Efficiencies of singlet oxygen production and rate constants for oxygen quenching in the S1 state of dicyanonaphthalenes and related compounds
Fujio
Tanaka
*a,
Kazuyuki
Tsumura
a,
Tomoaki
Furuta
a,
Kenichi
Iwamoto
a and
Masami
Okamoto
b
aDepartment of Chemistry, Graduate School of Science, Osaka Prefecture University, Gakuen-cho, Naka-ku, Sakai, Osaka, 599-8531, Japan. E-mail: fuji@c.s.osakafu-u.ac.jp
bFaculty of Technology and Design, Kyoto Institute of Technology, Matsugasaki, Sakyo-ku, Kyoto, 606-8585, Japan
First published on 29th October 2007
Abstract
The quantum yield of singlet oxygen (1O2 (1Δg)) production (ΦΔ) in the oxygen quenching of photoexcited states for 1,2-dicyanonaphthalene (1,2-DCNN), 1,4-dicyanonaphthalene (1,4-DCNN) and 2,3-dicyanonaphthalene (2,3-DCNN) in cyclohexane, benzene, and acetonitrile was measured using a time-resolved thermal lens (TRTL) technique, in order to determine the efficiency of singlet oxygen (1Δg) production in the first excited singlet state (S1), (fΔS). The efficiencies of singlet oxygen (1Δg) production from the lowest triplet state (T1), (fΔT), were nearly unity for all DCNNs in all the solvents. The values of fΔS were fairly large for 1,2-DCNN (0.33–0.57) and 1,4-DCNN (0.33–0.66), but were close to zero for 2,3-DCNN. Rate constants for oxygen quenching in the S1 state (kqS) obtained for these compounds were significantly smaller than diffusion-controlled rate constants. The kinetics for processes leading to production and no production of singlet oxygen is discussed on the basis of the values of fΔS and kqS. The results obtained regarding phenanthrene (PH), 9-cyanophenanthrene (9-CNPH), pyrene (PY) and 1-cyanopyrene (1-CNPY) are also discussed.
Introduction
The quenching of photoexcited molecules by oxygen and resultant production of singlet oxygen have been long investigated since they are common phenomena for almost all molecules and important in relation to applications such as photooxygenation and photodynamic therapy. Over two decades, the kinetics and mechanism for explaining the rate constant of oxygen quenching and the efficiency of singlet oxygen production have been elucidated fairly well.1–3 However, information on the production of singlet oxygen by the oxygen quenching in the S1 state is still scarce in comparison with that in the lowest excited triplet state (T1). The efficient production of singlet oxygen from the S1 state is rare in contrast to high efficiencies for the T1 state of most organic molecules and, hence, the approach to the kinetics and mechanism based on data of fΔS and kqS is restricted.4–16The three possible processes for the oxygen quenching in the S1 state are given in Scheme 1, in which 3(S1–3O2) represents an encounter complex and kdiff and k-diff are the diffusion-controlled rate constants for encounter and separation of the complex, respectively. The processes (1)–(3) are energy transfer producing T1 and 1O2, intersystem crossing without production of 1O2 and internal conversion producing neither T1 nor 1O2, respectively. kΔ, kST and kSS are the rate constants for the three processes, respectively. The process (1) is energetically possible when the energy gap between S1 and T1 is larger than the state energy of 1O2 (1Δg) (94 kJ mol–1). However, the process (1) is in competition with processes (2) and (3) and the efficiency of production of 1O2 is determined by the amplitude of kΔ relative to kST and kSS. According to data obtained so far, fΔS is zero or a low value even for many organic molecules with a larger S1–T1 energy gap than 94 kJ mol–1 and kqS for these molecules is nearly a diffusion-controlled rate constant (kdiff).9–12 It was found that 9,10-dicyanoanthracene, 9-cyanoanthracene, 9,10-dichloroanthracene and other molecules which had an electron-withdrawing group, i.e., a high oxidation potential, yielded a significant value of fΔS, and kqS for these molecules was distinctly smaller than kdiff.4–9,13–17 This inverse correlation between fΔS and kqS can be qualitatively explained in terms of a proposition that the process (2) is enhanced by charge transfer interaction between the S1 molecule and 3O2.5,9 It is also known that the process (3) becomes open in acetonitrile for molecules with a low oxidation potential owing to enhancement of charge transfer interaction in the polar solvent, whereas this process can be neglected in nonpolar solvents.10–12,18
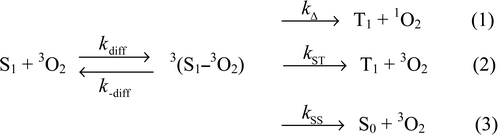 | ||
| Scheme 1 The three possible processes for the oxygen quenching in the S1 state. | ||
As for the oxygen quenching in the T1 state, dependences of rate constants for three processes leading to 1O2 (1Σg), 1O2 (1Δg) and 3O2 (3Σg) on the T1 energy and the charge transfer interaction have been investigated in detail on the basis of data of the efficiencies of 1O2 (1Σg, 1Δg) production and the rate constant of oxygen quenching in the T1 state (kqT).1,2,19 A similar approach based on data of fΔS and kqS for the oxygen quenching in the S1 state was made only for a few cases such as anthracene derivatives.9,13–16 For deep understanding about the elementary processes of (1)–(3), systematic data of fΔS and kqS about more compounds are needed.
