Rapid analysis of the essential oil components of dried Perilla frutescens (L.) by magnetic nanoparticle-assisted microwave distillation and simultaneous headspace solid-phase microextraction followed by gas chromatography-mass spectrometry†
Qing
Ye
* and
Dagui
Zheng
Key Laboratory of Applied Organic Chemistry, Higher Institutions of Jiangxi Province, Shangrao Normal University, Shangrao 334001, China. E-mail: sryq6333@163.cm; Fax: +86-7938150645
First published on 9th September 2009
Abstract
In this work, two solvent-free sample preparation techniques of microwave distillation (MD) and headspace (HS) solid-phase microextraction (SPME) were combined, and developed for the determination of essential oil compounds in dry traditional Chinese medicine (TCM). Amine-functionalized magnetite nanoparticles (AMNs) were added and mixed with the dried Perilla frutescens (L.) sample, which was used as a microwave absorption solid medium for dry distillation of the TCM. Using the proposed method, isolation, extraction and concentration of TCM essential oil compounds can be carried out in a single step. The AMN-assisted MD-HS-SPME parameters including fiber coating, microwave power, irradiation time, and the amount of added AMN were studied. The optimal analytical conditions were: fiber coating of 100 µm PDMS/DVB , microwave power of 230 W, irradiation time of 2 min, as well as the addition of 0.1 g AMN to the TCM sample. The proposed method is applied to the determination of essential oil in Perilla frutescens (L.) and the RSD values is less than 9%. To demonstrate the method feasibility, the conventional HS-SPME method was also used for the analysis of essential oil in the TCM. Experimental results show that more compounds were isolated and identified by AMN-assisted MD-HS-SPME than those by HS-SPME. It was found that the proposed method is an alternative tool for the fast analysis of essential oils in dry TCMs.
1 Introduction
Traditional Chinese medicines (TCMs) are invaluable drug resources. Because of their high pharmacological activity, low toxicity, and rare complications, TCMs have been used in clinical therapy of many diseases for several thousand years.1 For many TCMs, there are active components in their essential oils. Analysis of TCM essential oil is an important research subject. Essential oils are complex mixtures of volatile substances usually present at low concentrations. Their components can be identified by gas chromatography-mass spectrometry (GC-MS). Before such substances can be analyzed, they have to be extracted from the matrix. Various different methods can be used for that purpose, e.g.steam distillation (SD), Soxhlet extraction, and solvent extraction.2–7 However, losses of some volatile compounds, low extraction efficiency, and toxic solvent residue in the extract may be encountered using these extraction methods. Moreover, these extraction procedures are time-consuming. These disadvantages have led to the consideration of new techniques which use less solvent, time, and energy for the extraction of essential oils.Headspace (HS) SPME coupled to GC-MS has been shown to be a simple and solvent-free method for the analysis of essential oils in plant materials8–12 and TCMs.13–17 As we know, it is very slow for volatile components to be evaporated from TCMs, the solid samples, to the headspace; thus, a long time is required for headspace extraction using HS-SPME.13–17 For solid samples such as TCMs, heating can enhance the analyte concentrations in the headspace.18–21 Moreover, an increase in the sample temperature is generally beneficial in speeding the achievement of extraction equilibrium. However, by using traditional heating, sample temperature increase and headspace temperature also increased at the same time. High-temperature extraction can cause significant deterioration of the coating/sample distribution coefficient, resulting in a decrease in the equilibrium amount of analytes extracted.
Microwave heating involves internal heating based on conduction and dielectric polarization caused by microwave irradiation.22 It is therefore not only more efficient when compared to traditional heating but also may result in an external temperature much lower than that of the sample with control of the time and output power of the microwave irradiation. Microwave distillation (MD) coupled with HS-SPME was developed for the fast analysis of essential oil in fresh plant tissue in recent years. It has been demonstrated that MD-HS-SPME is a rapid, simple and effective method for the extraction of the essential oils in fresh plant tissues.23,24 Because there is adequate water within the fresh plant materials, essential oil can be evaporated by heating in situwater that can absorb microwaves. But fresh plant materials are not easy to be preserved, traditional Chinese medicines are dried before being preserved and used, and there is too little water to absorb microwave energy and heat the TCM samples. So essential oils in these TCMs can not be evaporated and extracted simply by the MD methods. Adding some microwave absorption solid medium to the sample can be a feasible way in SPME of essential oils from the dried plant materials. The types of material must have good microwave absorption capacity. Magnetite materials are excellent absorbers of microwave radiation.25–27 In the work detailed here, magnetite nanoparticle-assisted microwave distillation and simultaneous headspace solid-phase microextraction followed by GC-MS was developed for the analysis of essential oil in dry TCM. Amine-functionalized magnetite nanoparticles were used as a microwave absorption solid medium, and Perilla frutescens (L.) (Zhishu in Chinese) was used in this study, which has been used in Chinese traditional medicine as a diaphoretic, antipyretic, sedative, an antitussive, and for the treatment of intestinal disorders and allergies.28,29 The AMN-assisted MD-HS-SPME parameters were studied, and the method precision was also investigated.
