Rapid determination of ginsenoside Rg1, Re and Rb1 in ginseng samples by capillary electrophoresis
Yulu
Tian
,
Yanyan
Lu
,
Jing
Xie
,
Yuan
Cheng
,
Ruobing
Qi
,
Yangjie
Wu
and
Shusheng
Zhang
*
Chemistry Department, Key Laboratory of Chemical Biology and Organic Chemistry of Henan, Zhengzhou University, Daxue Road 75,, Zhengzhou, 450052, P. R. China. E-mail: zsszz@126.com; Tel: 86 371 67763224
First published on 29th October 2009
Abstract
A rapid and practical capillary electrophoresis (CE) method was developed for the determination of ginsenoside Rg1, Re and Rb1 in ginseng samples. Rg1, Re and Rb1 were extracted by ultrasonication with water-saturated n-butanol. The CE method was optimized with a running buffer of 20 mM borate in 30% MeOH (pH 9.67), and an applied separation voltage of +18 kV over a capillary of 50 μm i.d. × 50 cm (41.5 cm to the detector window), which gave a baseline separation of Rg1, Re and Rb1 within ca. 5 min. Under the detection at 203 nm, the method gave limits of quantification (S/N = 10) at about 5.2–7.3 μg ml−1 for Rg1, Re and Rb1, whereas the overall recoveries were larger than 83.0%. The proposed method has been successfully applied to measure three different kinds of ginseng samples (10 real samples) and the contents of Rg1, Re and Rb1 in actual samples were obtained and evaluated.
1. Introduction
Ginseng, King of Herbs, has been highly valued for its mystical properties and has a 5000-year long history as a traditional herbal medicine originating from ancient China.1 Given a scientific definition and classification, ginseng plant belongs to the Araliaceae family. Most of the studies of ginseng samples, including commercial pharmaceutical preparations, are mainly from five common species: Panax ginsengC.A. Meyer (Asian or Korea ginseng), Panax quinquefolius L. (American ginseng), Panax notoginseng (Burkill) F.H. Chen (Tianqi or Sanqi, Yunnan Province, China) and Panax japonicus C.A. Meyer (Chikusetsu, Japanese ginseng).2,3Ginseng extracts have wide pharmacological properties, such as antifatigue, antidiabetic, vasodilating and antidepressant, and are effective in stimulating the memory as well as in the prevention of cancer and the ageing process. Pharmacological effects of ginseng are attributed to ginsenosides which are widely regarded as the active components and are found in most ginseng products.4
The basic structures of ginsenosides contain triterpene aglycones and various sugar moieties.5 To date, at least 30 ginsenosides have been identified, but ginsenosides Rf, Rg1, Rd, Re, Rc, Rb2, and Rb1 are the most abundant.6 In the Chinese Pharmacopoeia, the content of Rg1, Re and Rb1 are compulsory to provide. Therefore, in this work, we mainly investigated these three ginsenosides (Rg1, Re and Rb1, Fig. 1).
