Nitroxyl radicals as low toxic spin-labels for non-invasive magnetic resonance imaging of blood–brain barrier permeability for conventional therapeutics†
Zhivko
Zhelev
a,
Rumiana
Bakalova
*a,
Ichio
Aoki
a,
Ken-ichiro
Matsumoto
b,
Veselina
Gadjeva
c,
Kazunori
Anzai
b and
Iwao
Kanno
a
aDepartment of Biophysics, Molecular Imaging Center, National Institute of Radiological Sciences (NIRS), 4-9-1 Anagawa, Inage-ku, Chiba 263-8555, Japan. E-mail: bakalova@nirs.go.jp; ra_bakalova@yahoo.com; Fax: +81-43-206-3276; Tel: +81-43-206-4067
bCenter for Heavy-ion Particle Therapy, National Institute of Radiological Sciences (NIRS), 4-9-1 Anagawa, Inage-ku, Chiba 263-8555, Japan
cDepartment of Chemistry and Biochemistry, Trakia University, Stara Zagora, Bulgaria
First published on 13th November 2008
Abstract
The present study describes a novel non-radioactive methodology for in vivo non-invasive, real-time imaging of blood–brain barrier (BBB) permeability for conventional drugs, using nitroxyl radicals as spin-labels and magnetic resonance imaging (MRI).
The non-invasive real-time imaging of blood–brain barrier (BBB) permeability for drugs is an indispensible step in the preclinical and clinical testing of new pharmaceuticals for brain diseases. The precise mapping of a drug in the brain has a significant impact on its dosing and prognostication of its target-specific effect.
The conventional methods for investigation of BBB permeability are usually invasive, time- and cost-consuming, often suffer from artefacts, and require a large number of experimental animals.1–6In vitro models of BBB (e.g., cell and tissue cultures, immobilized artificial membranes, etc.) often serve as a major approach for indirect evaluation of drug permeability for brain tissue. The development of new methodologies for in vivo imaging of BBB permeability which are non-invasive, environmentally friendly, with minimal animal loss and minimal risk for volunteers, is a major goal of the modern pharmaceutical industry.
Currently, radiopharmaceuticals combined with autoradiography or positron-emission tomography (PET) are the only option for non-invasive real-time imaging of BBB permeability.7–9 Despite this approach being highly sensitive and valuable, it suffers from several restrictions which prevent it from being widely applicable in preclinical and clinical trials of new pharmaceuticals. Radio-labelling carries a risk for human safety and requires special experimental equipment and facilities, which increases markedly the cost of this analysis. Radiotracers are usually used for labelling of diagnostic markers, but not for labelling of therapeutics and real-time imaging of their BBB permeability and distribution in the organism.
In the present study, we would like to introduce a novel non-radioactive and environmentally friendly alternative for non-invasive real-time imaging of BBB permeability for conventional drugs, using stable nitroxyl radicals as spin-labels and magnetic resonance imaging (MRI).
Nitroxyl radicals are well known from electron paramagnetic resonance (EPR ) studies.10–13 In 1984, it was reported that they have T1 contrast properties and could be applied in MRI.14 The nitroxyls are small molecules, sensitive to the redox status of biological samples and their use in life science research is limited predominantly to tissue oxygen and redox mapping in vitro and in vivo. 10–13,15–17 The paramagnetic nitroxyl radical could be reduced to diamagnetic hydroxylamine with the loss of the EPR signal or 1H-MRI relaxation time and thus could serve as a reduction sensor. However, the diamagnetic hydroxylamine could be re-converted via oxygenation to paramagnetic nitroxyl radical with the appearance of the EPR or MRI signal and thus could serve as an oxidation sensor. The rate constants of both processes could be used for evaluation of reduction/oxidation balance in cells and tissues.
Nitroxyl radicals are characterized by comparatively high T1 contrast properties, lower toxicity in comparison with the conventional MRI contrast agents (e.g., gadolinium derivatives), and some of them possess an excellent cell permeability. All these characteristics make them attractive for MRI diagnostics and their application in life science research could be extended beyond the limits mentioned above. In this context, we supposed that nitroxyl radicals could be appropriate spin-labels for non-invasive real-time MRI of BBB permeability for conventional therapeutics, which is an important step in their preclinical testing. Below, we would like to give a proof of the reality of this concept. In addition, MRI is characterized by much higher spatial resolution than EPR imaging (and even PET) and gives an excellent anatomical reference, which facilitates the exact localization of a nitroxyl probe in the organism.