2 Experimental
2.1 Plant materials, SPME fibers and MD-SPME apparatus
Perilla frutescens (L.) was purchased from Huangqingrenzhan Pharmacy in Shangrao, China. After being ground to a fine powder with particle size of 120 mesh, the Perilla frutescens (L.) sample was used in the study. The SPME fibers: 100 µm polydimethylsiloxane (PDMS), 65 µm polydimethylsiloxane/divinylbenzene (PDMS/DVB), 30 µm divinylbenzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS) and 85 µm polyacrylate (PA) were purchased from Supelco (Bellefonte, USA). The laboratory-made apparatus of AMN-assisted MD-HS-SPME is shown in Fig. 1. Its constituents are four parts, namely the microwave oven with a hole in the casing of the microwave unit plus with the usage of tin foil to prevent leakage of energy, a glass bottle containing the mixture of TCM and AMN, a condenser reducing the temperature of the headspace-fiber, and the solid-phase microextraction instrument, respectively.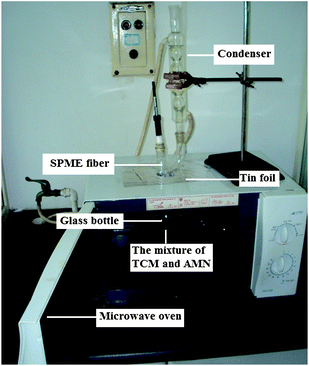 | ||
| Fig. 1 Extraction apparatus of AMN-assisted MD-HS-SPME. | ||
2.2 Preparation of the amine-functionalized magnetite nanoparticles
Add 3.0 g ferric chloride hexahydrate, 12.0 g sodium acetate, and 12.7 mL 1,6-hexanediamine to 90 mL glycol in turn. Then mix them to a transparent solution. The mixture was sealed in a PTFE-lined, stainless steel autoclave and was heated at 200 °C for 6 h. The product, which settled at the bottom of the autoclave, was washed with hot water and ethanol (three times) under ultrasonic conditions to remove the solvent and unbound 1,6-hexanediamine effectively, and then dried at 50 °C to gain the black powder. With the average diameter of 50 nm (see the SEM in Fig. S1, ESI† ), the large surface/volume ratio makes them absorb microwave irradiation better than microsize magnetic particles and have a higher load capacity.2.3 Optimization of AMN-assisted MD-HS-SPME conditions
SPME fiber coating, microwave power, irradiation time and the amount of AMN added can affect the extraction efficiency. In the work, these four parameters were studied. A mass of 1.0 g Perilla frutescens (L.) and 0.05 g AMN was introduced into a 25 mL glass bottle. At first, the SPME fiber coating was studied. The four fibers of PDMS, PDMS/DVB, DVB/CAR/PDMS, PA were tested, with the following microwave parameters: power of 380 W and irradiation of 2 min. Next, microwave power (120 W, 230 W, 380 W and 540 W) and irradiation time (1 min, 2 min, 3 min and 5 min) were simultaneously investigated, using the optimal fiber of PDMS/DVBfiber. Lastly, the amount of added AMN (0.05 g, 0.1 g, 0.15 g, and 0.2 g) was also investigated.2.4 GC-MS analysis