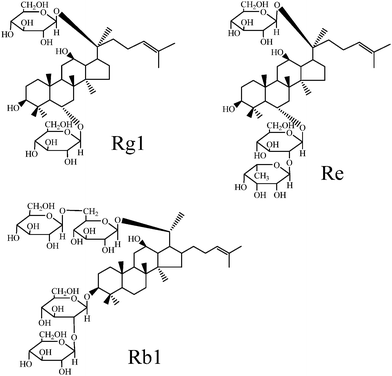 | ||
| Fig. 1 Chemical structures of ginsenoside Rg1, Re and Rb1. | ||
Because ginsenosides are valuable components with pharmacological effects, the method development for their determinations is very necessary. To date, several analytical methods have been reported and reviewed.5 These methods were accomplished by means of thin layer chromatography (TLC),7,8gas chromatography (GC),9–11high performance liquid chromatography (HPLC) with either ultraviolet (UV),12–18 fluorescence,19mass spectrometry (MS),20,21 or MS-MS detection.22–25 Among these methods, HPLC is the most useful tool for the separation and analysis of ginsenosides.5 This is due to the several advantageous features of HPLC: sensitivity, rapidity, consistent response and broad applicability to non-volatile polar compounds, and the compatibility to a variety of detection techniques. However, the HPLC method requires expensive columns and equipment, and consumes large amounts of toxic organic solvents, such as methanol and acetonitrile. Capillary electrophoresis (CE) is a very active research area in separation science since this technique often provides higher resolution power, shorter analysis time and lower operating cost than HPLC. It has been frequently reported in the analysis of traditional Chinese medicines.26,27 It is fair to say that the analysis of ginseng extracts and ginsenosides by CE remains at a moderately low level.28,29
This study developed a rapid, practical and reliable CE method to analyze ginsenoside Rg1, Re and Rb1 in ginseng roots and preparations. The main works includes: (i) to develop a practical extraction prior to the analysis of Rg1, Re and Rb1; (ii) to develop a practical CE method for the measurement of Rg1, Re and Rb1, and (iii) to determine Rg1, Re and Rb1 in actual ginseng samples using the developed method.
2. Experimental
2.1 Chemicals and solutions
Unless specified otherwise, all chemicals and solvents were of analytical reagent grade and purchased from Beijing Chemical Plant (Beijing, China). Acetonitrile (MeCN) and methanol (MeOH) were of HPLC grade and purchased from Shanghai Luzhong Reagent Plant (Shanghai, China). Ginsenoside Rg1, Rb1 and Re (99.0%) were purchased from China Pharmaceutical & Biological Products Control Institute (Beijing, China). Tris-(hydroxymethyl)aminomethane (Tris) was of biological reagent grade and purchased from China Medication Reagent Plant of Shanghai (Shanghai, China). Water was purified using a Milli-Q system (Millipore, Bedford, MA, USA).Stock standard solution of Rg1, Rb1 and Re was prepared by dissolving each standard in MeOH–water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) in order to give a concentration of 500 μg mL−1. Quantification of samples was made using calibration curves of Rg1, Rb1 and Re at a final concentration of 5, 10, 25, 50, 100 and 150 μg mL−1 in MeOH–water (1
1, v/v) in order to give a concentration of 500 μg mL−1. Quantification of samples was made using calibration curves of Rg1, Rb1 and Re at a final concentration of 5, 10, 25, 50, 100 and 150 μg mL−1 in MeOH–water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Each determination was performed in triplicate.
1). Each determination was performed in triplicate.
2.2 CE procedures
CE experiments were performed using the HP3DCE system (Agilent, Germany) with an on-column diode-array detector, an automatic sampler and a power supply ranging from −30 kV to +30 kV. The pH value was measured using a pH meter (Shanghai Weiye Factory, Shanghai, China). The separations were carried out with a bare fused-silica capillary (Yongnian Photo Fiber Factory, Hebei Province, China), with an inner diameter of 50 μm and a length of 50 cm (41.5 cm to the detector window). The initial new capillary was rinsed with 1.0 M NaOH for 20 min, water for 5 min and the running buffer solution for 20 min. Prior to each injection, the capillary was rinsed with the running buffer solution for 2 min. The capillary was thermostated at 25 °C, unless stated otherwise. A constant voltage of +18 kV was applied during electrophoresis. The sample injection was achieved using a dynamic pressure of 50 mbar for 5 s. The detection wavelength was set at 203 nm.2.3 Sample preparation
