Enrichment/isolation of phosphorylated peptides on hafnium oxide prior to mass spectrometric analysis†
José G.
Rivera
,
Yong Seok
Choi
,
Stefan
Vujcic
,
Troy D.
Wood
and
Luis A.
Colón
*
Department of Chemistry, University at Buffalo, The State University of New York, NS Complex, Buffalo, NY 14260-300, USA. E-mail: lacolon@buffalo.edu; Fax: +1 716 645 6963; Tel: +1 716 645 6800 ext. 2143
First published on 21st October 2008
Abstract
Hafnium oxide (hafnia) exhibits unique enrichment properties towards phosphorylated peptides that are complementary to those of titanium oxide (titania) and zirconium oxide (zirconia) for use with mass spectrometric analysis in the field of proteomics.
Protein phosphorylation has been known to be a significant regulatory mechanism in many organisms controlling a wide variety of biological functions.1 The determination of phosphorylation sites on proteins, however, may not necessarily be a trivial task. Traditional methods for the analysis of phosphoproteins include labeling of the protein with 32P to monitor phosphorylation, and Edman degradation chemistry on phosphopeptides to localize the site of phosphorylation.2 This approach suffers because of disadvantages associated with the use of a radioactive material. This is in addition to being time consuming and laborious, and typically requiring large amounts of purified protein; furthermore, Edman degradation produces no data from proteins/peptides with blocked N-termini (i.e., N-terminal acetylation), resulting in loss of sample.3
Methods based on mass spectrometry (MS) are emerging for the analysis of post-translational modifications, including phosphorylation; this is due to their higher sensitivity, selectivity, and speed of analysis when compared to most biochemical techniques.4 In spite of the advances in mass spectrometry to identify phosphopeptides, however, some difficulties still remain. For example, frequently the stoichiometric level of phosphorylation for a given protein is very low and hence difficult to identify. It has also been observed that the intensity of the signals due to phosphorylated peptides in the mass spectrometer is lower than those for the non-phosphorylated species, particularly when using positive ion mode monitoring. It is, therefore, advantageous to separate/isolate the phosphorylated peptides/proteins from non-phosphorylated species in a given sample to facilitate the detection and identification of phosphorylation events viaMS.
Commonly used isolation techniques for phosphorylated peptides include immobilized metal ion affinity chromatography (IMAC),5immunoprecipitation using phosphoprotein specific antibodies,6 or specific chemical modification strategies targeted for phosphorylated amino acids.7 Of these methods, immobilized IMAC combined with electrospray ionization (ESI) MS is the most widely used approach. However, enrichment and recovery of the phosphopeptides strongly depends on the type of metal ion used, column material, and the loading/eluting procedures utilized. Furthermore, IMAC requires additional metal ion loading and washing steps, increasing total sample analysis time, and it is tedious to configure in on-line applications. There is great interest in finding alternatives to IMAC.
Titanium oxide (titania), mostly in the particulate form, has emerged as a viable alternative medium for the isolation/enrichment of phosphopeptides; it appears that titania has particular affinity towards multiply phosphorylated species.8Zirconium oxide (zirconia) has also been shown to have enrichment properties towards phosphorylated peptides.9 These metal oxides have drawn attention due to their high affinity towards phosphate-containing compounds as a result of their strong Lewis acid–base interactions.10 Both, titania and zirconia have been used in both off-line9a,b and on-line chromatographic applications.8a,c,9b,c,e Although it has been reported that zirconia complements titania by having more affinity towards monophosphorylated peptides,9a recent published reports indicate that zirconia behaves very similar to titania, with less affinity towards monophosphorylated species.9c,d
Very recently, our research group reported the use of hafnium oxide (hafnia) in a monolithic format as a potential metal oxide support material for chromatographic applications.11Hafnium is another member of Group IV in the Periodic Table of the Elements with similar characteristics to those of zirconium;12 however, hafnia has not been explored much for chromatography, nor for the potential enrichment/isolation of phosphorylated peptides in the field of proteomics. Herein, we report on the unique property of hafnia material to enrich/isolate phosphorylated peptides. To the best of our knowledge, this is the first time that hafnia has been shown to be useful for the enrichment of phophorylated peptides.