Essential oil analysis was carried out by using a Finnigan Voyager GC-MS. The extracted compounds were separated on an HP-5MS capillary column (30 m × 0.25 mm I.D., 0.25 µm film). Desorption of the SPME fibers was performed in splitless mode for 3 min. The column oven temperature was programmed to rise from an initial temperature of 40 °C for 3 min, followed by a first ramp to 200 °C at 8 °C/min, and a second ramp to 300 °C at 15 °C/min, and 300 °C was maintained for 5 min. The injection temperature and ion source temperature were 250 and 230 °C, respectively. Helium was used as the carrier gas with a flow rate of 1 mL/min. The ionizing energy was 70 eV. All data were obtained by collecting the full-scan mass spectra within the scan range of 40–450 amu. The compounds were identified using the NIST Mass Spectral Search Program (National Institute of Standards and Technology, Washington, DC, USA).2.5 Determination of essential oil compounds in Perilla frutescens (L.) by the proposed method
The optimal parameters of PDMS/DVB fiber, microwave power of 230 W, irradiation time of 2 min and with 0.1 g of the added NMP were used for MD-HS-SPME of the essential oil in Perilla frutescens (L.) (1.0 g). The analytes extracted on the fiber were desorbed at the GC injector (250 °C for 3 min), and then analyzed by GC-MS.2.6 Determination of essential oil compounds in Perilla frutescens (L.) by HS-SPME and microwave-assisted extraction
In order to demonstrate the method feasibility, conventional HS-SPME was also used to analyze the essential oil compounds in Perilla frutescens (L.) (1.0 g). A PDMS/DVB fiber was used to extract and concentrate the essential oil in Perilla frutescens (L.). Headspace extraction was performed at 80 °C for 40 min. The extracted analytes were desorbed and analyzed by GC-MS.2.7 The precision of MD-HS-SPME
The method precision was studied by four replicate analyses of the essential oil in Perilla frutescens (L.) by MD-HS-SPME at the optimum conditions. The precision was expressed by relative standard deviation (RSD ). The peak areas of the essential oil compounds in the TCM obtained by replicate analyses were used for calculation of their RSD values.3 Results and discussion
3.1 Optimization of AMN-assisted MD-HS-SPME parameters
The four MD-SPME parameters of SPME fiber coating, microwave power, irradiation time and added amount of AMN were investigated. Firstly, the fiber coating was studied. AMN-assisted MD-HS-SPME was performed by using four different fibers of PDMS, PDMS/DVB, DVB/CAR/PDMS, PA, with the same conditions of 1.0 g Perilla frutescens (L.), a microwave power of 380 W, an irradiation time of 2 min, and 0.1 g AMN. The four main compounds of limonene (LI), 3,7-dimethyl-1,6-octadien-3-ol (OC), 4-methyl-1-[1-methylethyl]-3-cyclohexen-1-ol (CY), Perilla ketone (PE) (Table 1) present in the TCM sample were used for the determination of the optimal fiber coating. Fig. 2 is the peak areas of the four main compounds obtained by using the four different fibers. As seen from Fig. 2, PDMS/DVB fiber has more extraction efficiencies of OC, CY and PE than the other three fibers. For LI, PDMS/DVB fiber has only lower extraction efficiency than DVB/CAR/PDMS fiber. Comprehensively considering, the PDMS/DVB fiber was regarded as the optimal fiber, and used in the further work.![The effect of fiber coating on extraction efficiencies of limonene (LI), 3,7-dimethyl-1,6-octadien-3-ol (OC), 4-methyl-1-[1-methylethyl]-3-cyclohexen-1-ol (CY), Perilla ketone (PE).](/image/article/2009/AY/b9ay00035f/b9ay00035f-f2.gif) | ||
