Surface photochemistry of the herbicide napropamide. The role of the media and environmental factors in directing the fates of intermediates†
José P.
Da Silva
*a,
Edgar V.
Bastos
a,
Luis F. V.
Ferreira
b and
Richard G.
Weiss
c
aFCT, Universidade do Algarve, Campus de Gambelas, 8005-139, Faro, Portugal. E-mail: jpsilva@ualg.pt; Fax: +351 289 800 066; Tel: +351 289 800 900, ext. 7644
bCentro de Química-Física Molecular, Instituto Superior Técnico, 1049-001, Lisboa, Portugal
cDepartment of Chemistry, Georgetown University, Washington, DC 20057-1227, USA
First published on 31st October 2007
Abstract
The photochemical behaviour of the herbicide napropamide is studied on cellulose and silica surfaces, using steady-state and laser-flash diffuse reflectance techniques. The results are used to probe how the reaction sites of the host matrices influence the photo-reactive pathways. Napropamide undergoes reaction when irradiated with UV (lamps) or visible (sunlight) radiation on both solid supports. The nature of the intermediates and final products depend strongly on the presence or absence of molecular oxygen. The triplet state of napropamide adsorbed on cellulose is detected by both time-resolved luminescence and transient absorption spectroscopies. The triplet sate was not observed on silica, but transients which include the participation of molecular oxygen are detected during flash photolysis studies. The keto intermediates of the photo-Claisen rearrangement products are observed on both solids. Substituted 1-naphthols from photo-Claisen reactions and 1-naphthol are among the main reaction products. 1,4-Naphthoquinone is a major photoproduct in the presence of molecular oxygen and is expected to be prevalent when napropamide undergoes photodegradation in the environment (i.e., after being applied to plants and fields).
1. Introduction
Photochemical transformations are a principal means by which organic molecules such as herbicides and pesticides are removed from the environment.1 There are, however, literally hundreds of chemically different organic xenobiotics and they are dispersed on many diverse types of natural surfaces. Thus, it is difficult to determine how a specific molecule is degraded photochemically in nature because there may be several distinct routes which depend on specific environmental conditions. Fortunately, examination of the photochemistry of one specific chromophoric group common to several herbicides and pesticides inside or on the surfaces of representative media which approximate those in natural environments can serve as a model for the family. Napropamide [2-(α-naphthoxy)–N,N-diethylpropionamide] (Fig. 1) is such a herbicide and naphthyl is such a chromophore.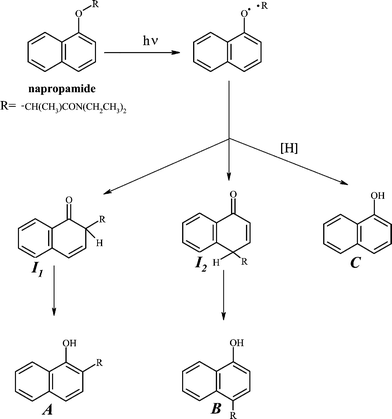 | ||
| Fig. 1 Simplified scheme for photo-Claisen reactions of napropamide. | ||
Napropamide has been reported to undergo photo-Claisen reactions in aqueous media.2,3 Photo-Claisen reactions of 1-naphthyl alkyl ethers are known to proceed mainly from their excited singlet states via homolytic scission of the C–O bond between the naphthoxy and alkyl moieties (Fig. 1).4 The 1-naphthoxy and alkyl geminate radical pairs thus formed undergo cage recombination, leading to the Claisen products, after tautomerization of the initial keto intermediates (I11 and I22, Fig. 1). The fraction of radical pairs that undergoes cage-escape usually results in the formation of 1-naphthol if the medium contains abstractable H-atoms.5 Although H-atom abstractions are slightly endoergonic from most of the C–H bonds in a polymer such as cellulose, they can occur when the naphthoxy radicals live for a long time and because there exist many ‘defect’ sites in this complex polymer that afford more attractive C–H bonds as H-atom donors. Additionally, when molecules of napropamide are either aggregated or in high local concentration, bimolecular formation of naphthol, involving abstraction of an H-atom from one molecule by a naphthoxy fragment of another, can become important. Regardless, the fate of the radical pairs is sensitive to the nature of their immediate environment, and that sensitivity can be monitored by the distributions of photoproducts obtained.4 Thus, the excited states and other intermediates involved in the photo-Claisen rearrangements (and related photo-Fries rearrangements of aromatic esters) are very useful in assessing the influences of different reaction environments on molecular motions over short distances.4–11)
The specific photo-Claisen products from napropamide are N,N-diethyl-2-(1-hydroxynaphthalene-2-yl)propionamide (A) and N,N-diethyl-2-(4-hydroxynaphthalene-1-yl)propionamide (B), as well as the cage-escape product, 1-naphthol (C). Although the photoreactions of napropamide have been examined on soil surfaces,12,13 we are unaware of any spectroscopic or photoproduct distribution studies employing it at solid–gas interfaces.
