Gallyl lanthanide complexes containing unsupported Ln–Ga (Ln = Sm, Eu, Yb or Tm) bonds†
Cameron
Jones
*,
Andreas
Stasch
and
William D.
Woodul
School of Chemistry, Monash University, Melbourne, PO Box 23, Victoria, 3800, Australia
First published on 12th November 2008
Abstract
A series of bis(gallyl) lanthanide(II) complexes, [LnII{Ga[(ArNCH)2]}2(tmeda)2] (Ln = Sm, Eu or Yb; Ar = C6H3Pri2-2,6), and a gallyl thulium(III) compound, [TmIII{Ga[(ArNCH)2]}{(ArNCH)2}(tmeda)], have been prepared; the first structural studies of Ga–Tm and Ga–Sm bonded complexes are described.
The chemistry of complexes containing metal–metal bonds is of much importance, both from fundamental and applications standpoints.1 With this in mind, in recent years we have been systematically examining the coordination and further chemistry of the anionic gallium(I) heterocycle, [:Ga(ArNCH)2]−1,2 which is a valence isoelectronic analogue of the important N-heterocyclic carbene (NHC) class of ligand. The majority of complexes derived from this ligand have been with p-3 or d-block4 metal fragments and these have highlighted similarities, but also differences, between 1 and both NHCs and cyclic boryl ligands. In 2006, this work was extended to group 2 with the preparation of compounds 2 and 3,5 which exhibited the first examples of gallium-group 2 bonds in molecular species. The next year, and in collaboration with Arnold et al., the first gallium–lanthanide bonded complex, 4, was prepared and shown by X-ray crystallographic and theoretical studies to have an unsupported, polar-covalent Nd–Ga bond.6 Wiecko and Roesky later reported the only other gallium–lanthanide complexes, [Cp*2Eu(GaCp*)2] and [Cp*2Yb(GaCp*)(THF)] (Cp* = C5Me5−),7,8 though unlike 4, these contain weak GaI→ LnII donor–acceptor bonds.
There is no known further chemistry for Ln–Ga bonded compounds. For this to advance with any pace, it seemed that the development of systems containing polar-covalent lanthanide(II)–gallium bonds would be necessary. Once such compounds are accessed, it would be of considerable interest to compare their redox chemistry with that of widely explored lanthanide(II) reducing agents such as SmI29 and SmCp*2.10 Here we detail preliminary results towards this end, namely the synthesis of three bis(gallyl) lanthanide(II) compounds and a related gallyl thulium(III) complex. X-Ray crystallographic studies on these compounds have led to the first structural elucidations of Ga–Sm and Ga–Tm bonds.
Early in this study, attempts were made to prepare complexes of the type [Ln{Ga[(ArNCH)2]}2(THF)n] (Ln = Sm, Eu or Yb) by reduction of the digallane(4), [{GaII[(ArNCH)2]}2], or the paramagnetic gallium(III) halide complex, [GaI2{(ArNCH)2}], with the elemental lanthanide in THF. Although this methodology was successfully used to prepare the group 2 complexes, 2 and 3, no reaction occurred with the lanthanide metals. Salt elimination reactions of [K(tmeda)][Ga{(ArNCH)2}] with half an equivalent of LnI2 in THF were then investigated, but all led to intractable mixtures of products. However, when the reactions were repeated in the presence of an excess of tmeda, the bis(gallyl) lanthanide complexes, 5 (dark green), 6 (orange) and 7 (red–orange), were reproducibly obtained in low to moderate isolated yields (Scheme 1).†
 | ||
| Scheme 1 Reagents and conditions: i, LnI2(THF)n, toluene/tmeda, −KI; ii, TmI2(THF)5, toluene/tmeda, −KI, −Ga(s). | ||
After Eu2+, Yb2+ and Sm2+, Tm2+ is the next most stable lanthanide dication (E° Tm3+/Tm2+ = −2.22 V) and has a rapidly emerging molecular chemistry.11 Given the thermal stability of 5–7, and the previously demonstrated reducing nature of the gallium heterocycle, [:Ga{(ArNCH)2}]−,3,4 it was thought possible that the thulium analogue of 5–7 might be a stable entity at room temperature. However, a comparable reaction with TmI2 reproducibly led to a low isolated yield of the red thulium(III)–gallyl complex, 8, the coordination sphere of which is completed by a molecule of tmeda and a doubly reduced diazabutadiene ligand. It seems likely that the intermediate in the reaction that gave 8 is the target thulium(II) complex, [Tm{Ga[(ArNCH)2]}2(tmeda)2], which was subject to an intramolecular reduction of one gallyl ligand by the thulium centre, leading to elimination of gallium metal.