ESI-MS was performed on tryptic digest samples of bovine β-casein, treated and non-treated with hafnia material to test the isolation/enrichment characteristics of hafnia towards phosphopeptides (see ESI for experimental details on the synthesis of the hafnia material and tryptic digestion†). Bovine β-casein has been reported to have five phosphorylated serine residues, and it is well documented that its tryptic digestion results in both monophosphophorylated and tetraphosphorylated peptides.13
For comparison, tryptic digest samples of β-casein were also treated with titania and zirconia materials. The spectra are shown in Fig. 1. A list is provided in Table 1 for the phosphopeptide sequences identified in the collected mass spectra (with S/N ≥ 3); the masses were identified in the proteolytic peptide database generated with the Peptide Mass tool on the ExPASy web site (http://us.expasy.com). It is worth mentionioning that the same peptide may appear on the spectrum at different mass/charge ratios as a result of the different charges generated during the ESI process.
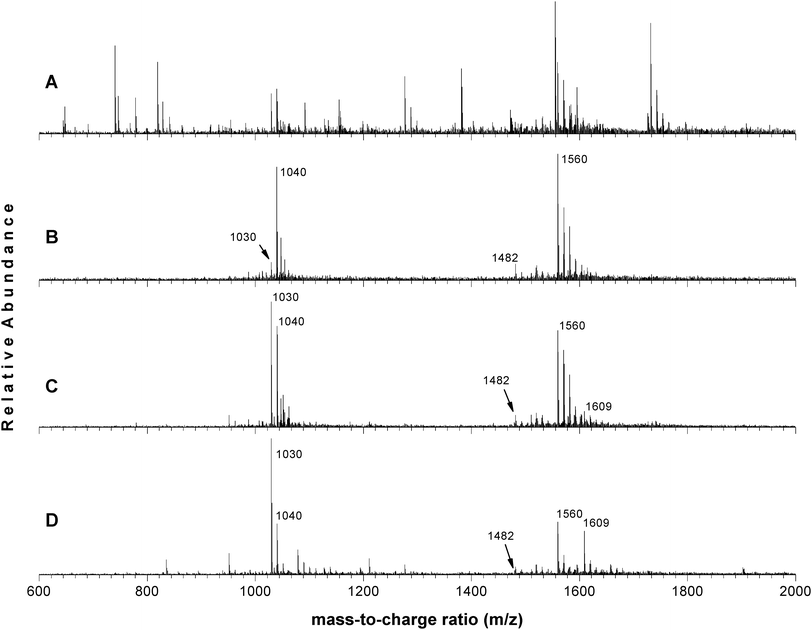 | ||
| Fig. 1 Mass spectra of the β-casein tryptic digest treated with different metal oxides: (A) non-treated, (B) ZrO2, (C) TiO2, and (D) HfO2. | ||
| m/z Observed | Charge state | Sequence | Phosphorylation:site | Monoisotopic mass |
|---|---|---|---|---|
| a Generated with the Peptide Mass tool on the ExPASy website (http://us.expasy.org). | ||||
| 1030.4 | −2 | 48–63 | 1P :50 | 2059.8 |
| 1040.4 | −3 | 16–40 | 4P :30,32,33,34 | 3120.3 |
| 1482.6 | −2 | 17–40 | 4P :30,32,33,34 | 2964.1 |
| 1560.6 | −2 | 16–40 | 4P :30,32,33,34 | 3120.3 |
The ESI-MS spectrum obtained for the non-treated β-casein tryptic digest sample (Fig. 1A) is dominated by non-phosphorylated peptides, which creates complexity in the MS spectrum. Mass spectra of the tryptic digest treated with the metal oxides are shown in Fig. 1B–D. When the tryptic digest was treated with the metal oxides prior to ESI-MS analysis, the selective enrichment/isolation of the phosphorylated peptides is clearly evident, shown by the increased signals and cleaner MS spectra. Interestingly, when samples of the digest were treated with the zirconia material (Fig. 1B), the peptides with the highest intensities corresponded to those of the tetraphosphorylated peptides (m/z of 1040, 1482 and 1560), along with up to three of their sodiated forms, and very little signal was observed for the single phosphorylated species (m/z of 1030). Titania, on the other hand (Fig. 1C), while showing higher selectivity towards the tetraphosphopeptide (m/z of 1040 and 1560), still enriched/isolated a significant amount of the monophosphorylated peptide (m/z of 1030). When the enrichment was performed using hafnia (Fig. 1D), both types of phosphopeptides appeared in the mass spectrum with similar intensities (after the abundance associated with m/z of 1040 and m/z of 1560 for the tetraphosphopeptide are summed). Under the experimental conditions used, both titania and zirconia appeared to be biased towards the tetraphosphorylated peptides over the monophosphorylated ones, with zirconia having very low affinity towards the monophosphopeptide. Hafnia, on the other hand, enriched both the single and tetraphosphopeptides. However, the degree of discrimination by hafnia towards the mono- or the tetraphosphosphorylated peptide was not as pronounced as with zirconia or even titania; both phosphopeptides were enriched nearly equally when using hafnia as the isolating/enrichment matrix.