The object of this paper is to estimate the rate constants for the three elementary processes of (1)–(3) from values of kqS and fΔS obtained for three DCNNs, 9-CNPH and 1-CNPY, in order to elucidate determining factors for the three rate constants of kΔ, kST and kSS.
Experimental
1,2-DCNN, 1,4-DCNN, 2,3-DCNN, 1,4-dimethylnaphthalene (1,4-DMN) (extra pure grade, Tokyo Kasei), 9-CNPH (guaranteed grade, Tokyo Kasei) and PH (zone refined, Tokyo Kasei) were used as received. PY (Wako Pure Chemicals Ltd.) was purified by column chromatography and recrystallization. 1-CNPY was given by Prof. K. Mizuno of Osaka Prefecture University. Cyclohexane, benzene and acetonitrile of spectroscopic grade (Dojin Kagaku) were used.Absorption and fluorescence spectra were measured with a spectrophotometer (Jasco UV/VIS 660) and a spectrofluorophotometer (Shimadzu RF-5300), respectively. The measured fluorescence spectra were corrected using fluorescence spectra of sulfuric acid solutions of quinine sulfate and 2-aminopyridine used as standard fluorescence solutions.20 The average fluorescence energy (EFav) and the fluorescence quantum yield (ΦF) were determined using the corrected fluorescence spectra. The value of ΦF0 = 1 for 9-cyanoanthracene in the deaerated solution was used as a standard value of ΦF in all the solvents used.9 Triplet energies were obtained from phosphorescence spectra in methanol–ethanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at 77 K in the absence or presence of methyl iodide. The fluorescence lifetime (τF) was measured with a time-correlated single photon counting instrument (Horiba NAES-700). The value of kqS was obtained from the values of τF in deaerated and air-saturated solutions and the concentration of O2 in the air-saturated solution.21
1) at 77 K in the absence or presence of methyl iodide. The fluorescence lifetime (τF) was measured with a time-correlated single photon counting instrument (Horiba NAES-700). The value of kqS was obtained from the values of τF in deaerated and air-saturated solutions and the concentration of O2 in the air-saturated solution.21
The TRTL system used for the determination of ΦΔ was described in a previous paper.9 The quantum yield of triplet state in the absence of oxygen (ΦT0) was also determined by the same TRTL instrument. A nitrogen laser (NDC JH-1000L) and a He–Ne laser (Spectra-Physics 117A) were used as excitation and probe light sources, respectively. The excitation light (337 nm) was focused at the center of a 1 cm square cuvette containing a sample solution. The probe light (632.8 nm) focused in front of the cuvette passed through the sample solution, and the intensity of the probe light passing through a band filter and pinhole (a diameter of 0.5 mm) was detected with a photomultiplier (Hamamatsu R928). The signal from the photomultiplier was fed into the 1 kΩ input of a preamplifier and recorded by a digital oscilloscope (YOKOGAWA DL1520L) with averaging of 256 signals. The concentration of sample solutions was adjusted to the absorbance of 0.30 at 337 nm (path length of 1 cm). In the TRTL measurement, the lens signal composes of a fast-rising component (Uf) and a slow-rising component (Us), as shown in Fig. 1.