Little useful information could be obtained from the 1H NMR spectra of 5, 6 and 8 due their paramagnetic nature.12 The spectrum of 7 is, however, more informative and displays a major set of resonances that is consistent with its solid state structure (vide infra). It also exhibits a more complex, minor set of resonances which we believe corresponds to the cis-isomer of the compound (major isomer : minor isomer ratio is ca. 85 : 15). The most compelling evidence for this proposal is that there are two chemically inequivalent sets of backbone protons for the gallyl ligands which resonate as an AB spin system. The fact that these protons are inequivalent suggests that the bulky heterocyclic ligands of the complex are “interlocked” and cannot rotate freely with respect to each other. Very similar spectra have been observed for square planar transition metal complexes, e.g. cis-[Pd{Ga[(ArNCH)2]}2(tmeda)].4a That the two isomers of 7 exist in equilibrium in solution was seemingly confirmed by dissolving several crystallographically authenticated samples of the trans-isomer of the compound in C6D6 which, in each case, led to spectra corresponding to identical isomeric mixtures. In addition, only trans-7 could be crystallised from these solutions, suggesting this is the thermodynamically favoured isomer. The low solubility of 7 in aromatic solvents at low temperature precluded a variable temperature NMR study of the equilibrium between the isomers. Furthermore, no signals were observed in the 171Yb NMR spectrum of 7, presumably because of significant peak broadening, arising from the coordination of the Yb atom of both isomers of 7 by two quadrupolar Ga centres (69Ga, 60% abundant, I = 3/2; 71Ga, 40% abundant, I = 3/2). The apparent isomerisation of 7 in solution is of some interest as observations of geometric isomerism in lanthanide complexes are rare, though not unknown.13
Compounds 5–7 were crystallographically characterised and found to be isostructural.‡ As a result, only the molecular structure of 5 is depicted in Fig. 1, though relevant geometric parameters for the other two compounds are included in the caption. Each compound has a distorted lanthanide octahedral geometry with the gallyl ligands trans- to each other (cf.3). The Ln–Ga distances in the compounds follow the expected trend (Sm–Ga ∼ Eu–Ga > Yb–Ga) based on the effective ionic radii for six-coordinate Ln2+ cations (Sm2+ 1.19 Å, Eu2+ 1.17 Å, Yb2+ 1.02 Å).14 In addition, given the similarity between the ionic radii of Yb2+ and Ca2+ (1.00 Å; six-coordinate14), it is of note that the Ca–Ga bonds in 3 (3.1587(6) Å) are only slightly shorter than the Yb–Ga bonds of 7. Although the Ln–Ga bonds in 5–7 are almost certainly very polar, they should possess some covalent character based on prior theoretical studies on 2–4.5,6 The Ln–Ga separations of the compounds in this study are, however, significantly greater than sums of the covalent radii for the atom pairs (Sm–Ga 3.20 Å, Eu–Ga 3.20 Å or Yb–Ga 3.09 Å).15 Although there are no known Sm–Ga bonded complexes to compare with, the Eu–Ga and Yb–Ga bonds in 6 and 7 are, not surprisingly, shorter than those in the adducts, [Cp*2Eu(GaCp*)2] (3.320 Å mean) and [Cp*2Yb(GaCp*)(THF)] (3.2872(4) Å).7
 | ||
| Fig. 1 Molecular structure of 5 (20% thermal ellipsoids; hydrogen atoms omitted). Symmetry operation: ′−x, −y, −z. Selected bond lengths (Å) and angles (°) for 5: Ga–Sm 3.3124(9), Sm–N 2.724 (mean), Ga–N 1.931 (mean); N–Ga–N 83.66(8); 6: Ga–Eu 3.3124(11), Eu–N 2.677 (mean), Ga–N 1.934 (mean); N–Ga–N 83.57(18); 7: Ga–Yb 3.226 (mean), Yb–N 2.596 (mean), Ga–N 1.939 (mean); N–Ga–N 83.74 (mean). | ||
