1b). It should be noted that no other anomeric signals in the spectrum changed when the sample was treated with H2O2. Therefore, it can be concluded that MTX is oxidised selectively to MSX by H2O2, and no other monosaccharide component of LAM is capable of sequestering H2O2. This observation was corroborated by experiments on methyl α-mannopyranoside and methyl α/β-arabinofuranoside which showed no oxidation by H2O2 under conditions that allowed complete conversion of MTX to MSX (data not shown).
 | ||
| Fig. 1 Anomeric (H-1) region of the 1H NMR spectra of LAM (a) before oxidation, and after successive treatment with (b) H2O2 and (c) MsrA–DTT. | ||
Mycobacterium tuberculosis has two genes encoding Msr enzymes: MsrA and MsrB which reduce S- and R-configured sulfoxides, respectively. Cell wall/membrane and soluble protein fractions were prepared according to Mueller-Ortiz et al.19 Immunoblotting experiments showed that while MsrB is confined to the cytosol of the bacterium, MsrA is present in both the soluble and membrane/cell wall fractions (Fig. 2). As deletion of the gene encoding MsrB is generally less important for anti-oxidative protection than MsrA,14 we chose to focus first on reduction of MSX by MsrA.
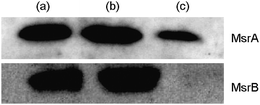 | ||
| Fig. 2 Immunoblot analysis of MsrA and MsrB in different fractions of Mtb cells: (a) whole Mtb cell, (b) the cytosolic fraction and (c) the cell wall/membrane fraction. | ||
MSX-LAM was treated with MsrA in the presence of excess dithiothreitol (DTT) as a co-reductant to regenerate the catalytically active form of the enzyme. The 1H NMR spectrum of the reaction mixture showed that the low field MSX anomeric signal disappeared to be replaced by the anomeric signal for MTX (Fig. 1c). The MTX anomeric signal is slightly larger than that for the remaining MSX as a consequence of incomplete oxidation of the original MTX residues (Fig. 1b). These results demonstrate that MSX is reduced stereoselectively by MsrA. A molecular model of methionine sulfoxide docked into the crystal structure of Mtb MsrA indicates that only the S-configured sulfoxide can be accommodated in the enzyme active site.20 Therefore, we would assign the remaining MSX signal to correspond to the R-sulfoxide. Treatment of MSX-LAM with DTT alone had no effect on the NMR spectrum, and hence did not modify the polysaccharide structure.
The stereoselective reduction of MSX presented us with an opportunity to determine the absolute stereochemistry of MTX while still attached to the polysaccharide. Inspection of a model of MSX docked into the MsrA crystal structure indicated that little of the MSX structure would be bound within the enzyme active site (Supporting information Fig. S1†). Therefore, either D- or L-configured MSX should be able to act as a substrate for the enzyme, but in each case only the S-configured sulfoxide should be reduced. The remaining R-sulfoxides would be a pair of diastereoisomers and would thus have different NMR spectra. We anticipated that only one of these isomers would match the spectrum of the natural polysaccharide.
The methyl α-furanosides of D- and L-MTX were prepared by chemical synthesis (see supporting information†) and oxidised to MSX by exposure to H2O2. No evidence for over-oxidation to the sulfone was observed. The 1H NMR spectra for D- and L-MSX were identical (Fig. 3a,d), as expected for pairs of enantiomers. The difference in chemical shift for the anomeric proton of methyl α-MSX from that of MSX-LAM (Fig. 1b) can be attributed to the different anomeric substituents.10 Upon treatment with MsrA and DTT, only the S-sulfoxide isomers were reduced to give mixtures of MTX and either the R-methylsulfinyl-D-xylofuranoside (Fig. 3b) or R-methylsulfinyl-L-xylofuranoside (Fig. 3c).21 As the downfield MSX anomeric signal is lost for both MSX-LAM (Fig. 1c) and methyl α-D-MSX (Fig. 3b), we can thus conclude that MTX is D-configured. This result provides independent corroboration of the findings of Lowary and co-workers who compared the NMR spectra of six synthetic MTX-mannosyl disaccharides with the published NMR spectra of LAM.11
 | ||
| Fig. 3 Anomeric (H-1) region of the 1H NMR spectra of methyl α-D-MSX (a) before and (b) after treatment with MsrA–DTT. Anomeric (H-1) region of the 1H NMR spectra of methyl α-L-MSX (d) before and (c) after treatment with MsrA–DTT. | ||
In conclusion, we have demonstrated that exposure of LAM to the biological oxidant H2O2 results in oxidation of only the MTX substituent. Stereoselective reduction of the S-sulfoxide isomer of MSX-LAM using MsrA provides proof of the absolute configuration of this novel sugar without the need to remove the sugar from the polysaccharide. While oxidation of MTX would almost certainly affect its biological function in vivo, this damage could be repaired, in part, by MsrA which is present in the mycobacterial cell wall/membrane fraction. Alternatively, there exists the possibility that MSX on the surface of M. tuberculosis may be reduced by host MsrA and MsrB when the bacteria reside inside macrophages. If so, then MSX would be the first natural non-protein substrate for these enzymes. Furthermore, this redox cycle of chemical oxidation and enzymatic reduction could also provide a mechanism for more general anti-oxidative protection, as has been established previously for methionine oxidation.12,15 Indeed, as H2O2 is a direct precursor of hydroxyl radicals in vivo, sequestration of H2O2 by MTX–MsrA could also reduce the production of OH˙ in the mycobacterial cell wall. Future studies will focus on testing this hypothesis in vivo.
