Screen printed electrochemical platforms for pH sensing
Dimitrios K.
Kampouris
,
Rashid O.
Kadara
,
Norman
Jenkinson
and
Craig E.
Banks
*
Faculty of Science and Engineering, School of Biology, Chemistry and Health Science, Division of Chemistry and Materials, Manchester Metropolitan University, Chester Street, Manchester, UK M1 5GD. E-mail: c.banks@mmu.ac.uk
First published on 7th September 2009
Abstract
We explore the possible use of screen printing technology for fabricating disposable electrochemical platforms for the sensing of pH. These screen printed pH sensors incorporate the pH sensitive phenanthraquinone moiety which undergoes a Nernstian potential shift with pH, and the pH insensitive dimethylferrocene which acts as an internal reference. This generic approach offers a calibration-less and reproducible approach for portable pH measurements with the possibility of miniaturisation allowing incorporation into existing sensing devices. The advantages, limitations and future prospects of this fabrication approach for producing electrochemical platforms for pH sensing are also discussed.
1. Introduction
There is an increasing need for pH sensors that are portable, robust, sensitive, and economical for use in a plethora of environments such as the food industry, agriculture, medical organisations and oil refinery.1,2 Traditionally the glass electrode, based on potentiometry, has been used for pH sensing but unfortunately suffers from instability and/or drift requiring frequent re-calibration.3 Pioneering work by Hickman et al. demonstrated that this may be overcome by the measurement of the potential difference between the response of a pH sensitive redox compound and a second pH insensitive redox compound providing a calibration-less protocol for pH sensing.4 This advantageous approach is developed in the hope of replacing the well-established glass electrode which, in addition to suffering from the problems highlighted above, is fragile, expensive and can be difficult to implement ‘in-the-field’. However there are limitations with the pKa of the chosen pH sensitive redox compound limiting the pH sensing range; for example, the use of anthraquinone allows only the sensing of pH over the range of 4 to 10.5Carbon materials are extensively used in electrochemical sensors, and particularly in pH sensing applications due to their good electrical conductivity, low cost, availability and suitability for functionalisation.1 A range of derivatisation strategies for functionalising carbon materials with pH sensing compounds have been reported such as covalent bonding of target pH-sensitive compounds via chemical or electrochemical activation, physical adsorption and co-polymers molecularly attached to nanomaterials such as carbon nanotubes.6,7 In these cases, the derivatised materials are then explored via immobilisation onto suitable electrode substrates.8 In other cases, films of the pH sensing compounds are preferred.2 Lawrence et al. point-out that such immobilisation strategies are useful for integrating these functionalised pH sensing materials but need to be developed such that they can readily be implemented in commercial pH sensors as these modified layers supported on electrode substrates are, in some instances, unstable; for example, in conditions where solution flows across the electrode surface may be routinely encountered.9 Additionally, the concept of producing such sensors on a large scale ready for commercialisation needs to be addressed. In response to these two problems, epoxy electrodes have been reported.9
Electrochemical platforms can be fabricated a number of ways such as pad printing, air bushing, direct pen and screen printing.10–12 Screen printing has been developed over many years and one of its best known applications is the production of low-cost disposable glucose sensors for diabetics to monitor blood glucose levels.13,14
To the author's surprise, there are no literature reports of using screen printing technology for fabricating electrochemical pH sensors despite its obvious advantages. We note that pad-printing has been briefly mentioned as a possible fabrication technique in a patent application15 but fails to provide any detailed information. In this embodiment, as the name suggests, a large pad, similar to a rubber stamp is used to transfer a thixotropic-type fluid from a stencil to the desired substrate. Pad-printing is a rather limited printing approach and has yet to reach its full commercial potential which is due to the complexity of the processes, poor reproducibility and the inability to produce large quantities of electrodes.