The molecular structure of 8 is shown in Fig. 2 and exhibits the first example of a structurally characterised Tm–Ga bond in a molecular compound. The doubly reduced diazabutadiene ligand is coordinated to the thulium centre in what can be described as a slipped η4-mode, as has been seen in related complexes, e.g. [Yb{(ArNCH)2}(C5Me5)(THF)].16,17 This gives rise to a heavily distorted trigonal bipyramidal coordination geometry (not taking into account the alkene–Tm interaction) with Ga(1) and N(5) in the axial positions. The Ln–Ga distance in the compound is markedly shorter than those in 4–7, and unlike those compounds, is well within the sum of the covalent radii for the two metals (3.12 Å).15
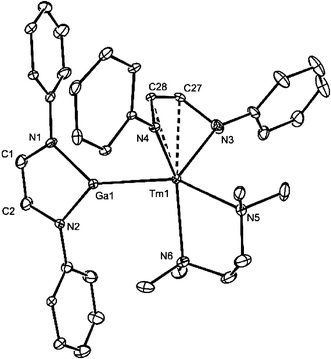 | ||
| Fig. 2 Molecular structure of 8 (20% thermal ellipsoids; hydrogen atoms, isopropyl groups and a second crystallographically independent molecule omitted). Selected bond lengths (Å) and angles (°): Tm(1)–Ga(1) 2.9742(16), Tm(1)–N(3) 2.163(10), Tm(1)–N(4) 2.171(8), Tm(1)–N(5) 2.481(9), Tm(1)–N(6) 2.512(10), Tm(1)–C(27) 2.547(11), Tm(1)–C(28) 2.563(10), N(3)–C(27) 1.452(15), N(4)–C(28) 1.409(14), C(27)–C(28) 1.344(16); N(3)–Tm(1)–N(4) 85.1(4), N(3)–Tm(1)–N(5) 92.2(4), N(4)–Tm(1)–N(5) 94.4(3), N(3)–Tm(1)–N(6) 128.3(4), N(4)–Tm(1)–N(6) 142.9(4), N(5)–Tm(1)–N(6) 71.4(3), N(3)–Tm(1)–Ga(1) 109.6(3), N(4)–Tm(1)–Ga(1) 97.6(2), N(5)–Tm(1)–Ga(1) 155.9(2), N(6)–Tm(1)–Ga(1) 86.7(2). | ||
In conclusion, a series of stable bis(gallyl) lanthanide(II) complexes have been prepared. In addition, an analogous bis(gallyl) thulium(II) complex has been implicated as an intermediate in the formation of a gallyl thulium(III) complex. Crystallographic studies on these complexes have given rise to the first structurally characterised GaTm or GaSm bonds. We are currently examining the use of 5–7 as reductants towards a range of unsaturated substrates and will report on this in due course.
We gratefully acknowledge financial support from the Australian Research Council (fellowships for CJ and AS), and the EPSRC Mass Spectrometry Service, Swansea.
Notes and references
- F. A. Cotton, C. A. Murillo and R. A. Walton, Multiple Bonds Between Metal Atoms, 3rd edn, Springer, New York, 2005 Search PubMed.
- R. J. Baker, R. D. Farley, C. Jones, M. Kloth and D. M. Murphy, J. Chem. Soc., Dalton Trans., 2002, 3844 RSC.
- See, for example: (a) S. P. Green, C. Jones, K. -A. Lippert, D. P. Mills and A. Stasch, Inorg. Chem., 2006, 45, 7242 CrossRef CAS; (b) R. J. Baker, C. Jones, D. P. Mills, D. M. Murphy, E. Hey-Hawkins and R. Wolf, Dalton Trans., 2006, 64 RSC; (c) R. J. Baker, C. Jones and M. Kloth, Dalton Trans., 2005, 2106 RSC; (d) R. J. Baker, C. Jones, M. Kloth and J. A. Platts, Angew. Chem., Int. Ed., 2003, 43, 2660 CrossRef CAS.
- See, for example: (a) C. Jones, D. P. Mills, R. P. Rose and A. Stasch, Dalton Trans., 2008, 4395 RSC; (b) C. Jones, R. P. Rose and A. Stasch, Dalton Trans., 2007, 2997 RSC; (c) S. P. Green, C. Jones, D. P. Mills and A. Stasch, Organometallics, 2007, 26, 3424 CrossRef CAS; (d) R. J. Baker, C. Jones and J. A. Platts, J. Am. Chem. Soc., 2003, 125, 10534 CrossRef CAS.
- C. Jones, D. P. Mills, J. A. Platts and R. P. Rose, Inorg. Chem., 2006, 45, 3146 CrossRef CAS.
- P. L. Arnold, S. T. Liddle, J. McMaster, C. Jones and D. P. Mills, J. Am. Chem. Soc., 2007, 129, 5360 CrossRef CAS.
- M. Wiecko and P. W. Roesky, Organometallics, 2007, 26, 4846 CrossRef CAS.
- N.B. Two Al–lanthanide and one Al–actinide donor–acceptor complexes have also been reported: (a) M. T. Gamer, P. W. Roesky, S. N. Konchenko, P. Nava and R. Ahlrichs, Angew. Chem., Int. Ed., 2006, 45, 4447 CrossRef CAS; (b) S. G. Minasian, J. L. Krinsky, V. A. Williams and J. Arnold, J. Am. Chem. Soc., 2008, 130, 10086 CrossRef CAS.
- G. A. Molander and C. R. Harris, Chem. Rev., 1996, 96, 307 CrossRef CAS , and references therein.