The procedure of sample preparation was carried out according to the literature.4 For the ginseng roots, capsules and tablets, twenty grams of the samples were chopped and crushed to produce a powder at 40 mesh. A portion of the sample (about 10.0 g) was accurately weighed and dissolved in 50 mL chloroform. After refluxing extraction in a Soxhlet Extractor for 3 h, the chloroform extract was discarded, and the residue together with the filter paper was returned to a 100-mL conical flask, and dissolved in 50 mL water-saturated n-butanol. It was then ultrasonicated for 30 min. The 25-mL filtrate was accurately collected and transferred to a 100-mL flask with 5-mL graduated bottom. It was evaporated to near dryness under a vacuum. The residue was accurately dissolved with 5 mL methanol under ultrasonication. The 1-mL solution was filtered with a 0.22 μm membrane for CE analysis. It is noteworthy to mention here that the prepared sample solutions should be diluted if required.2.4 Recovery
The efficiency for sample pretreatment was validated by a recovery investigation. In the initial step of the sample preparation, each standard of Rg1, Rb1 and Re (5.0 and 20 mg) was added to 10 g of the powdered ginseng samples (40 mesh). They were then treated as described in section 2.3 (sample preparation). Finally, the prepared sample solution (1 mL) was analyzed by the developed CE method, and the recovery was determined.3. Results and discussion
3.1 Choice of sample treatment methods
Ultrasonication extraction (UE) and microwave extraction are often used to extract Rg1, Rb1 and Re.30,31 Both extractions provide high extraction efficiency. In this work, UE was selected to extract Rg1, Rb1 and Re. At the same time, different extraction solvents (water, methanol–water, acetonitrile–water, ethanol–water and n-butanol–water) were investigated. The results showed that recoveries for Rg1, Rb1 and Re were about 80–85% using water, methanol–water (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5), acetonitrile–water (5
5), acetonitrile–water (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) and ethanol–water (5
5) and ethanol–water (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) as the extraction solvent, and over 95% using water-saturated n-butanol. Finally water-saturated n-butanol was selected to extract Rg1, Rb1 and Re from the ginseng samples. However, the matrices of the ginseng samples were complicated for direct analysis of Rg1, Rb1 and Re by the CE method. Therefore, before UE extraction, chloroform extraction by Soxhlet was performed to remove the lipids and pigments in the samples, which was very necessary to clean up the samples.
5) as the extraction solvent, and over 95% using water-saturated n-butanol. Finally water-saturated n-butanol was selected to extract Rg1, Rb1 and Re from the ginseng samples. However, the matrices of the ginseng samples were complicated for direct analysis of Rg1, Rb1 and Re by the CE method. Therefore, before UE extraction, chloroform extraction by Soxhlet was performed to remove the lipids and pigments in the samples, which was very necessary to clean up the samples.
3.2 Optimized CE separation
In the CZE method, the solvent (such as organic solvent) and the electrolyte are two important parameters for modulating the mobility of electroosmotic flow (μeof) and the effective mobility (μep), as well as selectivity and resolution.32–34MeOH, EtOH and MeCN are widely used as the organic media in CE, and borate and organic amines are commonly used as special electrolytes and buffering groups in CE. Thus, in this section the CE separation of ginsenosides was investigated using borate as the buffer system and MeOH as the organic solvent.In fact, both Tris and NH4Ac electrolytes are also well evaluated in the separation of ginsenosides. The results display that borate electrolyte can provide a more preferable separation than both Tris and NH4Ac. This is attributed to the complexing effect of borate with ginsenosides.
The solvent effect of the MeOH–H2O ratios on migration behaviors of ginsenosides is evaluated. The results display that with the increase of MeOH–H2O ratio the apparent mobilities (Fig. 2) decreased, and resolutions were not obviously improved. For rapid separation, the ratio of MeOH–H2O was selected at 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70, while the appropriate migration times for ginsenosides were achieved with near baseline separation.