Here, we examine both the nature of the transients and the distributions of the photoproducts from napropamide adsorbed on the solid surface of silica and at two locations on cellulose. This study provides mechanistic information about the interactions between napropamide and its intermediates and these solid surfaces, and insights into how napropamide and other systemic herbicides and pesticides with similar structures14 are degraded by solar radiation when they are applied to soils and plants.
2. Experimental
2.1 Materials
Napropamide (Chem Service, 99.0% (GC)), 1,4-naphthoquinone (Fluka, ≥99% (UV), 1-naphthol (Riedel-de Haën, 99.8% (GC)), microcrystalline cellulose powder DSO (Fluka, ≤5% loss on drying at 100 °C for 24 h; additive free according to supplier), silica gel 60 (60 Å pore size, Fluka, 6% mass loss after 5 h at 130 °C), methanol and acetonitrile (Merck Lichrosolv), and hexane (Merck, p.a.) were used without further treatment. Water was deionized and distilled.2.2 Sample preparations
The samples (2.5, 25, 50, 100 and 250 µmol napropamide g–1 support) were prepared using a solvent evaporation method.15 Adsorption of napropamide onto unactivated and activated (130 °C, 5 h) silica was achieved by adding the support to a hexane solution of napropamide and allowing them to equilibrate for 12 h while being stirred in a closed vessel. The mixtures were then stirred continuously in open vessels until the solvent had evaporated. Samples on cellulose were prepared in the same way, but using methanol as the solvent. The final concentration of napropamide was determined by extracting a known weight of sample with a known volume of methanol, followed by centrifugation, and comparing their HPLC chromatograms (relative peak areas) with those of standard solutions. This extraction procedure recovered 100% (within the experimental error) of the adsorbed napropamide based on the weights of the mixed species.Washed cellulose samples (to remove napropamide at external surfaces) were also prepared and analysed. Typically, 50 mg of sample was mixed with 5 mL of hexane and allowed to equilibrate during 1–2 min. The mixture was then filtered and the solvent was evaporated. This process resulted in the removal of 10–15% of the napropamide at 50 µmol g–1 initial loading.
2.3 Methods
After irradiation, a weighed amount (10–15 mg) of sample irradiated within a 1 cm2 area was placed in 1 mL of methanol, the mixture was shaken vigorously, and the solid was allowed to settle. The liquid was then analyzed by HPLC.
The amounts of napropamide and 1,4-naphthoquinone were determined by comparing the peak areas from injections of a known amount of the methanol extract with those from a calibration curve obtained with standard solutions. The concentrations of the other photoproducts detected at 300 nm were estimated by assuming that their extinction coefficients are that of 1-naphthol at this wavelength. The reported uncertainties are the standard deviations calculated for more than five measurements. The quantifications were performed only on samples irradiated in air equilibrated conditions. Analyses of silica and cellulose samples kept in the dark for more than three months showed no sign of napropamide degradation. Extracts of non-irradiated and irradiated samples were also analyzed by GC-MS.
