Metallopeptoids†
Galia
Maayan
,
Michael D.
Ward
* and
Kent
Kirshenbaum
*
Department of Chemistry and the Molecular Design Institute, New York University, 100 Washington Square East, New York, New York 10003-6688, USA. E-mail: mdw3@nyu.edu; kk54@nyu.edu
First published on 17th November 2008
Abstract
N -substituted glycine peptoid oligomers bearing hydroxyquinoline ligands form complexes with Cu(II) and Co(II) in which the chiral helical secondary structure of the foldamers is enhanced upon metal binding and establishes a stereogenic environment for metal coordination.
Peptoids—oligomers of N-substituted glycine—have emerged as intriguing mimics of polypeptides, particularly with respect to their ability to form well-defined folded architectures. Although peptoids are incapable of forming hydrogen-bonded networks along the backbone, many peptoid sequences exhibit a remarkable propensity for folding at small oligomer chain lengths, a property that has been ascribed to the influence of local steric and stereoelectronic interactions.1 Peptoid oligomers can be synthesized efficiently by solid-phase methods, allowing the facile introduction of a variety of side chains within the context of well-defined asymmetric environments. This feature enables the coordinated display of multiple chemical functionalities, potentially emulating binding sites and enzymatic sites in proteins. The conformation and attendant catalytic activity of proteins and polypeptides, however, often depends upon the coordination of metal species.2 Recent studies have investigated the interaction of metal ions with biomimetic oligomers (a.k.a. foldamers)3,4 and include peptoids that bind zinc.5 Further advances in mimicking natural systems require an improved understanding of the reciprocating influences of metal coordination and peptoid secondary structure. We have demonstrated that multidentate metal-binding ligands can be introduced as pendant groups in peptoid sequences.6 Herein we report the formation of metal complexes using these ligands, wherein helical secondary structure is enhanced upon metal binding and enforces a chiral environment about the metal center.
In order to explore the influence of metal binding on the conformation of chiral peptoids, hydroxyquinoline ligands were introduced as N-substituted pendant groups on glycine oligomer scaffolds. Chiral (S)-1-phenylethyl groups (Nspe) were introduced at other N-positions to enforce helicity resembling that of the polypro-type I helix, with a pitch of roughly three residues per turn.1a The hydroxyquinoline ligands were expected to bind divalent metal ions, producing tetra-coordinated metal species.7 Specifically, H115 and H226 (Fig. 1; H = hydroxyquinoline; 5 = pentamer, 6 = hexamer) were synthesized as model systems for comparison of inter- and intramolecular metal complex formation, respectively. These compounds were chosen because each contains four bulky chiral side chains, which are sufficient for achieving helicity in oligomers of this length.1c The pentamer H115, with one hydroxyquinoline site at the N-terminus, was expected to form a peptoid duplex upon metal binding (2:1 peptoid:metal). The hexamer H226 contains two hydroxyquinoline ligands, endowing this oligomer with the capacity to form an intramolecular 1:1 peptoid:metal complex (Fig. 1). The disposition of the ligands at positions i and i + 3 in the sequence matches the pitch of the helix and is designed to orient these groups in proximity on the same face of the scaffold, separated by one helical turn.
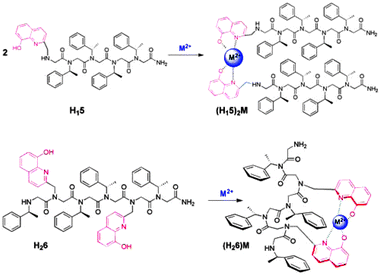 | ||
| Fig. 1 The expected 2:1 and 1:1 peptoid-metal complexes formed from H115 and H226, respectively. | ||
Oligomers H115 and H226 were synthesized using solid phase methods, with 8-hydroxy-2-quinolinemethylamine and (S)-(−)-1-phenylethylamine as synthons, employing “submonomer” protocols.6,8 The peptoids were cleaved from the solid support and purified by HPLC, with no detectable impurities (>98% purity). The molecular weights measured by electrospray mass spectrometry were consistent with the masses expected for H115 and H226.