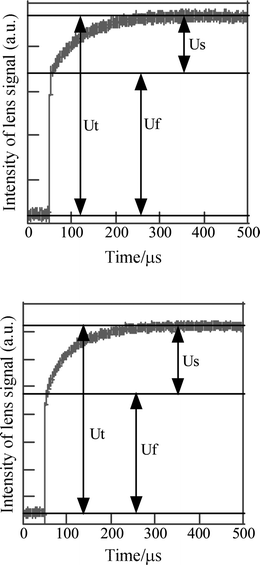 | ||
| Fig. 1 Time-resolved thermal lens signals of 1,4-dicyanonaphthalene in air-saturated acetonitrile (upper) and in deaerated acetonitrile (lower). | ||
The value of ΦΔ for solutions containing oxygen is determined from the ratio of Us to Ut by the following equation,9,22
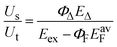 | (4) |
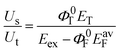 | (5) |
Deaeration of sample solutions was performed by freeze–pump–thaw cycles. Oxygen-rich solutions were prepared by replacing air in the sample cuvette for an adequate time by pure oxygen gas. All measurements were carried out at 25 ± 2 °C.
Results and discussion
Efficiencies of singlet oxygen production (fΔS) and rate constants of oxygen quenching (kqS) for dicyanonaphthalenes
The S1 energy (ES), the T1 energy (ET), the fluorescence quantum yield (ΦF0) and the triplet quantum yield (ΦT0) for three DCNNs are given in Table 1. As seen in Table 1, the energy gap of the S1 and T1 states (ΔES–T) is larger than EΔ (94 kJ mol–1) for all the three DCNNs and, hence, the production of singlet oxygen from the S1 state is energetically possible in the process (1), as well as the production from the T1 state. The sum of the quantum yields of fluorescence and triplet, (ΦF0 + ΦT0), is approximately unity in all the studied systems of compound and solvent, indicating that the S1 state is mainly deactivated by two processes of fluorescence and intersystem crossing.| Compound | Solvent | E S/kJ mol–1 | E T/kJ mol–1 | Φ F 0 | Φ T 0 | τ F 0/ns |
|---|---|---|---|---|---|---|
| a Accurate TRTL measurements on 2,3-DCNN in cyclohexane were impossible owing to low solubility. | ||||||
| 1,2-DCNN | CH | 347 | 226 | 0.36 | 0.57 | 7.80 |
| BZ | 340 | 226 | 0.49 | 0.39 | 9.54 | |
| AC | 337 | 226 | 0.49 | 0.35 | 12.0 | |
| 1,4-DCNN | CH | 355 | 221 | 0.26 | 0.67 | 3.00 |
| BZ | 344 | 221 | 0.63 | 0.24 | 9.25 | |
| AC | 345 | 221 | 0.69 | 0.23 | 9.77 | |
| 2,3-DCNN | CHa | 351 | 246 | 37.5 | ||
| BZ | 348 | 246 | 0.69 | 0.22 | 28.4 | |
| AC | 349 | 246 | 0.76 | 0.17 | 26.3 | |
| PH | CH | 345 | 25721 | 0.10 | 0.89 | 52.6 |
| BZ | 343 | 25721 | 0.17 | 0.87 | 51.6 | |
| AC | 344 | 25721 | 0.13 | 0.86 | 52.6 | |
| 9-CNPH | CH | 335 | 242 | 0.17 | 0.59 | 28.0 |
| BZ | 333 | 242 | 0.29 | 0.55 | 24.4 | |
| AC | 334 | 242 | 0.24 | 0.69 | 23.6 | |
| PY | CH | 322 | 20321 | 0.61 | 0.33 | 417 |
| BZ | 322 | 20321 | 0.69 | 0.30 | 318 | |
| AC | 321 | 20321 | 0.67 | 0.28 | 322 | |
| 1-CNPY | CH | 313 | 194 | 0.77 | 0.13 | 36.2 |
| BZ | 311 | 194 | 0.86 | 0.10 | 19.4 | |
| AC | 312 | 194 | 0.80 | 0.13 | 22.0 |
The measured value of ΦΔ and the efficiencies for 1O2 production in the S1 and T1 states (fΔS and fΔT) are related by the following equation, under the condition that the T1 state is completely quenched by O2,9,13