Details about the equipment used for ground state absorbance and calculation of the ground state absorbance spectra, the HPLC and GC-MS instruments and the conditions of their operation, and the laser apparatus are described in the ESI.†
3. Results and discussion
3.1 UV-vis absorption spectra
Napropamide and its aromatic photoproducts are able to undergo direct phototransformation in nature because their absorption spectra overlap partially the solar radiation striking the Earth's surface near sea level (> 290 nm1). The absorption spectra of napropamide on both silica and cellulose are similar to those obtained in solution (Fig. 2). The band between 250 and 330 nm corresponds to a π → π* transition of the naphthoxy group. Photoreaction increases the absorbance between 250 nm and ca. 500 nm, indicating the formation of new products (Fig. 2, spectrum 3), some of which have higher molar extinction coefficients than the herbicide itself.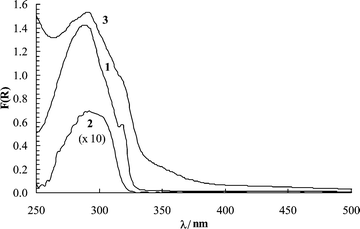 | ||
| Fig. 2 Remission function spectra at room temperature of non-irradiated napropamide on silica (1, 50 µmol g–1) and on cellulose (2, 2.5 µmol g–1) and irradiated in air on silica (3, 50 µmol g–1) for 1 min at 254 nm, 1 cm from the lamp surface. | ||
3.2 Time-resolved luminescence and transient absorption studies
Time-resolved luminescence spectra of napropamide on cellulose in air consisted of a broad band centred at about 520 nm that decays in the millisecond time range (Fig. 3). The emission spectra are similar to those reported by Pulgarín et al.19 and are assigned to napropamide phosphorescence. Room temperature phosphorescence (RTP) has been observed for several molecules adsorbed on cellulose, and it is a well-documented phenomenon, especially when the delivery agent for adsorption is a hydroxylic solvent that swells the polymer and allows the guest molecules to be placed well below the polymer surfaces.20 The probe molecules become entrapped between polymer chains, in a rigid environment that is somewhat protected from molecular oxygen. The slowed diffusion of oxygen to the sites where the molecules in their triplet states reside allows phosphorescence to be observed.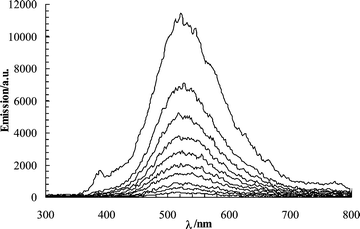 | ||
| Fig. 3 Emission spectra of napropamide on cellulose (50 µmol g–1) in air after pulsed laser excitation (337 nm, ∼1 mJ pulse–1, 600 ps pulse width, 500 ns gate width). Spectra were recorded at delays of 1.0, 3.0, 5.0, 7.0, 9.0, 11.0, 13.0, 15.0, 17.0, and 19.0 ms (from top to bottom) after the laser pulse. | ||
The triplet state of napropamide adsorbed on cellulose was also detected by transient absorption spectroscopy. The triplet–triplet absorption of similar naphthoxy derivatives has been reported to have a maximum near 430 nm.20–23 Naphthyl acetates show a somewhat lower absorption maximum at 417 nm.5 The transient absorption spectrum of napropamide on cellulose obtained at pulse end (∼20 ns) shows a sharp absorption band at 340 nm and the expected triplet–triplet absorption of napropamide centered around 425 nm (Fig. 4a). After 20 ms, the absorbance at 340 nm decreased while the one at 425 nm nearly vanished.
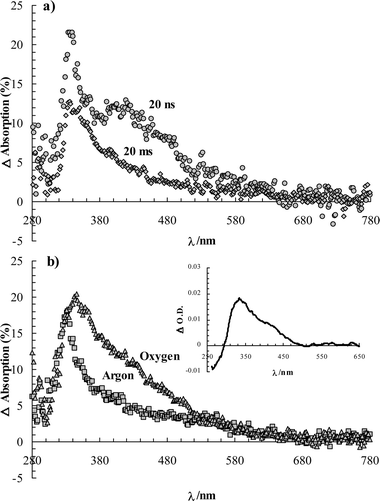 | ||
| Fig. 4 Transient absorption of napropamide (a) on cellulose (50 µmol g–1) in air-equilibrated conditions at ∼20 ns and 20 ms after a laser pulse, and (b) on silica (50 µmol g–1) under argon and oxygen atmospheres at ∼20 ns after the laser pulse (266 nm excitation, 6 ns FWHM, 30 mJ pulse–1, 500 ns gate width). The inset shows the transient absorption obtained for napropamide in and air-equilibrated aqueous solution at pulse end (O.D. ∼ 0.4, 266 nm, 30 mJ pulse–1, 500 ns gate width). | ||
By contrast, the characteristic triplet–triplet absorbance of the naphthoxy moiety was not detected on silica, even when the pulsed excitation was performed on a sample under argon atmosphere (Fig. 4b). Failure to observe the napropamide triplet state on silica (even under an argon atmosphere) indicates that factors besides the presence or absence of oxygen determine the stability of the triplet states and the efficiency of their emission.
The absorption maximum at 390 nm and a low intensity absorption band between 400 and 650 nm, expected for a 1-naphthoxy radical,24,25 were not apparent in transient absorption spectra from samples with cellulose or silica supports. The lifetime of the geminal radical pairs from lysis of napropamide may be too short to be detected after the ca. 20 ns of our laser pulses—1-naphthoxy/acetyl radical pairs combine in acetonitrile at room temperature in less than 1 ns26—or to the presence of other transients absorbing in the same spectral region.