| Fig. 2 The effect of fiber coating on extraction efficiencies of limonene (LI), 3,7-dimethyl-1,6-octadien-3-ol (OC), 4-methyl-1-[1-methylethyl]-3-cyclohexen-1-ol (CY), Perilla ketone (PE). | ||
| No. | Retention time (min) | Compounds | Molecular weight | Mass spectra (relative abundance) | Relative content (%) | RSD (%) | |
|---|---|---|---|---|---|---|---|
| MD-HS-SPME | HS-SPME | ||||||
| a ND = not determined. | |||||||
| 1 | 8.78 | 4-Methyl-[1-methylethyl] bicyclo[3.1.0]hexane | 136 | 93(100), 77(50),136(5) | 0.37 | 0.33 | 6.8 |
| 2 | 8.94 | α-Pinene | 136 | 93(100), 77(35), 121(10) | 0.21 | 0.28 | 7.2 |
| 3 | 9.85 | β-Phellandrene | 136 | 93(100), 77(40), 136(20) | 1.18 | 1.06 | 4.6 |
| 4 | 10.21 | β-Myrcene | 136 | 93(100), 69(80), 79(20) | 1.59 | 1.22 | 5.3 |
| 5 | 10.80 | [+]-4-Carene | 136 | 121(100), 93(90), 136(60), 77(40) | 0.84 | NDa | 7.8 |
| 6 | 10.99 | 1-Methyl-4-[1-methylethyl]benzene | 134 | 119(100), 91(20), 134(25), 115(5) | 1.26 | 0.77 | 4.5 |
| 7 | 11.11 | Limonene | 136 | 93(100), 68(100), 79(40), 119(40) | 11.34 | 7.24 | 4.1 |
| 8 | 11.16 | Eucalyptol | 154 | 81(100), 108(95), 111(80), 154(80) | 2.10 | 1.34 | 3.9 |
| 9 | 11.22 | 3,7-Dimethyl-1,3,6-octatriene | 136 | 93(100), 79(35), 67(10), 105(10) | 0.97 | 0.31 | 3.6 |
| 10 | 11.44 | 3,7-Dimethyl-1,3,7-octatriene | 136 | 93(100), 79(50), 105(20), 121(20) | 0.55 | 0.30 | 5.8 |
| 11 | 11.70 | 1-Methyl-4-[1-methylethyl]-1,4-cyclohexadiene | 136 | 93(100), 121(100), 136(85), 79(40) | 2.29 | 0.66 | 5.2 |
| 12 | 11.91 | β-Terpineol | 154 | 71(100), 93(80), 111(75), 81(70) | 0.46 | 0.48 | 4.8 |
| 13 | 12.32 | 1-Methyl-4-[1-methylethyl]cyclohexene | 136 | 93(100), 121(100), 136(85), 79(40) | 0.76 | NDa | 4.9 |
| 14 | 12.57 | 3,7-Dimethyl-1,6-octadien-3-ol | 154 | 71(100), 93(90), 55(50), 121(30) | 8.33 | 2.56 | 3.1 |
| 15 | 13.53 | Camphor | 152 | 95(100), 81(70), 108(40), 69(45) | 0.44 | 1.24 | 4.6 |
| 16 | 14.15 | 4-Methyl-1-[1-methylethyl]-3-cyclohexen-1-ol | 154 | 71(100), 111(80), 93(75), 86(30) | 4.96 | 0.65 | 6.2 |
| 17 | 14.39 | α-Terpineol | 154 | 59(100), 93(80), 121(70), 136(60) | 0.66 | NDa | 7.3 |
| 18 | 14.84 | 1S-α-Pinene | 136 | 93(100), 121(90), 136(80), 81(20) | 0.30 | NDa | 8.3 |
| 19 | 15.54 | Perilla ketone | 166 | 95(100), 110(80), 121(10), 80(10) | 28.66 | 29.19 | 3.5 |
| 20 | 15.63 | 3-Methyl-6-[1-methylethyl]-2-cyclohexen-1-one | 152 | 82(100), 110(95), 95(60), 137(25) | 1.04 | 0.50 | 4.7 |
| 21 | 16.11 | Isobornyl acetate | 196 | 95(100), 121(40), 136(38), 108(20) | 1.31 | 7.11 | 5.1 |
| 22 | 16.35 | exo-1,7,7-Trimethylbicyclo[2.2.1]heptan-2-ol | 154 | 95(100), 135(60), 121(20), 91(5) | 2.27 | 2.81 | 4.6 |
| 23 | 16.92 | Hotrienol | 152 | 82(100), 71(90), 67(40), 109(20) | 0.21 | NDa | 7.7 |
| 24 | 17.16 | α-Terpineol acetate | 196 | 121(100), 93(80), 136(70) | 2.10 | 0.92 | 4,8 |
| 25 | 17.32 | 3,7-dimethylocta-1,7-diene,3,6-diol | 170 | 67(100), 71(90), 82(50), 55(45) | 0.92 | NDa | 6.6 |
| 26 | 17.63 | 3,7-Dimethyl-2,6-octadien-1-ol acetate | 196 | 69(100), 93(25), 121(20), 136(15) | 0.83 | 0.48 | 6.2 |