70, while the appropriate migration times for ginsenosides were achieved with near baseline separation.
 | ||
| Fig. 2 Effect of methanol percentage of the buffer solvent on apparent mobility of the ginsenosides. Conditions: electrophoretic solution, 20 mM borate in MeOH–H2O (pH 9.67); applied voltage, +18 kV; capillary temperature, 25 °C; sample injection, 50 mbar × 5 s; detection wavelength, 203 nm. | ||
The pH value of the buffer (20 mM borate/NaOH) was altered by changing the amount of NaOH. The influence of pH value on the migration time was also significant. At the pH window of 8.5–11.0, the migration times of Rg1, Rb1 and Re could be obtained between 3.5 and 6.5 min. At pH 9.67, Rg1, Rb1 and Re can be separated with a resolution larger than 1.4 within 5 min. It is noteworthy that this CE method is much more rapid than the HPLC method (retention time >50 min).15
With the increase of the applied voltage, the migration time decreased, the joule heating increased, and the resolution possibly became poor. When the applied separation voltage was higher than +21 kV, the electrophoretic current was larger than 50 μA, the migration times of three ginsenosides were smaller than 4.0 min, and the resolution between Rg1 and Rb1 was less than 1.05. When the applied separation voltage was less than +15 kV, the migration times of three ginsenosides were longer than 8 min. Thus, taking the resolution, migration time and the peak shape into consideration, the optimal CE conditions were determined with MeOH–H2O (30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70, v/v) as solvent medium, 20 mM borate (pH 9.67) as the buffer system and +18 kV (with electrophoretic current of 35 μA) as the separation voltage. A typical electropherogram (Fig. 3A) of standard ginsenosides was obtained under the optimal conditions. It is noteworthy that three ginsenosides can be completely separated from the matrices of the roots, tablets and capsules after pretreatment.
70, v/v) as solvent medium, 20 mM borate (pH 9.67) as the buffer system and +18 kV (with electrophoretic current of 35 μA) as the separation voltage. A typical electropherogram (Fig. 3A) of standard ginsenosides was obtained under the optimal conditions. It is noteworthy that three ginsenosides can be completely separated from the matrices of the roots, tablets and capsules after pretreatment.
 | ||
Fig. 3 Typical electropherograms of (A) standard ginsenosides, (B) ginseng root No.1, (C) capsule No.3 and (D) tablet No.1. Conditions: electrophoretic solution, 20 mM borate in MeOH–H2O (30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70, v/v) (pH 9.67); others as Fig. 2. Concentration of standard ginsenosides in A: Rg1, 50 μg mL−1; Rb1, 50 μg mL−1; Re, 50 μg mL−1. Peaks: 1, Ginsenoside Rg1; 2, Ginsenoside Rb1; 3, Ginsenoside Re. 70, v/v) (pH 9.67); others as Fig. 2. Concentration of standard ginsenosides in A: Rg1, 50 μg mL−1; Rb1, 50 μg mL−1; Re, 50 μg mL−1. Peaks: 1, Ginsenoside Rg1; 2, Ginsenoside Rb1; 3, Ginsenoside Re. | ||
3.3 Methodology evaluation
Under the optimized CE conditions as used and described in Fig. 3, the linear ranges of the UV response at 203 nm were observed over the concentration range from 5.0 to 150 μg mL−1 for Rg1, Rb1 and Re. The regressions between peak area (Y) and concentration (X, μg mL−1) yielded the following equations: for Rg1, Y = 0.2389X + 0.4727 (n = 6, R2 = 0.9892); for Rb1, Y = 0.1608X + 0.4344 (n = 6, R2 = 0.9967); and for Re, Y = 0.2307X − 0.0837 (n = 6, R2 = 0.9895). The limits of quantification (LOQs, S/N = 10, a signal to noise ratio) were 5.20 μg mL−1 for Rg1, 7.30 μg mL−1 for Rb1 and 5.80 μg mL−1 for Re by calculating at a signal to noise ratio of 10 (S/N = 10). These LOQs allow Rg1, Rb1 and Re to be accurately detected in the ginseng samples at a lower content than 7.30 μg g−1 in the ginseng roots, capsules and tablets, respectively.The repeatability and recovery of the CE method were determined. At 20 μg ml−1, the RSDs of the peak area and migration time were 2.5% (n = 3) and 1.8% (n = 3) for Rg1, 3.2% (n = 3) and 2.6% (n = 3) for Rb1, and 2.2% (n = 3) and 1.9% (n = 3) for Re, respectively. The recovery of the method was examined by determining the fortified ginseng samples. The overall recoveries (as described in section 2.4) were obtained and are listed in Table 1. These data demonstrated the reliability of the CZE method.