Metal-free H115 and H226 exhibited absorption bands near λ = 245 nm and 307 nm in 4:1 methanol:water (the solvent of choice for solubility and spectral transparency). Upon addition of cupric acetate, binding of Cu2+ produced new absorption bands at λ = 260, 366 nm and λ = 263, 384 nm for H115 and H226, respectively (Fig. 2). Job plots constructed from UV titration of the peptoids with cupric acetate were consistent with 2:1 (H115)2Cu duplex and a 1:1 (H226)Cu complex that signaled the formation of an intramolecular tetracoordinated complex. Likewise, new absorption bands at λ = 262, 365 nm and λ = 266, 375 nm appeared upon addition of cobalt(II) acetate. The corresponding Job plots from UV titration revealed the formation of 2:1 (H115)2Co and 1:1 (H226)Co. The peptoid-to-metal ratio was corroborated further by electrospray mass spectrometry and MALDI-TOF (Table S1†). No evidence for the formation of higher order complexes with H226 (e.g. 2:2 complexes) was detected. The association constants for the two-step equilibrium responsible for formation of metal complexes with both peptoids were estimated from the UV titration experiments.9 The values for (H115)2Cu, as calculated by nonlinear regression curve fitting2 (see ESI†) are β1 (K1) = 3.75 ± 0.23 × 105 M−1 and β2 (K1*K2) = 5.45 ± 0.36 × 109M−2. The corresponding values for (H115)2Co are β1 = 7.4 ± 1.7 × 105 M−1 and β2 = 9.45 ± 2.2 × 109M−2. The association constants for (H226)Cu and (H226)Co reflected much stronger binding, with an estimated value of K1 > 1014 and K1 = 1.36 ± 0.18 × 1013 M−1, respectively. The larger binding constant for the (H226)M complexes suggests the existence of a preorganized binding environment due to the disposition of the hydroxyquinoline ligands on the helical backbone (see below). Indeed, differences in affinity that arise from a preorganization of the oligomer sequence have been shown to yield higher association constants in Zn2+-binding peptides.10 Moreover, the association constants for an unstructured achiral analog of (H226)Co incorporating N-methoxyethyl monomers (see ESI†), reflected weaker binding in comparison with the chiral H226 complex, with a value of K1 = 6.4 ± 2.2 × 1011 M−1.
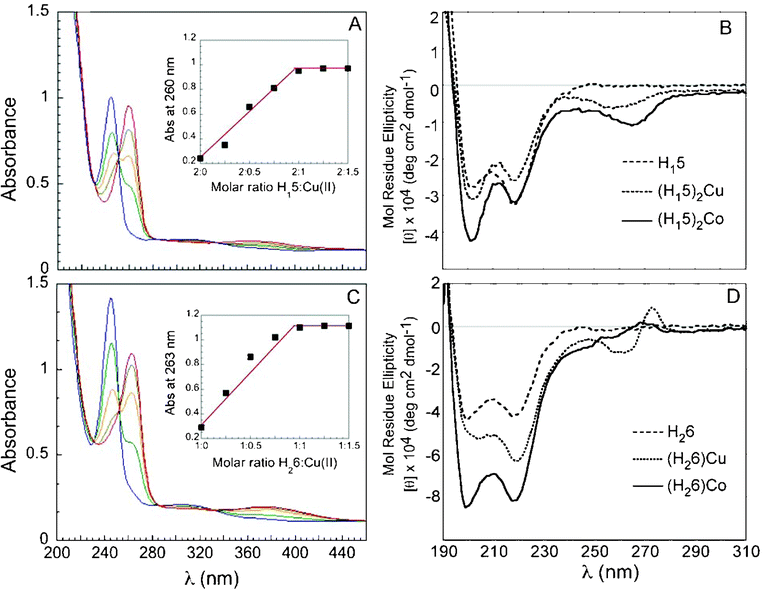 | ||
| Fig. 2 (A) UV-Vis spectra and Job plot for titration of H115 with Cu2+. (B) CD spectra for (H115), (H115)2Cu, and (H115)2Co. (C) UV-Vis spectra and Job plot for titration of H226 with Cu2+. (D) CD spectra for (H226), (H226)Cu, and (H226)Co. UV-Vis spectra: (A) 17 μM peptoid in MeOH:H2O (4:1) solution, (C) 40 μM peptoid in MeOH:H2O (4:1) solution. The peptoid solutions were titrated with 2 μL aliquots of a metal ion (5 mM in H2O), in multiple steps (blue = free ligand, red = metal complex); CD spectra: 100 μM MeOH:H2O (4:1) solutions. | ||
Circular dichroism (CD) measurements revealed substantial changes upon complex formation. Solutions of the metal complexes exhibited increases, relative to the metal-free peptoids, in the magnitude of the CD signals near 200 nm and 220 nm. In the case of (H115)2M this suggests an increase in conformational order, as the magnitude of the signal reflects the degree of helicity. Similar changes in CD spectra have been reported for peptide bundles formed upon metal complexation.2,11 Notably, no changes in CD intensity were observed for metal complexes of peptoid bundles with monodentate ligands.5 The increase in the CD signal was more dramatic for (H226)M, consistent with greater conformational constraint and enhanced secondary structure content due to intramolecular metal complexation.12,13Metal binding to H115 and H226 also produced new CD peaks between 240 and 280 nm, the region corresponding to the 8-hydroxyquinoline π–π* transition, which reflects the transmission of the stereogenic character of the peptoid scaffold to the metal center.2,14,15 These results indicate the reciprocating effects of metal binding—the chirality of the peptoid backbone establishes an asymmetric environment about the metal center while metal complexation enhances the helical character of the backbone.