For comparison purposes, the transient absorption of napropamide was also studied in aqueous solution (inset of Fig. 4b). As mentioned, formation of the photo-Claisen products involves recombination of the radical pair, leading initially to intermediates I11 and I22 (Fig. 1). The tautomerization of these keto intermediates to the isolated ‘enolic’ forms, A and B, in solution occurs over periods of microseconds to seconds, depending upon the availability of acid or base catalysts,11 The keto intermediates exhibit an absorption band near 320 nm.26 On this basis, we assign the spectral feature with a maximum at 330 nm to compounds I11 and I22. The negative absorption change at shorter wavelengths is due to depletion of napropamide. The absorptions between 320 and 360 nm observed on silica and cellulose must be due to the keto intermediates as well as photoproducts A and B, especially at longer times (See Figure S-1†). On the silica surface, the absorbance increases between 330 and 530 nm under air and oxygen atmospheres (Fig. 4b). It is ascribed to transients from reaction of naphthoxy radicals and molecular oxygen (vide infra).
3.3 Photoconversion and photoproducts
The direct conversion of napropamide to photoproducts occurs upon irradiation at 254 nm and also in sunlight (Table 1). The relative phototransformation rates are somewhat higher on silica than on cellulose, and they decrease slightly with increasing surface coverage in the range from 2.5 to 250 µmol g–1. The lower rates on cellulose may be due to absorption of some radiation by the support itself15,20 and to the availability of additional reaction pathways on silica involving molecular oxygen. However, similar photodegradation rates were found on unactivated (i.e., as received and, therefore, hydrated) and activated (i.e., preheated at 130 °C for 5 h to remove water and some hydroxyl surface sites) silica.| Silica a | Cellulose a,b | |||||||
|---|---|---|---|---|---|---|---|---|
| Initial concentration/µmol g–1 | 48.9 ± 2.2 | 48.4 ± 2.5 | ||||||
| Radiation | 254 nm | Sunlight | 254 nm | Sunlight | ||||
| a One standard deviation. b Samples prepared using methanol. c 1–3 PM | ||||||||
| Irradiation time | 5 min | 14 h | 0.5 hc | 1 day | 5 min | 14 h | 0.5 hc | 1 day |
| Final concentration/µmol g–1 | 40.5 ± 2.3 | 18.6 ± 2.5 | 35.2 ± 1.8 | 2.8 ± 2.3 | 43.3 ± 2.5 | 22.5 ± 1.5 | 37.3 ± 2.2 | 21.9 ± 2.9 |
The distributions of photoproducts from napropamide were first studied in aqueous solutions. The major products detected, A, B, and naphthol (C) (Fig. 3), are consistent with previous reports2,3 and account for nearly 100% of the consumed napropamide after ca. 15% conversion. At higher conversions, the initial photoproducts undergo secondary reactions, leading to unidentified species, and the mass balance becomes poorer.
At very low photoconversions (<5%) on cellulose and silica, the photoproducts, A, B, naphthol (C) and naphthoquinone (D) (Fig. 5), account for 60–75% of the consumed napropamide after irradiation at 254 nm or in sunlight. At higher conversions, the mass balance becomes poorer, indicating (as in aqueous media) that the initial photoproducts undergo efficient secondary reactions. The presence of photoproducts covalently bonded to the cellulose matrix especially,16,17,27 may contribute to the lack of a good mass balance. However, the absorption spectra of the extracted samples after irradiation are <5% as intense at all wavelengths than before extraction.
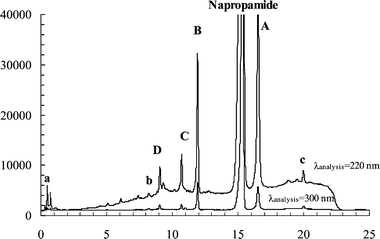 | ||
| Fig. 5 HPLC chromatograms at 220 and 300 nm as the detector wavelengths of the extracted product mixture from an irradiated (254 nm) sample of napropamide on silica (50 µmol g–1). Several unidentified minor products are apparent. The conversion of napropamide was ∼15%; peaks a, b, and c are unidentified products. | ||
The keto intermediates in Fig. 1 are known to be photochemically labile.26 The slower is their tautomerism, the more likely they can absorb a photon and undergo a secondary photochemical reaction. In fact, α-cleavage of the keto intermediates in the photo-Fries and photo-Claisen rearrangements to form dienic ketenes has been documented by flash photolysis studies.28 Thus, stabilization of the keto intermediates of napropamide on cellulose or silica may increase the fraction of reaction that does not yield eventually one of the classic photo-Claisen products (and account for the decreases in the mass balances with increasing conversion). HPLC analyses at 220 nm (Fig. 5) and other detection wavelengths of the extracts from napropamide irradiated on both solid supports indicate the formation of products containing only one aromatic ring (i.e., absorbing at 220 nm, but not at >300 nm; see Fig. S-2†). This observation is consistent with there being secondary photoreactions of the initially formed (major) photoproducts.