There is a clear lack of knowledge in possible fabrication strategies in this exciting area of sensor development and new types of pH sensitive and pH insensitive components are readily being developed and explored which can be incorporated into other sensing applications. For example, proof-of-concept for an anthracene-ferrocene moiety has been reported which is useful for the calibration-less sensing of pH but can also be simultaneously used in oxygen sensing processes2 as well as a pH sensor which also functions as a sulfide sensor.16 There is a lack of knowledge of using screen printing in this area which can beneficially aid those wishing to develop and explore these type of sensors in an academic environmental as well being able to easily implement to produce larger numbers for field testing. Additionally, the concept of screen printing can dramatically simplify the electronic components for in-the-field sensing since a common approach to printing is two working electrodes, one modified with a pH sensitive compound and another with a pH insensitive compound along with a common reference and common counter electrode. The electronics required for this, while achievable, are more challenging. On the other hand, screen printing can combine multi-components into one working electrode; we envisage and consequently explore this where the pH sensitive and pH insensitive moieties are combined within a single working electrode with a reference and counter electrode, providing a simple three electrode system which not only simplifiers the fabrication process but also the electronic components that are required. Given the advantages of using screen printing as described above, in this paper we consequently explore the fabrication of screen printed electrodes with redox pH active and redox pH inactive components; such an electrode has never been reported in the literature. We also discuss the advantageous, limitation and future prospects of this approach.
2. Experimental
All chemicals used were of analytical grade and were used as received without any further purification from Sigma Aldrich. All solutions were prepared with deionised water of resistivity not less than 18.2 MΩ cm−1. Voltammetric measurements were carried out using an in-house hand-held potentiostat which has the ability to perform a range of electrochemical measurements. All measurements were conducted using a three electrode configuration. Screen printed carbon electrodes were fabricated in-house with appropriate stencil designs using a microdek 1760RS screen printing machine (DEK, UK). The multistage printing process involved the sequential deposition of carbon-graphite, silver–silver chloride and dielectric inks (obtained from Gwent, Pontypool, UK) onto a polyester substrate material. Further modification of the working surface was carried out by mixing together 9,10-phenanthraquinone (referred herein as phenanthraquinone) and 1,1-dimethylferrocene (referred herein as dimethylferrocene) within the carbon-graphite ink and placed into a fan oven for 15 min at 40 °C to dry. Solutions of known pH in the range pH 1.0 to 13.0 were prepared in deionised water as follows: pH 1, 0.2 M potassium chloride and 0.2 M hydrogen chloride; pH 3, 0.1 M potassium hydrogen phthalate and 0.1 M hydrogen chloride; pH 5, 0.1 M potassium hydrogen phthalate and 0.1 M sodium hydroxide; pH 7, 0.1 M potassium dihydrogen phosphate and 0.1 M sodium hydroxide; pH 9, 0.025 M borax and 0.1 M hydrogen chloride; pH 11, 0.05 M disodium hydrogen phosphate and 0.1 M sodium hydroxide; pH 13, 0.2 M potassium chloride and 0.2 M sodium hydroxide. A three electrode connector (purchased from http://www.kanichi-research.com) was used to efficiently connect the SPE pH probe with the potentiostat. When the screen printed electrodes were used with the screen printed silver–silver chloride reference electrode to conduct measurements, 100 µL of the desired buffer solution was applied on to the electrode surface with the aid of a micropipette. If a saturated calomel electrode was used as a reference a small vial was used with a minimal amount of buffer solution. All experiments were performed at room temperature. The following parameters were used for the square wave voltammogramms: frequency 100 Hz, step potential 1 mV and amplitude 10 mV. For the cyclic voltammogramms a step potential of 1 mV and scan rates of 100 mV s−1 were employed.3. Results and discussion