| ΦΔ(F0/F) = (fΔS + fTOfΔT)[(F0/F) – 1] + fΔTΦT0 | (6) |
| Compound | Solvent | Φ Δ air b | f T O c | f Δ T | f Δ S | 10–9kqS/M–1 s–1 |
|---|---|---|---|---|---|---|
| a Accurate TRTL measurements on 2,3-DCNN in cyclohexane were impossible owing to low solubility. b Values of ΦΔ in air-saturated solutions. c The value of unity was assumed for DCNNs in the three solvents and for other compounds in cyclohexane and benzene. d The average of two reference values. e The value in cyclohexane was used. | ||||||
| 1,2-DCNN | CH | 0.78 | 1 | 0.97 ± 0.02 | 0.57 ± 0.02 | 14 |
| BZ | 0.59 | 1 | 0.99 ± 0.03 | 0.33 ± 0.04 | 15 | |
| AC | 0.72 | 1 | 1.01 ± 0.08 | 0.37 ± 0.10 | 21 | |
| 1,4-DCNN | CH | 0.68 | 1 | 0.91 ± 0.02 | 0.63 ± 0.04 | 12 |
| BZ | 0.52 | 1 | 1.00 ± 0.04 | 0.63 ± 0.05 | 14 | |
| AC | 0.59 | 1 | 0.96 ± 0.13 | 0.66 ± 0.16 | 19 | |
| 2,3-DCNN | CHa | 16 | ||||
| BZ | 0.59 | 1 | 0.96 ± 0.18 | 0.01 ± 0.01 | 16 | |
| AC | 0.64 | 1 | 0.96 ± 0.18 | 0.05 ± 0.05 | 22 | |
| 1,4-DMN | CH | 25 | ||||
| BZ | 30 | |||||
| AC | 36 | |||||
| PH | CH | 0.63 | 1 | 0.65 ± 0.05 | 0 | 24 |
| 0.87 ± 0.111 | 0.54,11 0.6211 | 0.0211 | ||||
| BZ | 0.42 | 1 | 0.46 ± 0.02 | 0 | 27 | |
| AC | 0.38 | 0.96 | 0.47 ± 0.04 | 0 | 35 | |
| 0.96 ± 0.1,12 0.4718 | 0.50 ± 0.0512 | 0.0012 | ||||
| 9-CNPH | CH | 0.87 | 1 | 1.06 ± 0.12 | 0 | 17 |
| BZ | 0.63 | 1 | 0.99 ± 0.13 | 0 | 21 | |
| AC | 0.67 | 0.85 | 0.93 ± 0.11 | 0 | 24 | |
| 0.85 ± 0.1,12 0.8518 | 0.80 ± 0.0812 | 0.04 ± 0.0412 | ||||
| PY | CH | 0.96 | 1 | 0.93d | 0.06 | 26 |
| 1.10 ± 0.111 | 0.86,11 0.9911 | 0.0011 | ||||
| BZ | 0.91 | 1 | 0.93e | 0.02 | 26 | |
| AC | 0.66 | 0.47d | 0.79 ± 0.0812 | 0.31 | 39 | |
| 0.49 ± 0.05,12 0.4518 | 0.30 ± 0.0312 | |||||
| 1-CNPY | CH | 0.82 | 1 | 1.08 ± 0.23 | 0.08 ± 0.08 | 21 |
| BZ | 0.55 | 1 | 1.00 ± 0.20 | 0.06 ± 0.06 | 21 | |
| AC | 0.59 | ≤1 | 1.00 ± 0.31 | ≥0.02 | 31 |
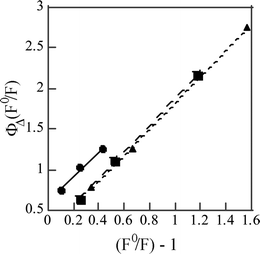 | ||
| Fig. 2 Plots to determine fΔS and fΔT according to eqn (6): 1,4-dicyanonaphthalene in (●) cyclohexane, (■) benzene, (▲) acetonitrile. | ||
As shown in Table 2, values of fΔT for three dicyanonaphthalenes were close to unity in all the solvents used, indicating that 1O2 is produced with an efficiency of about 100% in the oxygen quenching of triplet state. Wilkinson et al. measured values of fΔT for several naphthalene compounds and showed that fΔT increased with increasing oxidation potential. The fΔT values for 1-cyanonaphthalene with high oxidation potential were 1.03 (cyclohexane), 0.75 (benzene) and 0.74 (acetonitrile).24,25 Since the oxidation potential for DCNNs is expected to be higher than that for 1-cyanonaphthalene, the fΔT values close to unity obtained for three DCNNs in all the solvents are reasonable results.