| 27 | 17.91 | 1-Ethenyl-1-methyl-2,4-bis[1-methylethyl]cyclohexane | 204 | 69(100), 81(90), 93(80), 107(60) | 0.45 | 0.69 | 3.2 |
| 28 | 18.45 | Caryophyllene | 204 | 93(100), 133(100), 79(80), 69(80) | 2.23 | 10.58 | 2.9 |
| 29 | 18.82 | 1-[3-Methoxymethylphenyl]ethanol | 166 | 151(100), 69(60), 66(40), 93(35) | 1.21 | 2.37 | 3.7 |
| 30 | 18.99 | 7,11-Dimethyl-3-methyl-1,6,10-dodecatriene | 204 | 69(100), 93(70), 79(30), 133(30) | 0.70 | 2.07 | 3.9 |
| 31 | 19.30 | 1,2,3,4,4a,5,6,8a-Octahydronaphthalene | 204 | 161(100), 105(50), 119(45), 91(45) | 0.45 | 1.54 | 4.4 |
| 32 | 19.43 | 2,6-Dimethylbicyclo[3.1.1]hept-2-ene | 204 | 93(100), 119(95), 161(40), 105(40) | 2.65 | 8.99 | 4.9 |
| 33 | 19.65 | 4,7-Dimethyl-1,2,4a,5,6,8a-hexahydronaphthalene | 204 | 105(100), 161(60), 93(50), 204(30) | 0.67 | 1.17 | 7.1 |
| 34 | 19.89 | 4,7-Dimethyl-1,2,3,4,4a,5,6,8a-octahydronaphthalene | 204 | 105(100), 161(60), 93(50), 204(30) | 0.55 | 0.32 | 5.3 |
| 35 | 20.00 | 4,7-Dimethyl-1,2,3,5,6,8a-hexahydronaphthalene | 204 | 161(100), 119(70), 134(65), 204(65) | 1.55 | 1.81 | 4.3 |
| 36 | 20.35 | 1,2-Dihydro-1,1,6-trimethylnaphthalene | 172 | 157(100), 142(50), 71(50), 107(20) | 0.28 | NDa | 7.4 |
| 37 | 20.50 | 3,7,11-Trimethyl-1,6,10-dodecatrien-3-ol | 222 | 69(100), 93(80), 107(50), 55(30) | 0.71 | 1.19 | 5.9 |
| 38 | 20.92 | Spathulenol | 220 | 205(100), 57(100), 91(100), 71(95) | 0.50 | 0.54 | 4.9 |
| 39 | 21.03 | Caryophyllene oxide | 220 | 79(100), 93(80), 69(70), 109(60) | 1.75 | 3.33 | 3.5 |
| 40 | 21.40 | 1,5,5,8-Tetramethyl-12-oxabicyclo[9.1.0]dodeca-3,7-diene | 220 | 109(100), 67(90), 96(88), 138(80) | 0.33 | 0.44 | 3.7 |
| 41 | 21.61 | Isoaromadendrene epoxide | 220 | 159(100), 119(80), 91(80), 71(75) | 0.36 | 0.30 | 5.0 |
| 42 | 21.80 | α-Cadinol | 222 | 161(100), 95(100), 355(100), 121(85) | 0.45 | 0.67 | 4.2 |
| 43 | 22.27 | 1-[4-Hydroxy-3,5-dimethoxyphenyl]ethanone | 196 | 181(100), 196(30), 166(5) | 4.54 | NDa | 3.9 |
| 44 | 24.06 | 1-Hexadecyne | 222 | 68(100), 95(95), 82(85), 57(70) | 2.30 | 0.16 | 2.9 |
| 45 | 24.13 | 6,10,14-Trimethyl-2-pentadecanone | 268 | 58(100), 71(70), 85(40), 95(38) | 0.86 | 0.52 | 2.4 |
| 46 | 24.30 | 1-Ethynylcyclohexanol | 124 | 81(100), 95(90), 68(88), 57(80) | 0.28 | NDa | 7.7 |
| 47 | 24.48 | 9-Octadecyne | 250 | 82(100), 95(90), 57(70), 123(65) | 0.69 | NDa | 6.8 |
| 48 | 24.87 | Hexadecanoic acid methyl ester | 270 | 74(100), 87(70), 55(20), 143(15) | 0.32 | NDa | 8.1 |
Next, microwave power and irradiation time were studied. Fig. 3 shows the effect of microwave power and irradiation time on peak area sum of the four compounds (LI, OC, CY, and PE). It can be seen from Fig. 3 that the best extraction amount was obtained at 230 W, and the extraction reached balance in a short time of 2 min. This can be explained in that the microwave energy obtained at the power of 230 W and 2 min can evaporate the volatile oil compounds from the TCM. However, too high a power and long irradiation time may lead to a high temperature of the sample headspace, and decrease the SPME extraction efficiency.