| Known conc.a (mg/10 g) | Added conc. (mg/10 g) | Detected conc. (mg/10 g) | Av. Recovery (%) | RSD (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rg1 | Rb1 | Re | Rg1 | Rb1 | Re | Rg1 | Rb1 | Re | Rg1 | Rb1 | Re | Rg1 | Rb1 | Re |
| a Determined by CE method. | ||||||||||||||
| Root 1: | ||||||||||||||
| 27.5 | 14.7 | 5.30 | 5 | 5 | 5 | 31.9 | 19.3 | 9.7 | 84.0 | 91.3 | 88.0 | 4.7 | 3.3 | 4.9 |
| 31.5 | 19.4 | 9.5 | ||||||||||||
| 31.7 | 19.1 | 9.6 | ||||||||||||
| 20 | 20 | 20 | 45.2 | 32.2 | 24.2 | 86.0 | 87.6 | 91.3 | 3.2 | 2.0 | 3.1 | |||
| 44.1 | 32.6 | 23.1 | ||||||||||||
| 44.8 | 31.9 | 23.4 | ||||||||||||
| Capsule 3: | ||||||||||||||
| 30.0 | 52.0 | 21.0 | 20 | 20 | 20 | 46.3 | 68.2 | 38.5 | 83.1 | 84.8 | 83.0 | 4.5 | 4.1 | 4.7 |
| 47.5 | 69.6 | 37.2 | ||||||||||||
| 46.1 | 69.1 | 37.1 | ||||||||||||
| Tablet 3: | ||||||||||||||
| 15.0 | 33.0 | 13.0 | 20 | 20 | 20 | 32.6 | 51.0 | 29.5 | 86.3 | 85.5 | 85.5 | 2.0 | 5.2 | 3.1 |
| 31.9 | 49.2 | 30.5 | ||||||||||||
| 32.3 | 50.1 | 30.3 | ||||||||||||
3.4 Application
The contents of Rg1, Rb1 and Re in ginseng samples were determined by using the developed CE method. The electropherograms for three kinds of samples were shown in Fig. 3B, 3C and 3D. The content results are given in Table 2. Peaks were identified by three means: (i) by comparing the migration times of the unknown peaks with those of the standards eluted with the same conditions; (ii) by comparing their UV spectra with standard ginsenosides at the same conditions; and (iii) by adding standard ginsenosides to the sample solutions to observe the peak height increasing. From Fig. 3, it can be seen that the ginsenosides were well resolved within a 1-min time window, and they can be separated from the interferences. The results in Table 2 show that the content results of Rg1, Rb1 and Re by both CE and HPLC (similar to that in ref. 15) methods are comparable. Meanwhile, we also find that the contents of Rg1, Rb1 and Re of the samples are very different from different producers. The products with less ginsenoside content do not meet the requirement of National Standards of China (Rb1 ≥ 0.2%, Rg1 + Re ≥ 0.3%),4 which would lower their pharmacological effects and infringe consumers' benefits.| Samples | Rg1 | Rb1 | Re | |||
|---|---|---|---|---|---|---|
| CE (RSD) | HPLC (RSD) | CE (RSD) | HPLC (RSD) | CE (RSD) | HPLC (RSD) | |
| a Separation column, Agilent 250*4.0 mm i.d. Hypersil ODS; Mobile phase, binary gradient elution system consisted of water (A) and MeCN (B) and separation was achieved using the following gradient: 0–30 min, 20% B; 30–60 min, 20–45% B; 60–78 min, 45–75% B; 78–80 min, 75–100% B. The flow-rate, was 1 ml min−1; the injection volume, 20 μl; UV detection wavelength, 203 nm. | ||||||