Notably, Cu(hydroxyquinolate)2 adopts a trans-planar configuration (i.e. square planar with O trans to O and N trans to N).16 The trans-planar configuration is not possible in the intramolecular (H226)M complexes because of the parallel projection of the two hydroxyquinoline ligands from the peptoid scaffold and steric interactions that prohibit a square planar geometry. Consequently, the peptoid scaffold in H226 enforces an unusual coordination environment while transmitting its stereogenic character to the metal site. The metal center most likely adopts local C2 symmetry (i.e. pseudotetrahedral) about the Cu(II) and Co(II) centers. This is supported by molecular modeling, which revealed that formation of a pseudotetrahedral metal complex in (H226)Cu can be achieved without substantial repositioning of the ligands compared with the metal-free structure (Fig. 3). Moreover, the existence of this preorganized binding environment is consistent with the very large binding constants observed for the 1:1 complexes.
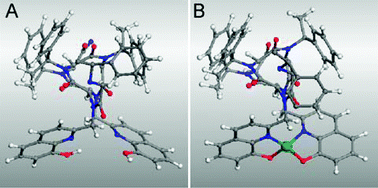 | ||
| Fig. 3 Energy-minimized structures of (A) metal-free H226 and (B) (H226)Cu.20,21Hydrogen atoms within the helix are omitted for clarity. | ||
In contrast, a trans-planar configuration should not be sterically prohibited in the unconstrained intermolecular (H115)2M complexes, which also exhibited CD signals in the π–π* region. The 8-hydroxyquinoline π–π* CD signal for (H226)Cu exhibited an exciton couplet,17–19 with a minimum at 260 nm and a maximum at 273 nm, crossing ε = 0 near 268 nm. In contrast, (H226)Co exhibited a very weak CD signal in this region with a corresponding weak excitonic couplet. The absence of a similar couplet for the intermolecular (H115)2Cu and (H115)2Co complexes and the close agreement between the CD minima and their respective λmax values, however, indicates the absence of exciton coupling. This most likely reflects reduced conformational constraints in the intermolecular complexes, which can diminish the effect of the dipole-dipole interactions responsible for an exciton couplet.
Collectively, these observations reveal that metal binding in designed peptoid sequences can introduce conformational constraints and chiral environments about the metal centers. We anticipate that this strategy can be extended to the synthesis of peptoid podands and other foldamers with unique secondary, tertiary or quaternary structures, promising new architectures with utility for asymmetric catalysis and materials science.
The authors gratefully acknowledge the support of the NSF (CAREER Award CHE-0645361), the NYU Molecular Design Institute, and the NIH (Research Facilities Improvement Grant C060RR-165720).
Notes and references
- (a) K. Kirshenbaum, A. E. Barron, R. A. Goldsmith, P. Armand, E. Bradley, K.-T. V. Truong, K. A. Dill, F. E. Cohen and R. N. Zuckermann, Proc. Natl. Acad. Sci., 1998, 95, 4303–4308 CrossRef CAS; (b) C. W. Wu, T. J. Sanborn, R. N. Zuckermann and A. E. Barron, J. Am. Chem. Soc., 2001, 123, 2958–2963 CrossRef CAS; (c) C. W. Wu, T. J. Sanborn, K. Huang, R. N. Zuckermann and A. E. Barron, J. Am. Chem. Soc., 2001, 123, 6778–6784 CrossRef CAS; (d) S.-B. Y. Shin, B. Yoo, L. Todaro and K. Kirshenbaum, J. Am. Chem. Soc., 2007, 129, 3218–3225 CrossRef CAS; (e) C. W. Wu, K. Kirshenbaum, T. J. Sanborn, J. A. Patch, K. Huang, K. A. Dill, R. N. Zuckermann and A. E. Barron, J. Am. Chem. Soc., 2003, 125, 13525–13530 CrossRef CAS; (f) B. C. Gorske, B. L. Bastian, G. D. Geske and H. E. Blackwell, J. Am. Chem. Soc., 2007, 129, 8928–8929 CrossRef CAS.