The fΔS values for 1,2-DCNN and 1,4-DCNN are 0.33–0.66, indicating that 1O2 is produced with considerable efficiencies from the S1 state in all the solvents. The kqS values of these compounds are lower by about one-half than those of 1,4-DMN which are considered as nearly diffusion-controlled rate constants (kdiff). This observation represents that kqS is smaller than kdiff when production of 1O2 is significant, in agreement with previous studies on anthracene derivatives.9 The fΔS value of 1,4-DCNN is not decreased in acetonitrile, whereas the remarkable decrease of fΔS was shown in changing solvent from cyclohexane to acetonitrile for 9-cyanoanthracene, 9,10-dicyanoanthracene and 9,10-dichloroanthracene.9,13,14 In contrast to 1,2-DCNN and 1,4-DCNN, the fΔS value of 2,3-DCNN is close to zero in both benzene and acetonitrile, whereas the kqS values of 2,3-DCNN are about one-half of kdiff analogously to 1,2-DCNN and 1,4-DCNN. The results obtained on fΔS and kqS of DCNNs demonstrate that the fΔS value varies largely from 0.01 to 0.66 though the value of (kqS/kdiff) varies slightly from 0.48 to 0.66.
Kinetics for oxygen quenching in the S1 state and rate constants of elementary processes (kΔ, kST, kSS)
Based on the kinetics for the oxygen quenching of the S1 state in Scheme 1, rate constants for elementary processes of (1)–(3) are derived from the values of fΔS and kqS. By assuming the stationary state for the encounter complex, eqn (7) is obtained,9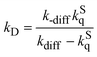 | (7) |
In this work, values of kqS obtained for 1,4-DMN in each solvent are used as kdiff in the corresponding solvents. Values of k-diff are estimated using eqn (8),3,27,28
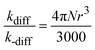 | (8) |
| kΔ = fΔSkD | (9) |
| kST = (fTO – fΔS)kD | (10) |
| kSS = (1 – fTO)kD | (11) |
The values of kΔ and kST in cyclohexane and benzene are obtained using fTO = 1, that is, neglecting kSS. In acetonitrile the value of fTO for DCNNs may be smaller than unity and, indeed, values of fTO measured for several molecules in acetonitrile are in the range from 0.25 to 1.0.12,18 The value of fTO is decreased with increasing charge transfer interaction and it is related to the free energy change (ΔETGS) in the electron transfer from the S1 state to O2 in acetonitrile. The value of ΔETGS is given by eqn (12),
| ΔETGS = F[E(M+˙/M) – E(O2/O2–˙)] – ES | (12) |
| Compound | Solvent | 10–9kdiffa/M–1 s–1 | 10–9k-diffb/s–1 | 10–9kDc/s–1 | 10–9kΔd/s–1 | 10–9kSTe/s–1 | 10–9kSSf/s–1 |
|---|---|---|---|---|---|---|---|
| a Values of kqS for 1,4-DMN, PH and PY were regarded as those of kdiff for corresponding compounds in respective solvents. b Values of k-diff were estimated by eqn (8). c Eqn (7). d Eqn (9). e Eqn (10). f Eqn (11). | |||||||
| 1,2-DCNN | CH | 25 | 75 | 95 | 54 ± 1 | 41 ± 1 | — |
| BZ | 30 | 90 | 90 | 30 ± 3 | 60 ± 3 | — | |
| AC | 36 | 108 | 151 | 56 ± 15 | 95 ± 15 | — | |
| 1,4-DCNN | CH | 25 | 75 | 69 | 43 ± 3 | 26 ± 3 | — |
| BZ | 30 | 90 | 79 | 50 ± 4 | 29 ± 4 | — | |
| AC | 36 | 108 | 121 | 80 ± 19 | 41 ± 19 | — | |
| 2,3-DCNN | CH | 25 | 75 | 133 | |||
| BZ | 30 | 90 | 103 | — | 102 ± 1 | — | |
| AC | 36 | 108 | 170 | — | 161 ± 9 | — | |
| 9-CNPH | CH | 24 | 62 | 150 | — | 150 | — |
| BZ | 27 | 70 | 250 | — | 250 | — | |
| AC | 35 | 91 | 200 | — | 170 | 30 | |
| 1-CNPY | CH | 26 | 68 | 286 | 23 ± 23 | 263 ± 23 | — |
| BZ | 26 | 68 | 286 | 17 ± 17 | 269 ± 17 | — | |
| AC | 39 | 101 | 391 | ≥8 | ≤380 |
As shown in Table 3, the values of kST for 2,3-DCNN are larger than those for 1,2-DCNN and 1,4-DCNN, and the value of kST is increased in acetonitrile for all the DCNNs in comparison with cyclohexane and benzene. This result is attributed to the effect of charge transfer interaction on kST. The oxidation potential of 2,3-DCNN is considered to be lower than those of 1,2-DCNN and 1,4-DCNN, because the reduction potential of 2,3-DCNN (–1.70 V) is lower than those of 1,2-DCNN (–1.36 V) and 1,4-DCNN (–1.31 V).30 Indeed, 2,3-DCNN is a weaker electron acceptor than 1,4-DCNN in the photo-induced electron transfer reaction in the S1 state,31 meaning that 2,3-DCNN is a stronger electron donor than 1,4-DCNN. As a result, the value of ΔETGS for 2,3-DCNN is smaller than for 1,2-DCNN and 1,4-DCNN because values of Es are almost same and, hence, the increased charge transfer interaction leads to larger kST values of 2,3-DCNN, resulting in small values of fΔS. The larger kST values in acetonitrile for all the DCNNs also are explained by increase of charge transfer interaction in a polar solvent.