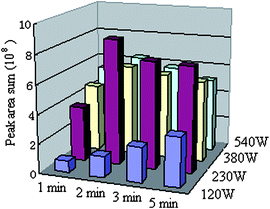 | ||
| Fig. 3 The effect of extraction time and microwave power on the extraction efficiency. | ||
The amount of added AMN in the sample has also been considered. A series of AMN amounts (0.05, 0.1, 0.15, 0.2 and 0.3) was investigated. The effect of AMN amount on the extraction efficiency is shown in Fig. 4. Fig. 4 shows that the best extraction efficiency was achieved using 0.1 g AMN. This can be explained in that the absorption microwave energy increases with the added AMN amount, which leads to more amounts of volatile compounds to evaporate from the TCM. However, too much microwave energy can make some volatiles in the TCM decompose, and the extraction efficiency decrease. Therefore, the optimal AMN-assisted MD-HS-SPME conditions are: PDMS/DVB fiber, a microwave power of 230 W, an irradiation time of 2 min, and 0.1 g AMN.
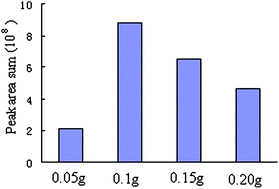 | ||
| Fig. 4 The effect of the added AMN amount on extraction efficiency. | ||
3.2 Determination of essential oil in Perilla frutescens (L.) by NMP-assisted MD-HS-SPME
The optimal parameters of PDMS/DVB fiber, microwave power of 230 W, irradiation time of 2 min and 0.1 g AMN were applied to the determination of essential oil in Perilla frutescens (L.) (1.0 g). The essential oil compounds in the TCM were isolated, extracted and concentrated by AMN-assisted MD-HS-SPME, and then the analytes extracted on the fiber were desorbed and analyzed by GC-MS. Fig. 5a is the GC-MS total ion chromatogram of the essential oil in Perilla frutescens (L.) by the proposed method. A total of 48 volatile compounds were identified, and are listed in Table 1. Their relative contents were calculated in relation to the extracts, and are also listed in Table 1. Perillaldehyde (or perilla ketone) is the main active constituent presented in the Perilla frutescens (L.), which has been reported in many researches (refs 30–33). Their components are identified by gas chromatography-mass spectrometry with steam distillation (SD) (or supercritical fluids extraction (SFE)) extracting essential oils from TCM. As seen from Table 1, perilla ketone is the most abundant constituent (accounting for more than 28% of the mass of all the compounds detected by the proposed method) and perilla aldehyde was not detected. It shows that the active constituents are perhaps different in the different Perilla frutescens (L.) sample. Compared with SD and SFE, the AMN-assisted MD-HS-SPME is a faster and lower-cost sample preparation technique. The proposed method has the potential for quality monitoring for Perilla frutescens (L.).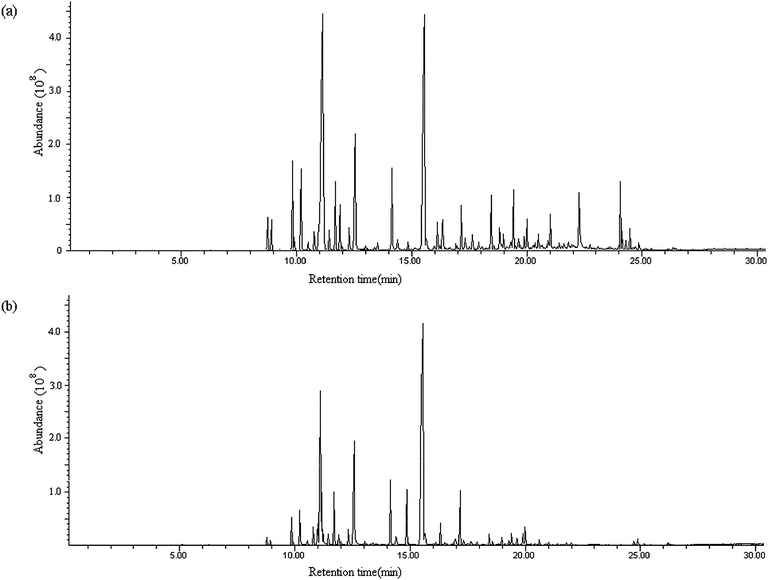 | ||
| Fig. 5 GC-MS total ion chromatograms of essential oil in the Perilla frutescens (L.) by GC-MS with AMN-assisted MD-HS-SPME (a) and HS-SPME (b), respectively. | ||
According to our previous work,34 the microwave-assisted extraction was also used to analyze the essential oil compounds in Perilla frutescens (L.). The extracted analytes were analyzed by GC-MS. Thirty-nine compounds were determined by HS-SPME. The analysis result is similar to the HS-SPME method.