Interestingly, several other peaks appeared in the spectrum of the tryptic digest sample treated with hafnia. We analyzed the relatively strong signal at m/z of 1609 viaMS/MS, which coincidently, it is also present in the spectrum for the sample treated with titania. The resultant spectrum of the MS/MS analysis of this particular species clearly showed a loss of H3PO4groups, evidenced by three successive loses of 98 Da. MS/MS analysis of the known tetraphosphorylated peptide having m/z of 1560, also provided similar fragmentation pattern (see electronic supplementary information). These data indicated that hafnium oxide had the ability to enrich/isolate phosphorylated species in our sample that were not prominent by either titania or zirconia. Further investigations, however, are underway to fully characterize and elucidate the origin of the new peaks observed.
Our results demonstrate that hafnia can be used to enrich phosphorylated peptides, with potential applications in the field of phosphoproteomics. The enrichment characteristics of the hafnia material used in our experiments show some differences compared to those of the commercially available materials titania and zirconia currently used in proteomics; our experiments indicate that hafnia can enrich mono- and tetraphosphorylated peptides while titania and zirconia showed a bias towards the tetraphosphorylated peptides. We envision the use of hafnia media for the enrichment of phosphopeptides in certain applications where the isolation of all types of phosphopeptides (e.g., mono- and tetraphosphorylated) is required. This can be important when characterizing phosphorylation modifications on a particular protein structure that is being assessed for the first time. In this regard, hafnia appears to be a complement to titania and zirconia materials. In other investigations, we are using more complex phosphoproteins to gain insight into the use of this new metal oxide material. In addition to characterizing the species that only appeared after using hafnia as the isolation material, experimentation is also underway on the use of hafnia for on-line LC-MS applications.
We acknowledge the financial support provided by The National Science Foundation, USA (CHE-0554677). We also thank Jerry Tso for technical assistance in obtaining the MS data. JGR acknowledges the Arthur A. Schomburg Graduate Fellowship and the Alliance for Graduate Education and the Professoriate Program (University at Buffalo) for financial support.
References
- (a) M. J. Hubbard and P. Cohen, Trends Biochem. Sci., 1993, 18, 172–177 CrossRef CAS; (b) C. Koch, D. Anderson, M. F. Moran, C. Ellis and T. Pawson, Science, 1991, 252, 668–674 CrossRef CAS; (c) G. Manning, D. B. Whyte, R. Martinez, T. Hunter and S. Sudarsanam, Science, 2002, 298, 1912–1934 CrossRef CAS.
- J. X. Yan, N. H. Packer, A. A. Gooley and K. L. Williams, J. Chromatogr., A, 1998, 808, 23–41 CrossRef CAS.
- M. Kinter and N. E. Sherman, Protein Sequencing and Identification Using Tandem Mass Spectrometry, John Wiley and Sons, New York, NY, 2000 Search PubMed.