On cellulose, the photoproduct distributions are similar at napropamide loadings between 2.5 µmol g–1 and 250 µmol g–1. On silica, increased loadings result in higher relative yields of product c (see Fig. 5 and Fig. S-2†). A possible explanation for this observation is that formation of product c involves a bimolecular process. A photoproduct with a high retention volume, assigned to a dimer of product A2, was previously reported from irradiations of napropamide in aqueous solutions. In support of this contention, the photoproduct distributions are similar when irradiations are conducted on activated or unactivated silica.
Products A and B were identified by comparison with their reported spectra while the structure for C was deduced from comparison of its properties with those of an authentic sample. An oxidation product, 1,4-naphthoquinone (D), was observed after irradiations on silica in an air atmosphere. Its structure was established by comparison of its HPLC retention volume and MS spectrum with those of an authentic sample. Irradiations of cellulose samples in the presence of oxygen also lead to D, but in lower relative yields. D is not detected after irradiations under an argon atmosphere on both solid surfaces. The presence of D also contributes to the aforementioned increased absorption of the irradiated samples observed in spectrum 3 of Fig. 2.
3.4 Role of environmental factors in the reaction pathways
The photochemical behaviour of napropamide on the solid surfaces is similar in some respects (and very different in others) to that reported in aqueous solutions. The formation of the Claisen products and 1-naphthol clearly indicate that the primary photoreaction step on the solid supports is homolytic cleavage of the napthoxy-alkyl C–O bond of napropamide (Fig. 1). The photoproduct distributions shown in Table 2 indicate that the local motions of the transients within the sites afforded by cellulose do not differ significantly from those in aqueous solutions. A major difference, in the enhanced yields of 1-naphthol, was expected because cellulose is known to be a good H-atom donor.5,15–18,20 The presence of some 1-naphthol upon irradiations of napropamide in water and on silica, two environments that lack easily abstractable H-atom sources, and the significantly less than 100% mass balance at even low conversions on cellulose and silica suggest that other reaction pathways for the formation of this compound can occur both in aqueous solution and on the surfaces studied here. Another source of easily abstractable H-atoms is napropamide itself, as well as other radicals or transients (such as the keto intermediates). H-abstraction by 1-naphthoxy radicals from napropamide molecules and species derived from it on silica and cellulose may account for the less than 100% mass balances for the products in Fig. 1 as reported in Table 1. However, we have not been able to detect the napropamide-like species which should be present if such processes are important. As found during photo-Claisen rearrangements of benzyl 1-naphthyl ether in another polymeric material, polyethylene films,4 the reaction sites afforded by cellulose appear to allow significant local motions by the napropamide radical pairs29; however, the high viscosity of the cellulose medium should retard processes that depend on bulk diffusion of large fragments from napropamide. Very different results have been observed when a matrix is rigid.9,10 In fact, the highest selectivity has been observed when the reaction sites possess cationic centers, suggesting that electrostatic interactions may be important, if available9| Medium | Irradiation | Conversion | Photoproduct distribution | |||
|---|---|---|---|---|---|---|
| A | B b , c | C b , c | D b , c | |||
| a Samples prepared using methanol. b Relative to product A. c One standard deviation. | ||||||
| Water | 254 nm | ∼15% | 1.00 | 0.45 ± 0.05 | 0.10 ± 0.05 | — |
| Cellulose a | 254 nm | <5% | 1.00 | 0.60 ± 0.10 | 0.40 ± 0.10 | 0.50 ± 0.15 |
| Cellulose a | >290 nm | <5% | 1.00 | 0.80 ± 0.10 | 0.35 ± 0.10 | 1.30 ± 0.15 |
| Silica | 254 nm | <5% | 1.00 | 0.75 ± 0.10 | 0.10 ± 0.05 | 0.90 ± 0.15 |
| Silica | >290 nm | <5% | 1.00 | 0.60 ± 0.10 | 0.10 ± 0.05 | 1.95 ± 0.20 |
Similar behaviour was found on silica. The results suggest that a geminal radical pair on this solid support is not impeded from undergoing the rotational and short-distance translational motions that lead to formation of the keto tautomers of products A and B, but as with cellulose, processes that depend on translational diffusion of the napropamide fragments over long distances should not be important. The higher local mobility expected at sites on silica is also consistent with the failure to detect the triplet state of napropamide molecules adsorbed on its surface even under an argon atmosphere; deactivation processes can compete efficiently with intersystem crossing by napropamide singlets and subsequent emission from the triplets. The formation of product c only on silica is also in agreement with the possibility of additional reaction channels for the transients on silica surfaces.