3.3 Determination of essential oil in Perilla frutescens (L.) by HS-SPME
To demonstrate the method feasibility, the essential oil in Perilla frutescens (L.) (1.0 g) was also extracted and concentrated by conventional HS-SPME using a PDMS fiber at 80 °C for 40 min. Then the compounds extracted were desorbed, analyzed and identified by GC-MS according to the same method described above. Fig. 5b was the GC-MS total ion chromatogram of the essential oil in Perilla frutescens (L.). Thirty-seven compounds were determined by HS-SPME (Table 1). Their relative contents were also calculated by peak area ratio, and are listed in Table 1.3.4 Precision of MD-HS-SPME
To obtain the method precision, four replicate analyses of the essential oil in Perilla frutescens (L.) were performed by AMN-assisted MD-SPME at the optimum conditions. The RSD values were calculated by the peak areas obtained by replicate analyses (Table 1). As seen from Table 1, RSD values of less than 9% show that AMN-assisted MD-SPME couples with GC-MS has good precision.4 Conclusions
In this work, AMN-assisted MD-HS-SPME-GC-MS was successfully developed for the analysis of essential oil from dried TCM. The optimal analytical conditions were: a fiber coating of 100 µm PDMS/DVB, a microwave power of 230 W, an irradiation time of 2 min, as well as the addition of 0.1 g AMN to the TCM sample. The proposed method is applied to the determination of essential oil in Perilla frutescens (L.) and the RSD value is less than 9%. Forty-eight compounds were identified in the Perilla frutescens (L.) using the proposed method. It has been shown that AMN-assisted MD-HS-SPME-GC-MS is a simple, fast and solvent-free method for the determination of essential oil compounds in dried plant materials such as TCMs.Acknowledgements
This research was financially supported by the Research Program of Science and Technology from Education Department of Jiangxi Province, China (No.: GJJ08524).References
- K. C. Wen, C. Y. Huang and F. L. Lu, J. Chromatogr., A, 1993, 631, 241–248 CrossRef CAS.
- F. Q. Guo, Y. Z. Liang, C. J. Xu, X. N. Li and L. F. Huang, J. Pharm. Biomed. Anal., 2004, 35, 469–478 CrossRef CAS.
- H. J. Li, X. Y. Meng, Y. Wu, Y. L. Bao and Y. X. Li, Chem. Res. Chin. Univ., 2004, 20, 311–313 Search PubMed.
- J. Q. Yu, J. C. Lei, H. D. Yu, X. Cai and G. L. Zou, Phytochemistry, 2004, 65, 881–884 CrossRef CAS.
- L. F. Huang, B. Y. Li, Y. Z. Liang, F. Q. Guo and Y. L. Wang, Anal. Bioanal. Chem., 2004, 378, 510–517 CrossRef CAS.
- F. Q. Guo, Y. Z. Liang, C. J. Xu and L. F. Huang, J. Chromatogr., A, 2003, 1016, 99–110 CrossRef CAS.
- R. Li and Z. T. Jiang, Flavour Fragrance J., 2004, 19, 311–313 CrossRef CAS.
- M. Kovacevic and M. Kac, J. Chromatogr., A, 2001, 918, 159–167 CrossRef CAS.