- W. J. Evans, J. Organomet. Chem., 2002, 652, 61 CrossRef CAS , and references therein.
- M. N. Bochkarev, Coord. Chem. Rev., 2004, 248, 835 CrossRef CAS.
- The solution state magnetic moments (Evans method, C6D6, 298 K) for 5 (3.3 μB), 6 (7.3 μB) and 8 (7.0 μB), all lie in the expected ranges: W. J. Evans and M. A. Hozbor, J. Organomet. Chem., 1987, 326, 299 Search PubMed.
- S. Beaini, G. B. Deacon, E. E. Delbridge, P. C. Junk, B. W. Skelton and A. H. White, Eur. J. Inorg. Chem., 2008, 4586 CrossRef.
- R. D. Shannon, Acta Crystallogr., Sect. A, 1976, 32, 751 CrossRef ; N.B. the value for Sm2+ was obtained by extrapolation.
- B. Cordero, V. Gomez, A. E. Platero-Prats, M. Reves, J. Echeverria, E. Cremades, F. Barragan and S. Alvarez, Dalton Trans., 2008, 2832 RSC.
- A. A. Trifonov, I. A. Borovkov, E. A. Fedorova, G. K. Fukin, J. Larionova, N. O. Druzhkov and V. K. Cherkasov, Chem. Eur. J., 2007, 13, 4981 CrossRef CAS.
- A related complex, [{CeIII[(ArNCH)2](tmeda)(μ-I)}2], was obtained in very low yield from the reaction of [CeI3(THF)4] with [K(tmeda)][:Ga{(ArNCH)2}]. See ESI† for details of its crystal structure.
Footnotes |
| † Electronic supplementary information (ESI) available: Full synthetic and spectroscopic details; ORTEP diagrams for 6 and 7, and full crystallographic information for [{CeIII[(ArNCH)2](tmeda)(μ-I)}2]. CCDC 705275–705279. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/b817933f |
‡ Crystal data for 5: C64H104Ga2N8Sm, M = 1275.34, monoclinic, space groupC2/c, a = 29.344(6), b = 13.855(3), c = 21.633(4) Å, β = 131.48(3)°, V = 6589(2) Å3, Z = 4, Dc = 1.286 g cm−3, F(000) = 2672, μ(Mo-Kα) = 1.734 mm−1, 123(2) K, 9610 unique reflections [R(int) = 0.0226], R (on F) = 0.0312, wR (on F2) = 0.0802 (I > 2σI).6: C64H104EuGa2N8, M = 1276.95, monoclinic, space groupC2/c, a = 29.408(6), b = 14.089(3), c = 21.746(4) Å, β = 131.73(3)°, V = 6724(2) Å3, Z = 4, Dc = 1.261 g cm−3, F(000) = 2676, μ(Mo-Kα) = 1.758 mm−1, 150(2) K, 6552 unique reflections [R(int) = 0.0425], R (on F) = 0.0553, wR (on F2) = 0.1474 (I > 2σI).7: C64H104Ga2N8Yb, M = 1298.03, monoclinic, space groupP21/c, a = 21.866(4), b = 14.060(3), c = 21.602(4) Å, β = 95.47(3)°, V = 6611(2) Å3, Z = 4, Dc = 1.304 g cm−3, F(000) = 2704, μ(Mo-Kα) = 2.254 mm−1, 123(2) K, 13377 unique reflections [R(int) 0.0442], R (on F) = 0.0459, wR (on F2) = 0.01029 (I > 2σI).8·Et2O: C62H98GaN6OTm, M = 1182.11, triclinic, space groupP![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , a = 16.791(3), b = 19.556(4), c = 20.300(4) Å, α = 90.45(3), β = 99.45(3), γ = 90.31°, V = 6575(2) Å3, Z = 4 (two crystallographically independent molecules are present in the asymmetric unit; there are no significant geometric differences between them), Dc = 1.194 g cm−3, F(000) = 2480, μ(Mo-Kα) = 1.790 mm, 123(2) K, 22752 unique reflections [R(int) 0.0715], R (on F) = 0.0853, wR (on F2) = 0.2011 (I > 2σI). , a = 16.791(3), b = 19.556(4), c = 20.300(4) Å, α = 90.45(3), β = 99.45(3), γ = 90.31°, V = 6575(2) Å3, Z = 4 (two crystallographically independent molecules are present in the asymmetric unit; there are no significant geometric differences between them), Dc = 1.194 g cm−3, F(000) = 2480, μ(Mo-Kα) = 1.790 mm, 123(2) K, 22752 unique reflections [R(int) 0.0715], R (on F) = 0.0853, wR (on F2) = 0.2011 (I > 2σI). |
| This journal is © The Royal Society of Chemistry 2009 |