The formation of 1,4-naphthoquinone from irradiations on cellulose in air, although in lower yield than on silica, was somewhat unexpected based on the time-resolved luminescence spectra. However, this result is more reasonable if one considers that some napropamide molecules reside at or very near the surfaces of cellulose matrices and others are buried within the matrix. Napropamide molecules at sites near the surfaces are much more exposed to molecular oxygen, and their intermediates can lead to formation of 1,4-naphthoquinone. Based on Beer’s law, electronic excitation (and, therefore, reaction) of napropamide molecules in these sites is more probable than in deeper, more constrained sites. Thus, the relative yield of 1,4-naphthoquinone on cellulose is expected to be (and is) highest at the lowest conversions: at ∼5% conversion, the A/D product ratio is one order of magnitude lower than at 25% conversion. To determine the role of non-entrapped napropamide molecules on the formation of product D, we irradiated a cellulose sample whose surfaces had been washed with hexane, a solvent which is unable to penetrate into the interior of bulk cellulose samples,20 but is able to remove surface-adsorbed napropamide molecules. As expected, only traces of product D were detected after irradiation of these samples.
Based upon these observations, the nature of the initial photoproducts from herbicides and pesticides is expected to be strongly dependent on their ability (as well as that of their intermediates) to diffuse on or to an air–solid surface (such as soil or a leaf). The diffusion of systemic herbicides and pesticides (structurally related to napropamide) to locations below plant surfaces should decrease the ability of molecular oxygen to come into contact with 1-naphthoxy radical intermediates before they follow a different reaction course and, thereby, decrease the yield of 1,4-naphthoquinone.
The formation of 1,4-naphthoquinone was also observed when 1-methoxynaphthalene was irradiated on a silica surface;30 it was attributed to reaction of a ground-state molecule with singlet oxygen. Radicals, such as 1-naphthoxy, are also known to be very reactive towards molecular oxygen.31 It is, therefore, likely that the formation of 1,4-naphthoquinone results from reaction of molecular oxygen with the naphthoxy radical or with 1-naphthol itself.32 The transformation of the oxygen adducts to yield 1,4-naphthoquinone can occur via several plausible routes.32
4. Conclusions
When irradiated by UV lamps or in sunlight on cellulose and silica supports, the herbicide napropamide undergoes interesting phototransformations. Homolytic cleavage of the 1-napthoxy-alkyl C–O bond, leading to formation of photo-Claisen products and 1-naphthol, is a major reaction pathway. In the presence of molecular oxygen, 1,4-naphthoquinone becomes a major product. Silica and cellulose restrict longer translational motions of napropamide and its fragments but allow the photochemically-generated radical pairs to move in a relatively unencumbered fashion within a reaction site; the rotational and diffusional motions within a reaction site are limited to a few Å. The constrained (sub-bulk surface) reaction sites supplied by cellulose upon entrapment of napropamide retard contact between molecular oxygen and either the excited states of napropamide or its radical intermediates and, thus, the formation of 1,4-naphthoquinone.These results provide valuable insights into how napropamide and other α-naphtoxy herbicides and pesticides are transformed by sunlight after their deposition on natural surfaces. Based upon our observations, the diffusion of systemic herbicides and pesticides into the interior regions of plants after their deposition on surfaces lowers the ability of molecular oxygen to come into contact with 1-naphthoxy radical intermediates and, therefore, should decrease the amount of the initial material which is converted to 1,4-naphthoquinone. To confirm this hypothesis, other model surfaces need to be explored. Thus, we intend to investigate the photochemical behaviour of napropamide and its intermediates on ca. surfaces with cationic centers,9 since they are more similar than silica to the environments found in soil.