- M. Gochin, V. Khorosheva and M. A. Case, J. Am. Chem. Soc., 2002, 124, 11018–11028 CrossRef CAS.
- (a) Z. Dong, R. J. Karpowicz, S. Bai, G. P. A. Yap and J. M. Fox, J. Am. Chem. Soc., 2006, 128, 14242–14243 CrossRef CAS; (b) D. J. Hill, M. J. Mio, R. B. Prince, T. S. Hunges and J. S. Moore, Chem. Rev., 2001, 101, 3893–4011 CrossRef CAS.
- (a) B. P. Gilmartin, K. Ohr, R. K. McLaughlin, R. Koerner and M. E. Williams, J. Am. Chem. Soc., 2005, 127, 9546–9555 CrossRef CAS; (b) D.-L. Popescu, T. J. Parolin and C. Achim, J. Am. Chem. Soc., 2003, 125, 6354–6355 CrossRef CAS.
- B.-C. Lee, T. K. Chu, K. A. Dill and R. N. Zuckermann, J. Am. Chem. Soc., 2008 Search PubMed ASAP; DOI: 10.1021/ja802125x.
- G. Maayan, B. Yoo and K. Kirshenbaum, Tetrahedron Lett., 2008, 49, 335–338 CrossRef CAS . The acylation steps performed after the incorporation of the hydroxyquinoline were slightly modified in order to make the reaction more efficient (see ESI†).
- D. V. Nicolau and S. J. Yoshikawa, Mol. Graphics Mod., 1998, 16, 83–96 Search PubMed.
- T. S. Burkoth, A. T. Fafarman, D. H. Charych, M. D. Connolly and R. N. Zuckermann, J. Am. Chem. Soc., 2003, 125, 8841–8845 CrossRef CAS.
- R. B. Prince, T. O. and J. S. Moore, Angew. Chem., Int. Ed., 1999, 38, 233–236 CrossRef CAS.
- R. P. Cheng, S. L. Fisher and B. Imperiali, J. Am. Chem. Soc., 1996, 118, 11349–11356 CrossRef CAS.
- M. A. Case and G. L. McLendon, Chirality, 1998, 10, 35–40 CAS.
- J. M. Holub, H. Jang and K. Kirshenbaum, Org. Lett., 2007, 9, 3275–3278 CrossRef CAS.
- M. R. Ghadiri, C. Soares and C. Choi, J. Am. Chem. Soc., 1992, 114, 825–831 CrossRef.
- M. Lieberman, M. Tabet and T. Sasaki, J. Am. Chem. Soc., 1994, 116, 5035–5044 CrossRef CAS.
- In contrast, a CD signal was not observed for the 1:1 Cu complex of an achiral analog of H226 prepared with N-methoxyethyl substituents.
- (a) G. J. Palenik, Acta Cryst., 1964, 17, 687 CrossRef CAS; (b) R. C. Hoy and R. H. Morriss, Acta Cryst., 1967, 22, 476 CrossRef CAS.
- C. R. Cantor and P. R. Schimmel, Biophysical Chemistry II: Techniques for the Study of Biological Structure and Function, W. R. Freeman and Company, New York, 1980 Search PubMed.
- X. Huang, N. Fujioka, G. Pescitelli, F. E. Koehn, R. T. Williamson, K. Nakanishi and N. Berova, J. Am. Chem. Soc., 2002, 124, 10320–10335 CrossRef CAS.
- Based on the relative intensities of the two branches of the exciton couplet, the angle between the transition dipole moments of the hydroxyquinoline chromophores is approximately 72°.
- Geometry optimization was performed with the Universal Force Field in the Accelrys VS software modeling suite.
- P. Armand, K. Kirshenbaum, A. Falicov, R. L. Dunbrack, Jr, K. A. Dill, R. N. Zuckermann and F. E. Cohen, Folding and Design, 1997, 2, 369–375 Search PubMed.
Footnote |
| † ]Electronic supplementary information (ESI) available: Synthesis and characterization details, additional CD and UV spectra. See DOI: 10.1039/b810875g |
| This journal is © The Royal Society of Chemistry 2009 |