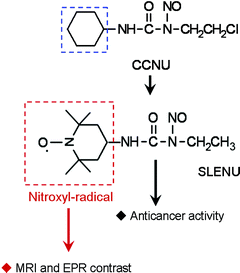
We synthesized a TEMPO-labelled analogue (SLENU) of the conventional anticancer drugLomustine [1-(2-chloroethyl)-3-cyclohexyl-1-nitrosourea, CCNU] (Fig. 1). Lomustine significantly improves the health-related quality of life of patients treated for brain and other tumours.18 Both TEMPO radical and Lomustine are permeable for BBB and cell membranes.15,19
Formally, the Lumustine molecule can be separated into two parts (Fig. 1). The nitrosourea ensures the anticancer effect and could not be modified. We exchanged the cyclohexyl part of the molecule with TEMPO radical. This substitution did not influence the anticancer effect of the drug or its toxicological characteristics (e.g., LD50).21,22SLENU was synthesized, purified and characterized as described previously.20 The present study was designed to clarify: (i) whether the TEMPO-labelling of nitrosourea will affect its BBB permeability and localization in the brain tissue; and (ii) whether nitroxyl radicals are appropriate spin-labels of conventional drugs for in vivo dynamic MR imaging of their BBB permeability.
The first step was to ensure that the TEMPO–nitrosourea bond was stable and that there was no dissociation between the two compounds in the blood. Thus, the detection of MRI signal enhancement of the nitroxyl radical could indicate the localization of nitrosourea in the brain tissue in vivo. In buffer, the EPR spectra of TEMPO and SLENU were distinguished by the third line in the triplet, which was shorter for SLENU (Fig. 2A). In blood, the difference between the amplitudes of line 3 became larger, however, both spectra (of TEMPO and SLENU) had the same profiles. The lower amplitude of line 3 in the EPR spectrum of SLENU could be explained by the limited motion of the conjugated nitroxyl radical (in SLENU), in comparison with free TEMPO radical. No changes in the shape of the EPR spectrum of SLENU were detected during long-term incubation in blood (Fig. 2B). This indicated that there was no dissociation of the TEMPO–nitrosourea covalent bond.
 | ||
| Fig. 2 (A) Normalized EPR spectra of SLENU and TEMPOL in PBS. In blood, the EPR spectra of both substances have the same profile. (B) Amplitudes of Line 3 during long-term incubation of TEMPOL or SLENU in freshly isolated blood. For comparison, the amplitude of Line 3 in PBS is shown. | ||
SLENU was injected intravenously in healthy mice via the tail vein and 1H-MR imaging of the brain was performed on a 7.0 Tesla horizontal MRI instrument (Fig. 3). The injected dose was below the toxic limit. The MRI-signal dynamic of SLENU (Fig. 3) in the brain and surrounding tissues followed almost the same kinetics and distribution as non-modified TEMPO radical (Fig. 1S, Supplementary Information† ). SLENU was rapidly transported and randomly distributed into the brain tissue. Obviously, the exchange of the cyclohexyl part of Lomustine with TEMPO radical did not suppress the BBB permeability of the drug. Both drugs (SLENU and TEMPOL) manifested a similar permeability. The data from diffusion-weighted MRI confirmed this assumption. SLENU was detected in the brain tissue (cortex, thalamus, hypothalamus), as well as in the brain ventricles. After free-water signal suppression by a diffusion-weighted MRI technique with motion probing gradients, the MRI signal remained in the tissue (data are not shown).
 | ||
| Fig. 3 MRI signal dynamic of SLENU in the brain after intravenous injection in mice. Each image was obtained within a 20-s interval, using Gradient-echo T1-weighted MRI. In the image, the red colour represents an extraction of the signal between every single slide and the averaged baseline signal (first five slides – before injection). In the chart, the red and black colours represent an MRI signal dynamic in the brain or entire area, respectively. | ||
The MRI signal enhancement of nitroxyl radical disappeared quickly (within 2–3 min) after passing of SLENU from the blood vessels in the brain tissue. Presumably, this is due to the high permeability of SLENU for cell membranes, which is accompanied by rapid reduction of nitroxyl radical to the respective hydroxylamine in the brain cells and loss of MRI signal enhancement. In the surrounding tissues, the MRI signal had a better stability and a slightly longer half-life.
The fast detection of MRI signal enhancement could be considered as an advantage, because of the shortening of the time of the analytical and diagnostic process.
The present study is just a first trial for using nitroxyl radicals for spin-labelling of conventional drugs and non-invasive, dynamic MR imaging of their BBB permeability. We have tried to show the advisability of this concept. Novel synthetic strategies are necessary to improve the contrast and stability of nitroxyl label in the brain tissue without affecting its BBB permeability, which will allow higher spatial resolution of signal-to-noise ratio and will facilitate real-time MRI data reconstruction and quantitative analysis . The exchange of TEMPO radical with 2,2,5,5-tetramethyl-pyrrolidinyl-1-oxyl (PROXYL radical) is a promising strategy for increasing the contrast and stability of an MRI signal in the brain17 and other tissues (Fig. 2S, Supplementary Information† ).
It is necessary to note that nitroxyl radicals have low toxicity and are comparatively harmless to living organisms. TEMPOL—one of the most famous commercially available nitroxyls, is in phase I of clinical trials, as a preventer of alopecia in radiation-treated cancer patients.23 The combined application of nitroxyls and conventional chemotherapeutics increases the anticancer effect and suppresses the multidrug resistance.24 Therefore, the nitroxyl-labelling could be considered as environmentally friendly and with minimal risk for humans. There is one more advantage in nitroxyl-labelling and imaging. The dynamic of MRI signal enhancement gives additional information concerning the oxi-redox status of the brain tissue. This information could be useful for planning of conventional chemo- and radiotherapy of cancer and other diseases. The described approach is applicable not only for non-invasive imaging of BBB permeability for conventional drugs, but also for imaging of their localization in other organs and tissues, using MRI.