Initial experiments were performed to explore the effect of varying the weight to weight ratio of the electroactive constituents in the graphite-carbon ink. Ratios of 5 : 15 of dimethylferrocene and phenanthraquinone to ink produced an optimal response; lesser amounts produced smaller voltammetric signals while larger amounts affected the screen printing process and resulted in no voltammetric signals due to the absence of conductive pathways. Cyclic voltammetry was performed on the screen printed pH probes where well defined and easily quantifiable peaks were observed in a pH 7 buffer solution with a single reduction peak at ∼ −0.21 V with a corresponding oxidation wave at ∼ −0.14 V (both vs.SCE) due to the quinone/hydroquinone electrochemical process. This peak-to-peak separation (100 mV s−1) of 64 mV indicates a quasi-reversible system in accordance with previous studies.8,9 A further oxidation peak is observed at higher potentials at ∼ +0.42 V (vs.SCE) which corresponds to electrochemical oxidation of dimethylferrocene in accordance with previous studies.17 Further investigation of the screen printed pH probe was conducted. The pH probe was modified with a droplet of pH 7 buffer and voltammetrically cycled for several scans. This droplet was washed off and replaced with a fresh one with the voltammetric response recorded. The corresponding voltammetric profile was found to be less than the first confirming that the electroactive species dissolves from the electrode surface into the aqueous droplet. This is as expected given the solubility of the electroactive species in aqueous solutions. In our approach this is not problematic since each sensor is designed to be used only once, but could be if one wanted to monitor the change in pH of a solution over a long period of time. We do envisage that suitable pH material may be derivatised through a variety of protocols1,3,5 which can then be incorporated into the screen printed pH electrode to provide reusable electrodes or where pH is required to be monitored over a period of time.Next, using a saturated calomel electrode (SCE) as a reference, a carbon counter and SPE pH probe as the working electrode the square-wave voltammetric response was explored over the entire pH range of 1 to 13. The peak potential of the voltammetric profiles was observed in relation to pH in a Nernstian fashion as governed by eqn (1):18
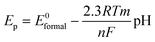 | (1) |
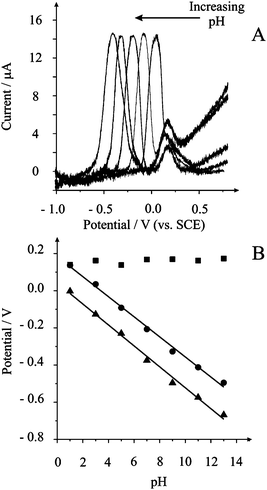 | ||
| Fig. 1 A. Typical voltammetric profiles obtained using the pH probe in pH buffer solutions of 3, 5, 7, 9 and 11. B. Plot of peak potential against pH for the dimethylferrocene species (squares), the phenanthraquinone species (circles) and the difference in potential between the two (triangles) at each pH studied. | ||
The observed shifts in peak potential are in good agreement with the theoretically predicted value of 58 mV per pH at 22 °C viaeqn (1). At the low pH region of pH 1 it is observed that the peak potential of the phenanthraquinone overlaps detrimentally with that of the pH inactive dimethylferrocene response indicating that the combination of these two species do not allow sensing at this pH range, viz lower than pH 2. However, this combination does allow pH values of up to pH 13 to be measured which is further than those recently reported which typically do not extend beyond pH 10.2,3,6 It is important to realise that each point on the plot of peak potential against pH is the result of individual electrodes highlighting the reproducibility of the screen printing process. Given the observed peak potentials of the pH active moiety and that of the pH inactive component, which are both not higher than ∼ +0.4 V (see above), interferences such as ascorbic acid, sulfite and catechol, which occur at considerably higher potentials,9 are not expected to interfere with the sensor.
For the screen printed pH probe to be used in-the-field for various pH sensing applications and possibly to be miniaturised and incorporated into existing sensors, a standalone screen printed pH probe is more appropriate, that is, a pseudo silver–silver chloride reference electrode. Fig. 2 depicts an SEM image of such a screen printed electrode. Closer inspection of the pH probe's working electrode with SEM, as shown in Fig. 2, indicates a relatively smooth electrode surface10 which may account for the excellent reproducibility observed above. The response of this integrated SPE pH probe was explored over the pH range of 1 to 13. The corresponding plot of the peak potential difference between the phenanthraquinone and the dimethylferrocene against pH was found to produce a value of 57 mV (±3) per pH unit at 22 °C (ΔEp = −0.057pH + 0.0307, R2 = 0.994). This experimentally observed shift is again in good agreement with the theoretical value of the 58 mV per pH at 22 °C. Based on the uncertainty estimation for a sample of pH 7.01 (n = 5), the uncertainty was found to correspond to ±0.12 (within 95% confidence). The reference material used on the screen printed electrode is produced from silver–silver chloride paste and acts as a pseudo reference electrode. The reference electrode is susceptible to the presence of chloride which alters the potential of both the pH active and pH inactive compounds yet the difference between the two is the most important and functions as an excellent calibration-less pH sensor. At high salinity (0 M to 5 M NaCl) it has been shown9 that as the chloride concentration increases, the peak potentials, the pH active component moves towards the ferrocene peak as a function of salt concentration which is consistent with a change in the pH of the solution. Given the similarity of our pH active and pH inactive moieties incorporated into the screen printed electrochemical platform, the sensor could be utilised in environmental monitoring such as seawater.