| Root 1 | 0.28 (2.2) | 0.27 (1.1) | 0.15 (1.7) | 0.15 (1.9) | 0.05 (2.6) | 0.05 (2.1) |
| Root 2 | 0.21 (2.5) | 0.22 (2.0) | 0.10 (2.3) | 0.11 (1.3) | unfound | unfound |
| Root 3 | 0.30 (1.8) | 0.29 (1.4) | 0.22 (2.5) | 0.23 (1.7) | 0.11 (1.8) | 0.10 (1.6) |
| Root 4 | 0.10 (2.8) | 0.10 (2.1) | unfound | unfound | unfound | unfound |
| Capsule 1 | 0.22 (2.9) | 0.21 (2.0) | 0.46 (1.2) | 0.47 (1.3) | 0.27 (2.9) | 0.26 (0.8) |
| Capsule 2 | 0.11 (2.3) | 0.12 (1.5) | 0.43 (2.0) | 0.45 (1.9) | 0.15 (1.8) | 0.15 (1.2) |
| Capsule 3 | 0.30 (2.6) | 0.32 (2.2) | 0.52 (1.6) | 0.50 (1.3) | 0.21 (2.5) | 0.22 (1.8) |
| Tablet 1 | 0.15 (1.9) | 0.14 (1.2) | 0.15 (2.2) | 0.15 (2.1) | 0.17 (1.9) | 0.17 (1.2) |
| Tablet 2 | 0.17 (1.5) | 0.17 (1.8) | 0.24 (2.8) | 0.23 (2.2) | 0.33 (2.1) | 0.34 (1.9) |
| Tablet 3 | 0.15 (2.4) | 0.16 (2.1) | 0.33 (2.1) | 0.34 (1.8) | 0.13 (2.6) | 0.12 (2.2) |
4. Conclusion
In summary, a rapid analytical method for ginsenoside Rg1, Rb1 and Re in ginseng samples was developed by CE coupling with a practical pretreatment. The method featured simpleness, sensitivity, rapidity and economy, and can be used for quality control of ginseng and its medical preparations. More importantly, it avoids using sophisticated apparatus, and can easily be conducted in laboratories with limited facilities such as Factory laboratories and monitoring centers in China.Acknowledgements
The authors would like to show their appreciation of the support of Henan Innovation People Grant and NSF of China (20575060 and 20875083). They also thank for Dr. Julie Rimes for assistance with editing the text.References
- A. R. Harding, Ginseng and Other Medicinal Plants, A.R. Harding Publishing Co., Columbus, Ohio, 1931 Search PubMed.
- S. Y. Hu, The genus Panax (Ginseng) in Chinese medicine, Econ. Bot., 1976, 30, 11–28.
- T. K. Yun, Y. S. Lee and Y. H. Lee, Anticarcinogenic effect of Panax ginseng C.A. Meyer and identification of active compounds, J. Korean Med. Sci., 2001, 16(Suppl), S6–18 CAS.
- The Pharmacopoeia Committee of Peoples of Republic of China, Traditional Chinese medicinal materials: Gingseng. Chinese Pharmacopoeia.Beijing: Chemical Industry Press, 2005, pp 7–8 Search PubMed.
- N. Fuzzati, Analysis methods of ginsenosides, J. Chromatogr., B, 2004, 812, 119–133 CrossRef CAS.
- R. M. Corbit, J. F. S. Ferreira, S. D. Ebbs and L. L. Murphy, Simplified extraction of ginsenosides from American ginseng (Panax quinquefolius L.) for high-performance liquid chromatography–ultraviolet analysis, J. Agric. Food Chem., 2005, 53, 9867–9873 CrossRef CAS.