- (a) R. S. Annan, M. J. Huddleston, R. Verma, R. Deshaies and S. A. Carr, Anal. Chem., 2001, 73, 393–404 CrossRef CAS; (b) D. T. McLachlin and B. T. Chait, Curr. Opin. Chem. Biol., 2001, 5, 591–602 CrossRef CAS; (c) B. A. Garcia, J. Shabanowitz and D. F. Hunt, Methods, 2005, 35, 256–264 CrossRef CAS; (d) M. Mann, S. E. Ong, M. Gronborg, H. Steen, O. N. Jensen and A. Pandey, Trends Biotechnol., 2002, 20, 261–268 CrossRef CAS.
- (a) D. C. Neville, C. R. Rozanas, E. M. Price, D. B. Gruis, A. S. Verkman and R. R. Townsend, Protein Sci., 1997, 6, 2436–2445 CAS; (b) T. S. Nuhse, A. Stensballe, O. N. Jensen and S. C. Peck, Mol. Cell. Proteomics, 2003, 2, 1234–1243 CrossRef; (c) M. C. Posewitz and P. Tempst, Anal. Chem., 1999, 71, 2883–2892 CrossRef CAS.
- (a) S. B. Ficarro, O. Chertihin, V. A. Westbrook, F. White, F. Jayes, P. Kalab, J. A. Marto, J. Shabanowitz, J. C. Herr, D. F. Hunt and P. E. Visconti, J. Biol. Chem., 2003, 278, 11579–11589 CrossRef CAS; (b) A. Pandey, A. V. Podtelejnikov, B. Blagoev, X. R. Bustelo, M. Mann and H. F. Lodish, Proc. Natl. Acad. Sci. U. S. A., 2000, 97, 179–184 CrossRef CAS.
- D. T. McLachlin and B. T. Chait, Anal. Chem., 2003, 75, 6826–6836 CrossRef CAS.
- (a) I. Kuroda, Y. Shintani, M. Motokawa, S. Abe and M. Furuno, Anal. Sci., 2004, 20, 1313–1319 CAS; (b) M. R. Larsen, T. E. Thingholm, O. N. Jensen, P. Roepstorrf and T. J. D. Jørgensen, Mol. Cell. Proteomics, 2005, 47, 873–886 CrossRef; (c) M. W. H. Pinkse, P. M. Uitto, M. J. Hilhorst, B. Ooms and A. J. R. Heck, Anal. Chem., 2004, 76, 3935–3943 CrossRef CAS.
- (a) H. K. Kweon and K. Hakansson, Anal. Chem., 2006, 78, 1743–1749 CrossRef CAS; (b) G. T. Cantin, T. R. Shock, S. K. Park, H. D. Madhani and J. R. Y. III, Anal. Chem., 2007, 79, 4666–4673 CrossRef CAS; (c) S. S. Jensen and M. R. Larsen, Rapid Commun. Mass Spectrom., 2007, 21, 3635–3645 CrossRef CAS; (d) H. Zhou, R. Tian, M. Ye, S. Xu, S. Feng, C. Pan, X. Jiang, X. Li and H. Zou, Electrophoresis, 2007, 28, 2201–2215 CrossRef CAS; (e) M. Cuccurullo, G. Schlosser, G. Cacace, L. Malorn and G. Pocsfalvi, J. Mass Spectrom., 2007, 42, 1069–1078 CrossRef CAS.
- R. G. Pearson, J. Chem. Educ., 1968, 45, 581 CrossRef CAS.
- D. C. Hoth, J. G. Rivera and L. A. Colón, J. Chromatogr., A, 2005, 1079, 392–396 CrossRef CAS.
- F. A. Cotton and G. Wilkinson, Advanced Inorganic Chemistry, John Wiley & Sons, New York, 1988, p. 777 Search PubMed.
- B. R. Dumas, G. Brignon, F. Grosclaude and J.-C. Mercier, Eur. J. Biochem., 1972, 25, 505–514 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details on the synthesis of the hafnia; procedure for isolation/enrichment and elution of the phosphopeptides; and MS/MS spectra for the peak with m/z 1560 and 1600. See DOI: 10.1039/b813162g |
| This journal is © The Royal Society of Chemistry 2009 |