Acknowledgements
J. P. S. thanks FCT for a Post-Doctoral fellowship SFRH/BPD/15589/2001 and R. G. W. thanks the U.S. National Science Foundation for financial support.References
- A. Leifer, The Kinetics of Environmental Aquatic Photochemistry, Theory and Practice ACS Professional Reference Book, Maple Press Company, York, PA, 1988 Search PubMed.
- L. L. Chang, B. Y. Giang, K.-S. Lee and C. K. Tseng, Aqueous photolysis of napropamide, J. Agric. Food Chem., 1991, 39, 617–621 CrossRef CAS.
- J. P. Aguer, P. Boule, F. Bonnemoy and J. M. Chezal, Phototransformation of napropamide [N,N-diethyl-2-(1naphthyloxy)propionamide] in aqueous solution: influence of the toxicity of solutions, Pestic. Sci., 1998, 54, 253–257 CrossRef CAS.
- W. Q. Gu, M. Warrier, B. Schoon, V. Ramamurthy and R. G. Weiss, Understanding the influence of active (Zeolite) and passive (polyethylene) reaction cages on photo-Claisen rearrangements of aryl benzyl ethers, Langmuir, 2000, 16, 6977–6981 CrossRef CAS.
- W. Gu, S. Bi and R. G. Weiss, Photo-Fries rearrangements of 1-naphthyl esters in the glassy and melted states of poly(vinyl acetate). Comparisons with reactions in less polar polymers and low-viscosity solvents, Photochem. Photobiol. Sci., 2002, 1, 52–59 RSC.
- M. S. Syamala and V. Ramamurthy, Modification of photochemical reactivity by cyclodextrin complexation-selectivity in photo-Claisen rearrangement, Tetrahedron, 1988, 44, 7223–7233 CrossRef CAS.
- K. Pitchumani, M. Warrier and V. Ramamurthy, Remarkable product selectivity during photo-fries and photo-claisen rearrangements within zeolites, J. Am. Chem. Soc., 1996, 118, 9428–9429 CrossRef CAS.
- I. F. Molokov, Y. P. Tsentalovich, A. V. Yurkovskaya and R. Z. Sagdeev, Investigation of the photo-Fries rearrangement reactions of 1- and 2-naphthyl acetates, J. Photochem. Photobiol., A, 1997, 110, 159–165 CrossRef CAS.
- W. Q. Gu, M. Warrier, V. Ramamurthy and R. G. Weiss, Photo-Fries reactions of 1-naphthyl esters in cation-exchanged zeolite Y and polyethylene media, J. Am. Chem. Soc., 1999, 121, 9467–9468 CrossRef CAS.
- S. Koodanjeri, A. R. Pradhan, L. S. Kaanumalle and V. Ramamurthy, Cyclodextrin-mediated regioselective photo-Fries reaction of 1-naphthyl phenyl acylates, Tetrahedron Lett., 2003, 44, 3207–3210 CrossRef.
- J. G. Xu and R. G. Weiss, Combinations of chiral and prochiral singlet radical pairs in reaction cavities of polyethylene films. Control and analysis of radical tumbling and translation, Photochem. Photobiol. Sci., 2005, 4, 348–358 RSC.
- S. G. Donaldson and G. C. Miller, Coupled transport and photoreaction of napropamide in soils undergoing evaporation from a shalow water table, Environ. Sci. Technol., 1996, 30, 924–930 CrossRef CAS.
- J. P. Aguer, L. Cox, C. Richard, M. C. Hermosín and J. Cornejo, Sorption and photolysis studies in soil and sediment of the herbicide napropamide, J. Environ. Sci. Health, Part B, 2000, 35, 725–738 CrossRef CAS.
- C. Tomlin, The Pesticide Manual, Incorporating the Agrochemicals Handbook, 10th edn, British Crop Protection Council Publ., Surrey, U.K., 1994 Search PubMed.
- A. M. Botelho do Rego, L. F. Vieira Ferreira, Photonic and electronic spectroscopies for the characterization of organic surfaces and organic molecules adsorbed on surfaces, In: Handbook of Surfaces and Interfaces of Materials, Ed. H. S. Nalwa;, Academic Press, New York, 2001 Search PubMed.
- L. F. Vieira Ferreira, J. P. Da Silva, I. Ferreira Machado, T. J. F. Branco and J. C. Moreira, Surface photochemistry: dibenzo-p-dioxin adsorbed onto silicalite, cellulose and silica, J. Photochem. Photobiol., A, 2007, 186, 254–262 CrossRef CAS.