- J. Corthout, T. Naessens, S. Apers and A. J. Vlietinck, Quantitative determination of ginsenosides from Panax ginseng roots and ginseng preparations by thin layer chromatography–densitometry, J. Pharm. Biomed. Anal., 1999, 21, 187–192 CrossRef CAS.
- R. J. Vanhaelen-Fastre, M. L. Faes and M. H. Vanhaelen, High-performance thin-layer chromatographic determination of six major ginsenosides in Panax ginseng, J. Chromatogr., A, 2000, 868, 269–276 CrossRef CAS.
- E. Bombardelli, A. Bonati, B. Gabetta and E. M. Martinelli, Gas-liquid chromatographic method for determination of ginsenosides in Panax ginseng, J. Chromatogr., A, 1980, 196, 121–132 CrossRef CAS.
- J. F. Cui, M. Garle, I. Bjoerkhem and P. Eneroth, Determination of aglycones of ginsenosides in ginseng preparations sold in Sweden and in urine samples from Swedish athletes consuming ginseng, Scand. J. Clin. Lab. Invest., 1996, 56, 151–160 CrossRef CAS.
- J. F. Cui, I. Bjoerkhem and P. Eneroth, Gas chromatographic-mass spectrometric determination of 20(S)-protopanaxadiol and 20(S)-protopanaxatriol for study on human urinary excretion of ginsenosides after ingestion of ginseng preparations, J. Chromatogr., B, 1997, 689, 349–355 CrossRef CAS.
- P. Pietta, P. Mauri and A. Rava, Improved high-performance liquid chromatographic method for the analysis of ginsenosides in Panax Ginseng extracts and products, J. Chromatogr., A, 1986, 356, 212–219 CrossRef CAS.
- T. G. Petersen and B. Palmqvist, Utilizing column selectivity in developing a high-performance liquid chromatographic method for ginsenoside assay, J. Chromatogr., A, 1990, 504, 139–149 CrossRef.
- M. Bonfill, I. Casals, J. Palazon, A. Mallol and C. Morales, Improved HPLC determination of ginsenosides in the ginseng, Biomed. Chromatogr., 2002, 16, 68–72 CrossRef CAS.
- A. J. Lau, S. O. Woo and H. L. Koh, Analysis of saponins in raw and steamed Panax notoginseng using high-performance liquid chromatography with diode array detection, J. Chromatogr., A, 2003, 1011, 77–87 CrossRef CAS.
- L. Li, J. L. Zhang, Y. X. Sheng, G. Ye, H. Z. Guo and D. A. Guo, Liquid chromatographic method for determination of four active saponins from Panax notoginseng in rat urine using solid-phase extraction, J. Chromatogr., B, 2004, 808, 177–183 CrossRef CAS.
- L. Li, J. L. Zhang, Y. X. Sheng, G. Ye, H. Z. Guo and D. A. Guo, Simultaneous quantification of six major active saponins of Panax notoginseng by high-performance liquid chromatography-UV method, J. Pharm. Biomed. Anal., 2005, 38, 45–51 CAS.
- H. K. Lee, H. L. Koh, E. S. Ong and S. O. Woo, Determination of ginsenosides in medicinal plants and health supplements by pressurized liquid extraction (PLE) with reversed phase high performance liquid chromatography, J. Sep. Sci., 2002, 25, 160–166 CrossRef CAS.
- D. Shangguan, H. Han, R. Zhao, Y. Zhao, S. Xiong and G. Liu, New method for high-performance liquid chromatographic separation and fluorescence detection of ginsenosides, J. Chromatogr., A, 2001, 910, 367–372 CrossRef CAS.
- T. W. D. Chan, P. P. H. But, S. W. Cheng, I. M. Y. Kwok, F. W. Law and X. X. Xu, Differentiation and authentication of Panax ginseng, Panax quinquefolius, and ginseng products by using HPLC/MS, Anal. Chem., 2000, 72, 1281–1287 CrossRef CAS.