The technical support of Dr Antoaneta Zheleva (Trakia University, Bulgaria) and Ms Sayaka Shibata (NIRS-Chiba, Japan) is gratefully acknowledged.
Notes and references
- L. Zhang, H. Zhu, T. I. Oprea, A. Golbraikh and A. Tropsha, Pharm. Res., 2008, 25, 1902 CrossRef CAS.
- D. D. Shen, A. A. Artru and K. K. Adkison, Adv. Drug Delivery Rev., 2004, 56, 1825 CrossRef CAS.
- D. Pan, M. Iyer, J. Liu, Y. Li and A. J. Hopfinger, J. Chem. Inf. Comput. Sci., 2004, 44, 2083 CrossRef CAS.
- A. K. Dash and W. F. Elmquist, J. Chromatogr., B: Anal. Technol. Biomed. Life Sci., 2003, 797, 241 CrossRef CAS.
- M. Gumbleton and K. L. Audus, J. Pharm. Sci., 2001, 90, 1681 CrossRef CAS.
- D. M. Killian, L. Gharat and P. J. Chikhale, Drug Delivery, 2000, 7, 21 CrossRef CAS.
- V. Josserand, H. Pélerin, B. de Bruin, B. Jego, B. Kuhnast, F. Hinnen, F. Ducongé, R. Boisgard, F. Beuvon, F. Chassoux, C. Daumas-Duport, E. Ezan, F. Dollé, A. Mabondzo and B. Tavitian, J. Pharmacol. Exp. Ther., 2006, 316, 79 CAS.
- N. J. Abbott, D. C. Chugani, G. Zaharchuk, B. R. Rosen and E. H. Lo, Adv. Drug Delivery Rev., 1999, 37, 253 CrossRef CAS.
- R. Weissleder and U. Mahmood, Radiology, 2001, 219, 316 Search PubMed.
- K. Takeshita and T. Ozawa, J. Radiat. Res. (Tokyo), 2004, 45, 373 Search PubMed.
- B. P. Soule, F. Hyodo, K. Matsumoto, N. L. Simone, J. A. Cook, M. C. Krishna and J. B. Mitchell, Antioxid. Redox Signaling, 2007, 9, 1731 Search PubMed.
- L. Valgimigli, G. F. Pedulli and M. Paolini, Free Radical Biol. Med., 2001, 31, 708 CrossRef CAS.
- H. Utsumi and K. Yamada, Arch. Biochem. Biophys., 2003, 416, 1 CrossRef CAS.
- V. Afzal, R. C. Brasch, D. E. Nitecki and S. Wolff, Invest. Radiol., 1984, 19, 549 CrossRef CAS.
- K. Matsumoto, F. Hyodo, A. Matsumoto, A. P. Koretsky, A. L. Sowers, J. B. Mitchell and M. C. Krishna, Clin. Cancer Res., 2006, 12, 2455 CrossRef CAS.
- F. Hyodo, K. Matsumoto, A. Matsumoto, J. B. Mitchel and M. C. Krishna, Cancer Res., 2006, 66, 9921 CrossRef CAS.
- F. Hyodo, K.-H. Chuang, A. G. Goloshevsky, A. Sulima, G. L. Griffiths, J. B. Mitchell, A. P. Koretsky and M. C. Krishna, J. Cereb. Blood Flow Metab., 2008, 1 Search PubMed.
- M. J. B. Taphoorn, M. J. Van den Bent, M. E. L. Mauer, C. Coens, J-Y. Delattre, A. A. Brandes, P. A. E. Smitt, H. J. J. A. Besrnsen, M. Frenay, C. C. Tijssen, D. Lacombe, A. Allgeier and A. Bottomley, J. Clin. Oncol., 2007, 25, 5723 CrossRef.
- N. Bodor and P. Buchwald, Adv. Drug Delivery Rev., 1999, 36, 229 CrossRef CAS.
- V. Gadjeva and R. Koldamova, Anti-Cancer Drug Des., 2001, 16, 247 CAS.
- A. M. Zheleva and V. G. Gadjeva, Int. J. Pharm., 2001, 212, 257 CrossRef CAS.
- V. Gadjeva, Eur. J. Med. Chem., 2002, 37, 295 CrossRef CAS.
- J. M. Metz, D. Smith, R. Mick, R. Lustig, J. Mitchell, M. Cherakuri, E. Glatstein and M. Hahn, Clin. Cancer Res., 2004, 10, 6411 CrossRef CAS.
- R. Ravizza, E. Cereda, E. Monti and M. B. Gariboldi, Int. J. Oncol., 2004, 25, 1817 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details. See DOI: 10.1039/b816878d |
| This journal is © The Royal Society of Chemistry 2009 |