In the present result, the kΔ value is also an important determining factor for fΔS. The kΔ values of (3.0–8.0) × 1010 s–1 for 1,2-DCNN and 1,4-DCNN are fairly large compared with values of (0.9–1.5) × 1010 s–1 obtained for 9-cyanoanthracene in a previous paper.9 These large kΔ values, together with small kST values, lead to considerably high values of fΔS for 1,2-DCNN and 1,4-DCNN. It is also owing to the increased kΔ value that fΔS value of 1,4-DCNN is not decreased in acetonitrile regardless of the increase of kST. On the other hand, the small fΔS values for 2,3-DCNN are attributed not only to large kST values but also to small kΔ-values. If kΔ values for 2,3-DCNN were similar to those for 1,2-DCNN and 1,4-DCNN, fΔS values of 2,3-DCNN would result in significant values of 0.23–0.33 regardless of large kST values. In practice, however, fΔS values of 2,3-DCNN are nearly zero because of the small kΔ values. Why are kΔ values of 2,3-DCNN smaller than those of 1,2-DCNN and 1,4-DCNN? For 2,3-DCNN, the energy difference between S1 and T1 is larger than 94 kJ mol–1 and the process (1) in Scheme 1 is energetically possible. At present, there is no distinct explanation about the difference of kΔ value between two classes of DCNNs. In the simplified mechanism of Scheme 1, kΔ and kST include rate constants for all kinetic processes which follow the formation of encounter complex, such as the formation of exciplex. Therefore, the process associated with the exciplex might be a cause for the difference in the value of kΔ.
f Δ S, kqS, kΔ, kST and kSS for phenanthrene, pyrene and their cyano-derivatives
In relation to the present results regarding DCNNs, measurements of fΔS and kqS were carried out for 9-CNPH and 1-CNPY in the same three solvents, together with PH and PY. The photophysical parameters are listed in Table 1. The energy gaps between S1 and T1 for PH and 9-CNPH are smaller than the energy of 1O2 (94 kJ mol–1), and, hence, the production of 1O2 in process (1) of Scheme 1 is energetically impossible. For PH, 9-CNPH and 1-CNPY, values of fΔT were obtained from the intercept of plot of eqn (6). The determination of fΔT for PY by eqn (6) was impossible owing to the long fluorescence lifetime of PY, because the plot based on eqn (6) needs measurements at lower concentrations of oxygen but their measurements make obscure the discrimination between a fast rise and a slow rise in the TRTL signal. Therefore, the literature values were used as fΔT for PY. For the determination of fΔS, the value of fTO for the four compounds in cyclohexane and benzene was reasonably taken as unity. The literature values of fTO were used for PH, 9-CNPH, PY in acetonitrile, and the minimum value of fΔS for 1-CNPY in acetonitorile was obtained using fTO = 1 since the value of fTO is unknown.As seen in Table 2, the values of fΔT for PH in cyclohexane and acetonitrile and for 9-CNPH in acetonitrile are nearly equal to those of Abdel et al.11,12 The low fΔT values of 0.46–0.65 for PH are attributed to both high energy of T1 and low oxidation potential.1 The high fΔT values close to unity for 9-CNPH and 1-CNPY are attributed to the high oxidation potential, analogously to DCNNs.