 | ||
| Fig. 2 SEM images of the SPE pH probe. The left image shows a fully integrated sensor where the centre circle is the working electrode containing the pH active and pH inactive compounds; on the bottom right of this mage is the pseudo silver–silver chloride reference electrode with the outer track being the counter electrode. The image on the right shows close inspection of the working electrode. | ||
4. Conclusions
We have reported a novel electrochemical pH probe based on screen printing technology which utilises the potential difference between two compounds, one pH active and the other pH inactive where the difference in the electrode potential provides a calibrant for the pH measurement of aqueous solutions. This screen printed pH sensing approach avoids the need for using/developing insoluble electrochemical species since the electrodes are specifically designed for ‘single shot’ usage where fouling of the electrode surface may occur, as likely encountered in real (bio)samples; due to the low cost of each sensor we believe this is where the future of screen printed electrode pH sensors lie. It is envisaged that users can readily change both the pH active and pH inactive species for specified applications. Such an approach provides an economical, reproducible and calibration-less approach for the portable sensing of pH. Researchers working in this area who are developing new compounds for this pH sensing technology can use this fabrication approach for evaluation purposes with the knowledge that this can be scaled up for commercialisation. The ‘in-the-field’ testing of pH systems reported in the literature are limited and it is hoped that this approach to screen printing, which has the advantages of readily producing large numbers of electrodes, will be overcome.References
- H. Kahlert, J. Solid State Electrochem., 2008, 12, 1255 CrossRef CAS.
- V. G. H. Lafitte, W. Wang, A. S. Yashina and N. S. Lawrence, Electrochem. Commun., 2008, 10, 1831 CrossRef CAS.
- G. G. Wildgoose, M. Pandurangappa, N. S. Lawrence, L. Jiang, T. G. J. Jones and R. G. Compton, Talanta, 2003, 60, 887 CrossRef CAS.
- J. J. Hickman, D. Ofer, P. E. Laibinis, G. M. Whitesides and M. S. Wrighton, Science, 1991, 25, 688 CrossRef.
- C. G. R. Heald, G. G. Wildgoose, L. Jiang, T. G. J. Jones and R. G. Compton, ChemPhysChem, 2004, 5, 1794 CrossRef.
- N. S. Lawrence and K. L. Robinson, Talanta, 2007, 74, 365 CrossRef CAS.
- G. G. Wildgoose, H. C. Leventis, I. Streeter, N. S. Lawrence, S. J. Wilkins, L. Jiang, T. G. J. Jones and R. G. Compton, ChemPhysChem, 2004, 5, 699.
- H. C. Leventis, I. Streeter, G. G. Wildgoose, N. S. Lawrence, L. Jiang, T. G. J. Jones and R. G. Compton, Talanta, 2004, 63, 1039 CAS.
- N. S. Lawrence, M. Pagels, S. F. J. Hackett, S. McCormack, A. Meredith, T. G. J. Jones, G. G. Wildgoose, R. G. Compton and L. Jiang, Electroanalysis, 2007, 19, 424 CrossRef CAS.
- R. O. Kadara, N. Jenkinson and C. E. Banks, Sens. Actuators, B, 2009, 138, 556 CrossRef.
- R. O. Kadara, N. Jenkinson, B. Li, K. H. Church and C. E. Banks, Electrochem. Commun., 2008, 10, 1517 CrossRef CAS.
- C. E. Walker, Z. Xia, Z. S. Foster, B. J. Lutz and Z. Hugh Fan, Electroanalysis, 2008, 20, 663 CrossRef CAS.
- K. Grennan, A. J. Killard and M. R. Smyth, Electroanalysis, 2001, 13, 745 CrossRef CAS.
- A. L. Hart, A. P. F. Turner and D. Hopcroft, Biosens. Bioelectron., 1996, 11, 263 CrossRef CAS.
- S. P. Mccormack, R. G. Compton, G. G. Wildgoose and N. S. Lawrence, 2006, WO 2005085825.
- K. L. Robinson and N. S. Lawrence, Electroanalysis, 2006, 18, 677 CrossRef CAS.
- C. E. Banks, A. S. Yashina, G. J. Tustin, V. G. H. Lafitte, T. G. J. Jones and N. S. Lawrence, Electroanalysis, 2007, 19, 2518 CrossRef CAS.
- R. G. Compton and C. E. BanksUnderstanding Voltammetry, World Scientific Ltd, 2008 Search PubMed.
- K. L. Robinson and N. S. Lawrence, Electrochem. Commun., 2006, 8, 1055 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2009 |