- J. P. Da Silva, L. F. Vieira Ferreira, A. M. Da Silva and A. S. Oliveira, Photochemistry of 4-chlorophenol on cellulose and silica, Environ. Sci. Technol., 2003, 37, 4798–4803 CrossRef CAS.
- J. P. Da Silva and L. F. Vieira Ferreira, Surface photochemistry of pesticides: An approach using diffuse reflectance and chromatography techniques, Environ. Sci. Technol., 2004, 38, 2849–2856 CrossRef CAS.
- J. A. M. Pulgarín and L. F. G. Bermejo, Determination of the pesticide napropamide in soil, pepper and tomato by micelle-stabilized room-temperature phosphorescence, J. Agric. Food Chem., 2002, 50, 1002–1008 CrossRef.
- L. F. Vieira Ferreira, J. C. Netto-Ferreira, I. V. Khmelinskii, A. R. Garcia and S. M. B. Costa, Photochemistry on surfaces: matrix isolation mechanisms for study of interactions of benzophenone adsorbed on microcrystalline cellulose investigated by diffuse reflectance and luminescence techniques, Langmuir, 1995, 11, 231–236 CrossRef CAS.
- K. Hara and K. Baba, Photodissociation of α-naphthol in solution: influence of hydrogen bonding, J. Chem. Soc., Faraday Trans. 2, 1975, 71, 1100–1108 RSC.
- H. Shizuka, H. Hagiwara and M. Fukushima, Laser flash photolysis study of the hydrogen atom transfer reaction from triplet 1-naphthol to ground benzophenone, J. Am. Chem. Soc., 1985, 107, 7816–7823 CrossRef CAS.
- M. Yamaji, T. Sekiguchi, M. Hoshino and H. Shizuka, Proton induced electron transfer reaction from triplet methoxynaphthalenes to benzophenone via triplet exciplex, J. Phys. Chem., 1992, 96, 9353–9359 CrossRef CAS.
- M. Lukeman, D. Veale, P. Wan, V. R. N. Munasinghe and J. E. T. Corrie, Photogeneration of 1,5-naphthoquinone methides via excited-state (formal) intramolecular proton transfer and photodehydration of 1-naphthol derivatives in aqueous solution, Can. J. Chem., 2004, 82, 240–253 CrossRef CAS.
- P. K. Das, M. V. Encinas, S. Steenken and J. C. Scaiano, Reaction of tert-butoxy radicals with phenols. Comparison with reactions of carbonyl triplets, J. Am. Chem. Soc., 1981, 103, 4162–4166 CrossRef CAS.
- T. Mori, M. Takamoto, H. Saito, T. Furo, T. Wada and Y. Inoue, Isolation of cyclohexadienone intermediates in the photo-Fries rearrangement of 2,4-dimethylnaphth-1-yl and 2,4-dimethylnaphth-2-yl 2,4,6-trimethylbenzoates, Chem. Lett., 2004, 33, 254–255 CrossRef CAS.
- W. D. Burgos, J. T. Novak and D. F. Berry, Reversible sorption and irreversible binding of α-naphthol to soil: elucidation of processes, Environ. Sci. Technol., 1996, 30, 1205–1211 CrossRef CAS.
- M. C. Jiménez, M. A. Miranda, J. C. Scaiano and R. Tormos, Two-photon processes on the photo-Caisen and photo-Fries rearrangements. Direct observation of dienic ketenes generated by photolysis of transient cyclohexa-2,4-dienones, Chem. Commun., 1997, 1487–1488 RSC.
- R. G. Weiss, V. Ramamurthy and G. S. Hammond, Photochemistry in Organized and Confining Media: A Model, Acc. Chem. Res., 1993, 26, 530–536 CrossRef CAS.
- M. E. Sigman, J. T. Barbas, E. A. Chevis and R. Dabestani, Spectroscopy and photochemistry of 1-methoxynaphthalene on SiO2, New J. Chem., 1996, 20, 243–248 Search PubMed.
- B. Maillard, K. U. Ingold and J. C. Scaiano, Rate constants for the reactions of free radicals with oxygen in solution, J. Am. Chem. Soc., 1983, 105, 5095–5099 CrossRef CAS.
- O. Suchard, R. Kane, B. J. Roe, E. Zimmerman, C. Jung, P. A. Waske, J. Mattay and M. Oelgemöller, Photooxygenations of 1-naphthols: an environmentally friendly access to 1,4-naphthoquinones, Tetrahedron, 2006, 62, 1467–1473 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and product spectra. See DOI: 10.1039/b713369c |
| This journal is © The Royal Society of Chemistry and Owner Societies 2008 |