Values of fΔS for PH and 9-CNPH, which were obtained by eqn (6), were approximately zero, except for PH in acetonitrile, and the value of fΔS for PH in acetonitrile is slightly negative for the fTO value of 0.9612 and is 0.16 ± 0.02 for the fTO value of 0.47.18 The value of fΔS obtained by Abdel et al., who determined fTO = 0.96, was zero.7 The value of 0.80 ± 0.06 as fTO for PH in acetonitrile is obtained from the present results when fΔS = 0 is assumed in eqn (6). The fTO value of 0.4718 seems to be too low, as long as fΔS = 0 is accepted. As a result, it is concluded that values of fΔS for PH and 9-CNPH are zero in the three solvents, in agreement with the prediction from the S1–T1 energy gap. The fΔS values were approximately zero for PY in cyclohexane and benzene, and the fΔS value for PY in acetonitrile was 0.29 ± 0.03. These values are in good agreement with those of Abdel et al.10–12 For 1-CNPY, values of fΔS were close to zero in cyclohexane and benzene, and fΔS ≥ 0.02 was estimated in acetonitrile. The value of fΔS was 0.17 ± 0.08 when fTO was assumed as 0.85 equal to the value for 9-CNPH because ΔETGS of 1-CNPY is considered to be similar to that of 9-CNPH.32 Taking these values of fΔS into account, it is probable that the value of fΔS for 1-CNPY in acetonitrile is not larger than that for PY. Analogously to DCNNs, values of kqS for 9-CNPH and 1-CNPY are significantly smaller than those for PH and PY which are nearly equal to kdiff. The value of fΔS ≈ 0.3 for PY in acetonitrile is noticeable because this is inconsistent with a general tendency that 1O2 is produced in the system having a smaller kqS value than kdiff.
Using the values of kqS for PY and PH as those of kdiff, respectively, values of kΔ, kST and kSS for 9-CNPH and 1-CNPY are obtained by eqn (7)–(11) and shown in Table 3. The values of kST obtained for 9-CNPH and 1-CNPY are large in comparison with DCNNs, suggesting that ΔETGS for these compounds is smaller than that for DCNNs. Values of fΔS close to zero for 1-CNPY in cyclohexane and benzene are due to these large values of kST. Values of ΔETGS are estimated to be –74, –116 and –128 kJ mol–1 for 9-CNPH, PH and PY, respectively.12 The increase of kST, as well as kSS in polar solvents, by larger charge transfer interaction in comparison with 9-CNPH and 1-CNPY, results in kqS of a nearly diffusion-limit for PH and PY. Taking into account kST values of (1.5–2.7) ×1011 s–1 for 9-CNPH and 1-CNPY, it is generally predicted that values of kST for many compounds having large negative values of ΔETGS and large kqS values equal to kdiff, such as PH and PY, are larger than 2 × 10 11 s–1.
Finally, the unusual relation between fΔS and kqS for PY in acetonitrile is mentioned because it cannot be explained only by the three processes in Scheme 1. As described above, the fΔS value of PY in acetonitrile is ca. 0.3 though the kqS value is in a diffusion limit. A similar result is also reported for perylene in acetonitrile,10,12i.e.fΔS = 0.27 ± 0.03 and kqS = 3.8 × 1010 M–1 s–1, and the production of 1O2 by oxygen quenching in the S1 state was explained from a viewpoint of the energy level of the second triplet state (T2). However, the fΔS value of PY is zero in cyclohexane and benzene. Therefore, the explanation based on the T2 level cannot be accepted since the ordering of S1, T1 and T2 levels is considered to be same in all the solvents. A different mechanism from the process (1), for example the process leading to S0 and 1O2 through the charge transfer state, might exist for production of 1O2 in acetonitrile for sensitizers with large negative values of ΔETGS.
Conclusions
The significant production of 1O2 in the oxygen quenching of the S1 state was confirmed for 1,2-DCNN and 1,4-DCNN, whereas 1O2 was scarcely produced for 2,3-DCNN. The value of kqS was smaller than a diffusion-controlled rate constant (kdiff) for the three DCNNs. The value of kST increased with increasing charge transfer interaction. It was shown that the dependence of kΔ on the compound and solvent, as well as those of kST and kSS, is important as a determining factor for fΔS and kqS. The unusual relation between fΔS and kqS for PY in acetonitrile, i.e. the significant production of 1O2 (fΔS ≈ 0.3) and a large value of kqS close to kdiff, was pointed out.References
- C. Schweitzer and R. Schmidt, Chem. Rev., 2003, 103, 1685 CrossRef CAS.
- R. Schmidt, Photochem. Photobiol., 2006, 82, 1161 CrossRef CAS.
- J. Saltiel and B. W. Atwater, Adv. Photochem., 1988, 14, 1 CAS.
- D. C. Dobrowolski, P. R. Ogilby and C. S. Foote, J. Phys. Chem., 1983, 87, 2261 CrossRef.
- M. Kristiansen, R. D. Scurlock, K.-K. Iu and P. R. Ogilby, J. Phys. Chem., 1991, 95, 5190 CrossRef CAS.
- Y. Usui, N. Shimizu and S. Mori, Bull. Chem. Soc. Jpn., 1992, 65, 897 CAS.