- H. Y. Ji, H. W. Lee, H. K. Kim, H. H. Kim, S. G. Chang, D. H. Sohn, J. Kim and H. S. Lee, Simultaneous determination of ginsenoside Rb1 and Rg1 in human plasma by liquid chromatography–mass spectrometry, J. Pharm. Biomed. Anal., 2004, 35, 207–212 CrossRef CAS.
- X. Wang, T. Sakuma, E. B. Asafu-Adjaye and G. K. Shiu, Determination of ginsenosides in plant extracts from Panax ginseng and Panax quinquefolius L., Anal. Chem., 1999, 71, 1579–1584 CrossRef CAS.
- W. Li, C. Gu, H. Zhang, D. V. C. Awang, J. F. Fitzloff, S. S. S. Fong and R. B. van Breemen, Use of high-performance liquid chromatography-tandem mass spectrometry to distinguish Panax ginseng C.A. Meyer (Asian ginseng) and Panax quinquefolius L., Anal. Chem., 2000, 72, 5417–5422 CrossRef CAS.
- Q. C. Ji, M. R. Harkey, G. L. Henderson, M. E. Gershwin, J. S. Stern and R. M. Hackman, Quantitative determination of ginsenosides by high-performance liquid chromatography-tandem mass spectrometry, Phytochem. Anal., 2001, 12, 320–326 CrossRef CAS.
- H. Zhang, Y. Wu and Y. Cheng, Analysis of ‘SHENMAI’ injection by HPLC/MS/MS, J. Pharm. Biomed. Anal., 2003, 31, 175–183 CrossRef CAS.
- K. L. Li and S. J. Sheu, Determination of flavonoids and alkaloids in the scute-coptis herb couple by capillary electrophoresis, Anal. Chim. Acta, 1995, 313, 113–120 CrossRef CAS.
- K. T. Wang, H. T. Liu, Y. K. Zhao, X. G. Chen, Z. D. Hu, Y. C. Song and X. Ma, Separation and determination of alantolactone and isoalantolactone in traditional Chinese herbs by capillary electrophoresis, Talanta, 2000, 52, 1001–1005 CrossRef.
- J. Qin, F. C Leung, Y. Fung, D. Zhu and B. Lin, Rapid authentication of ginseng species using microchip electrophoresis with laser-induced fluorescence detection, Anal. Bioanal. Chem., 2005, 381, 812–819 CrossRef CAS.
- J. Cao, L. Yi, P. Li and Y. Chang, On-line concentration of neutral analytes by complexation and acetonitrile sweeping in nonionic microemulsion electrokinetic chromatography with direct ultraviolet detection, J. Chromatogr., A, 2009, 1216, 5608–5613 CrossRef CAS.
- Y. W. Jian, D. L. Li and C. Footim, Ultrasound assisted extraction of ginseng saponins from ginseng roots and cultured ginseng cells, Ultrason. Sonochem., 2001, 8, 347–352 CrossRef CAS.
- Y. S. Youn, Y. K. Ming and S. C. Yuan, Microwave assisted extraction of ginsenosides from ginseng root, Microchem. J., 2003, 74, 131–139 CrossRef CAS.
- G. Laurent, M. Myriam and V. Jean-Luc, Determination of electroosmotic flow in nonaqueous capillary electrophoresis, J. Chromatogr., A, 2005, 1068, 75–80 CrossRef CAS.
- S. P. Porras and E. Kenndler, Are the asserted advantages of organic solvents in capillary electrophoresis real? A critical discussion, Electrophoresis, 2005, 26, 3203–3209 CrossRef CAS.
- M. Vaher and M. Koel, Specific background electrolytes for nonaqueous capillary electrophoresis, J. Chromatogr., A, 2005, 1068, 83–90 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2009 |