We acknowledge the Royal Society, the San Antonio Area Foundation, the University of Leeds and NIH for funding. D.C. is supported by NIH/NIAID AI 37139. W.B.T. is a recipient of a Royal Society University Research Fellowship. Mr J. Willis is thanked for assistance in protein production.
Notes and references
- R. P. Tripathi, N. Tewari, N. Dwivedi and V. K. Tiwari, Med. Res. Rev., 2005, 25, 93 CrossRef CAS.
- WHO, Global Tuberculosis Control: Surveillance, Planning, Financing, World Health Organization, Geneva, 2008.
- P. J. Brennan and D. C. Crick, Curr. Top. Med. Chem., 2007, 7, 475 CrossRef CAS.
- L. Kremer and G. S. Besra, in Tuberculosis and the Tubercle Bacillus, ed. S. T. Cole, American Society for Microbiology, Washington, DC, 2005, p. 287 Search PubMed.
- D. Chatterjee and K.-H. Khoo, Glycobiology, 1998, 8, 113 CrossRef CAS; V. Briken, S. A. Porcelli, G. S. Besra and L. Kremer, Mol. Microbiol., 2004, 53, 391 CrossRef CAS; J. Nigou, M. Gilleron and G. Puzo, Biochimie, 2003, 85, 153 CrossRef CAS.
- J. Chan, X. Fan, S. W. Hunter, P. J. Brennan and B. R. Bloom, Infect. Immun., 1991, 59, 1755 CAS.
- J. A. Imlay, Annu. Rev. Biochem., 2008, 77, 755 CrossRef CAS.
- A. Treumann, X. Feng, L. McDonnell, P. J. Derrick, A. E. Ashcroft, D. Chatterjee and S. W. Homans, J. Mol. Biol., 2002, 316, 89 CrossRef CAS.
- P. Ludwiczak, M. Gilleron, Y. Bordat, C. Martin, B. Gicquel and G. Puzo, Microbiology (Reading, U. K.), 2002, 148, 3029 CAS; Y. Guerardel, E. Maes, V. Briken, F. Chirat, Y. Leroy, C. Locht, G. Strecker and L. Kremer, J. Biol. Chem., 2003, 278, 36637 CrossRef CAS.
- W. B. Turnbull, K. H. Shimizu, D. Chatterjee, S. W. Homans and A. Treumann, Angew. Chem., Int. Ed., 2004, 43, 3918 CrossRef CAS.
- M. Joe, D. Sun, H. Taha, G. C. Completo, J. E. Croudace, D. A. Lammas, G. S. Besra and T. L. Lowary, J. Am. Chem. Soc., 2006, 128, 5059 CrossRef CAS.
- R. L. Levine, L. Mosoni, B. S. Berlett and E. R. Stadtman, Proc. Natl. Acad. Sci. U. S. A., 1996, 93, 15036 CrossRef CAS.
- S. Boschi-Muller, A. Olry, M. Antoine and G. Branlant, Biochim. Biophys. Acta, 2005, 1703, 221.
- S. J. Sasindran, S. Saikolappan and S. Dhandayuthapani, Future Microbiol., 2007, 2, 619 Search PubMed.
- C. R. Picot, I. Petropoulos, M. Perichon, M. Moreau, C. Nizard and B. Friguet, Free Radical Biol. Med., 2005, 39, 1332 CrossRef CAS.
- T. Douglas, D. S. Daniel, B. K. Parida, C. Jagannath and S. Dhandayuthapani, J. Bacteriol., 2004, 186, 3590 CrossRef CAS.
- J. Moskovitz, H. Weissbach and N. Brot, Proc. Natl. Acad. Sci. U. S. A., 1996, 93, 2095 CrossRef CAS.
- B. Hamasur, G. Källenius and S. B. Svenson, FEMS Immunol. Med. Microbiol., 1999, 24, 11 CrossRef CAS; I. Verma, A. Rohilla and G. K. Khuller, Lett. Appl. Microbiol., 1999, 29, 113 CrossRef CAS.
- S. L. Mueller-Ortiz, A. R. Wanger and S. J. Norris, Infect. Immun., 2001, 69, 7501 CrossRef CAS.
- A. B. Taylor, D. M. Benglis, Jr, S. Dhandayuthapani and P. J. Hart, J. Bacteriol., 2003, 185, 4119 CrossRef CAS.
- X-ray crystallography could not be used to confirm the relative configurations of the products as the sulfoxides were syrups. Therefore, the configurations of the sulfoxides are assigned on the basis of the known substrate specificity of MsrA (see ref. 13).
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details for oxidation and reduction experiments and for the preparation of methyl α-D- and -L-MSX. See DOI: 10.1039/b817483k |
| This journal is © The Royal Society of Chemistry 2009 |