- R. C. Kanner and C. S. Foote, J. Am. Chem. Soc., 1992, 114, 678 CrossRef CAS.
- R. D. Scurlock and P. R. Ogilby, J. Photochem. Photobiol., A, 1993, 72, 1 CrossRef CAS.
- F. Tanaka, T. Furuta, M. Okamoto and S. Hirayama, Phys. Chem. Chem. Phys., 2004, 6, 1219 RSC.
- A. A. Abdel-Shafi and F. Wilkinson, J. Phys. Chem. A, 2000, 104, 5747 CrossRef CAS.
- A. A. Abdel-Shafi, D. R. Worrall and F. Wilkinson, J. Photochem. Photobiol., A, 2001, 142, 133 CrossRef CAS.
- A. A. Abdel-Shafi and D. R. Worrall, J. Photochem. Photobiol., A, 2005, 172, 170 CrossRef CAS.
- F. Wilkinson, D. J. McGarvey and A. F. Olea, J. Am. Chem. Soc., 1993, 115, 12144 CrossRef CAS.
- A. F. Olea and F. Wilkinson, J. Phys. Chem., 1995, 99, 4518 CrossRef CAS.
- C. Wirp, H. Gusten and H.-D. Brauer, Ber. Bunsen-Ges. Phys. Chem., 1996, 100, 1217 CAS.
- C. Wirp, J. Bendig and H.-D. Brauer, Ber. Bunsen-Ges. Phys. Chem., 1997, 101, 961.
- K. Kikuchi, C. Sato, M. Watanabe, H. Ikeda, Y. Takahashi and T. Miyashi, J. Am. Chem. Soc., 1993, 115, 5180 CrossRef CAS.
- C. Sato, K. Kikuchi, K. Okamura, Y. Takahashi and T. Miyashi, J. Phys. Chem., 1995, 99, 16925 CrossRef CAS.
- R. Schmidt, J. Phys. Chem. A, 2006, 110, 5990 CrossRef CAS.
- Shin Jikken Kagaku Koza, Chem. Soc. Jpn., Maruzen, Tokyo, 1976, vol. 4, ch. 8 Search PubMed.
- S. L. Murov, I. Carmichael and G. L. Hug, Handbook of Photochemistry, Marcel Dekker, Inc., New York, 1993 Search PubMed.
- G. Rossbroich, N. A. Garcia and S. E. Braslavsky, J. Photochem., 1985, 31, 37 CrossRef CAS.
- M. Terazima, T. Hara and N. Hirota, J. Phys. Chem., 1993, 97, 10554 CrossRef CAS.
- F. Wilkinson, D. J. McGarvey and A. F. Olea, J. Phys. Chem., 1994, 98, 3762 CrossRef CAS.
- In ref. 24, the value of fΔT for 1-cyanonaphthalene in cyclohexane was obtained using 0.92 for naphthalene as a reference value, and it was suggested that this reference value may be higher than the true value. The values of fΔT for naphthalene obtained by the present TRTL measurement in the 4-methoxyacetophenone–naphthalene system26 were 0.83 ± 0.02 (cyclohexane), 0.71 ± 0.01 (benzene) and 0.61 ± 0.02 (acetonitrile), respectively. When these values and ratios of fΔT for 1-cyanonaphthalene/naphthalene in ref. 24 are used, the values of fΔT for 1-cyanonaphthalene are 0.93 (cyclohexane), 0.86 (benzene) and 0.73 (acetonitrile), respectively.
- A. A. Gorman, A. A. Krasnovsky and M. A. J. Rodgers, J. Phys. Chem., 1991, 95, 598 CrossRef CAS.
- A. P. Darmanyan, W. Lee and W. S. Jenks, J. Phys. Chem. A, 1999, 103, 2705 CrossRef CAS.
- L. Eberson, Adv. Phys. Org. Chem., 1982, 18, 79–185 CAS.
- A. Bondi, J. Phys. Chem., 1964, 68, 441 CrossRef CAS.
- Y. Kubo, K. Kiuchi and I. Inamura, Bull. Chem. Soc. Jpn., 1999, 72, 1101 CrossRef CAS.
- L. Brancaleon, D. Brousmiche and L. J. Johnston, Can. J. Chem., 1999, 77, 787 CrossRef CAS.
- N. J. Peacock and G. B. Schuster, J. Am. Chem. Soc., 1983, 105, 3632 CrossRef CAS.
| This journal is © The Royal Society of Chemistry and Owner Societies 2008 |