- A. Cornu, A. P. Carnat, B. Martin, J. B. Coulon, J. L. Lamaison and J. L. Berdague, J. Agric. Food Chem., 2001, 49, 203–209 CrossRef CAS.
- G. Flamini, P. L. Cioni and I. Morelli, J. Chromatogr., A, 2003, 998, 229–233 CrossRef CAS.
- A. F. Lagalante and M. E. Montgomery, J. Agric. Food Chem., 2003, 51, 2115–2120 CrossRef CAS.
- G. H. Xiong, C. Goodridge, L. M. Wang, Y. Chen and J. Pawliszyn, J. Agric. Food Chem., 2003, 51, 7841–7847 CrossRef CAS.
- X. H. Zheng, C. H. Deng, G. X. Song and Y. M. Hu, Chromatographia, 2004, 59, 729–732 CrossRef CAS.
- S. Shen, Y. F. Sha, C. H. Deng, X. M. Zhang, D. X. Fu and J. K. Chen, J. Chromatogr., A, 2004, 1047, 281–287 CAS.
- C. H. Deng, G. X. Song, Y. M. Hu and X. M. Zhang, Chromatographia, 2003, 57, 357–361 CAS.
- C. H. Deng, N. Li and X. M. Zhang, J. Chromatogr., A, 2004, 1059, 149–155 CrossRef CAS.
- C. H. Deng, G. X. Song, Y. M. Hu and X. M. Zhang, Chromatographia, 2003, 58, 289–294 CAS.
- C. H. Deng, J. Ji, X. C. Wang and X. M. Zhang, J. Sep. Sci., 2005, 28, 1237–1243 CrossRef CAS.
- C. H. Deng, X. H. yang and X. M. Zhang, Talanta, 2005, 68, 6–11 CrossRef CAS.
- C. H. Deng and X. M. Zhang, J. Sep. Sci., 2005, 28, 1137–1142 CrossRef CAS.
- C. H. Deng, N. Yao, B. wang and X. M. Zhang, J. Chromatogr., A, 2006, 1103, 15–21 CrossRef CAS.
- D. M. P. Mingos and D. R. Baghurst, Chem. Soc. Rev., 1991, 20, 1–47 RSC.
- C. H. Deng, X. Q. Xu, N. Yao and X. M. Zhang, Anal. Chim. Acta, 2006, 556, 289–294 CrossRef CAS.
- H. Ye, J. Ji, C. H. Deng, N. Yao, N. Li and X. M. Zhang, Chromatographia, 2006, 63, 591–594 CrossRef.
- Z. M. Wang, L. Ding, T. C. Li, X. Zhou, L. Wang, H. Q. Zhang, L. Liu, Y. Li, Z. H. Liu, H. J. Wang, H. Zeng and H. He, J. Chromatogr., A, 2006, 1102, 11–17 CrossRef CAS.
- W. Y. Chen and Y. C. Chen, Anal. Chem., 2007, 79, 8061–8066 CrossRef CAS.
- D. K. Kim, M. S. Amin, S. Elborai, S. H. Lee, Y. Koseoglu, M. Zahn and M. Muhammed, J. Appl. Phys., 2005, 97, 10J510 CrossRef.
- E. A. Omer, M. E. Khattab and M. E. Ibrahim, Flavour Fragrance J., 1998, 13, 221–225 CrossRef CAS.
- M. Tada, R. Masumotoand and K. Chib, Phytochemistry, 1996, 43, 803–804 CrossRef CAS.
- K. H. C. Baser, B. Demirci and A. A. Dönmez, Flavour Fragrance J., 2003, 18, 122–123 CrossRef CAS.
- Q. Meng, F. Y. Fang, H. M. Liang and G. F. Chen, Academic Journal of Guangdong College of Pharmacy, 2004, 20, 590–591 Search PubMed.
- Z. H. Wu, C. M. Wu and Y. Xu, Amino Acids & Biotic Resources, 2003, 25, 18–20 Search PubMed.
- Y. Z. Ling, China Condiment, 2005, 11, 18–20 Search PubMed.
- Q. Ye and C. H. Deng, Anal. Lett., 2008, 41, 2387–2401 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: SEM image of amine-functionalized magnetite nanoparticles. See DOI: 10.1039/b9ay00035f |
| This journal is © The Royal Society of Chemistry 2009 |
