Photochemistry of metal complexes of 3-hydroxyflavone: towards a better understanding of the influence of solar light on the metal–soil organic matter interactions
Stefano
Protti
ab,
Alberto
Mezzetti
*a,
Christine
Lapouge
a and
Jean-Paul
Cornard
a
aLaboratoire de Spectrochimie Infrarouge et Raman UMR CNRS 8516, Université de Sciences et Technologies de Lille, Bat. C5, Cité Scientifique, 59655, Villeneuve d'Ascq, France. E-mail: alberto.mezzetti@univ-lille1.fr; Fax: +33 3 20 43 67 55; Tel: +33 3 20 43 66 03/+33 3 20 43 69 26
bDepartment of Organic Chemistry, University of Pavia, Via Taramelli 19, 27100, Pavia, Italy. Fax: +39 0382 98 73 23; Tel: +39 0382 98 73 16
First published on 8th November 2007
Abstract
The photo-stability of 3-hydroxyflavone (3HF) and its complexes with Al(III), Zn(II) and Pb(II) in methanol was investigated by a multi-disciplinary strategy, combining UV-Vis, fluorescence, Raman and FT-IR spectroscopies, DFT and time-dependent DFT calculations and a traditional organic photochemistry approach. We found that the presence of metals can slow down (in the case of Pb(II)) or even block (in the case of Al(III)) the photo-degradation of 3HF. The Zn(II) complex shows a reactivity comparable to free 3HF. In all cases (except the Al(III) complex) the photo-product is 3-hydroxy-3-phenyl-1,2-indandione, the same as reported in literature for 3HF photochemistry in methanol. This means that metallic cations do not modify the photochemical mechanism of the reaction. In addition, in the case of Zn(II) and Pb(II) complexes, the photo-produced 3-hydroxy-3-phenyl-1,2-indandione was found to exist in free form, i.e. without metal complexation. This means that similar photo-reactions occurring in soil organic matter under solar irradiation entail desorption of free Zn2+ and Pb2+ cations (the most toxic form) in the environment. The presence of external parameters—presence of water and oxygen—has also been studied. Interestingly, opposite effects have been found for free 3HF and Zn(II) and Pb(II) complexes. Tentative explanations for these effects are provided, relying on a detailed investigation on the structure and on the photophysical properties of the three complexes.
1. Introduction
Soil Organic Matter (SOM) is a fundamental soil constituent, originating from decaying plant or animal products that have been converted to relatively stable product called humus. The latter represents the end product of the degradation of biota residues, and has the major influence on the chemical properties of the soils. Humic substances (HSs) are a series of acidic polyelectrolytes of moderately high molecular weight, derived from the secondary degradation processes involving micro-organisms, and represent the main part of SOM. They play an important role in the retention and transport of many metal ions in soils, most of them showing phytotoxic and cytotoxic activity.1The toxicity of metals largely depends on the chemical and physical parameters of the soils. In general, metal free ions are the most toxic form, because the assumption of metals by living cells requires a decomplexation of the chelated form.2 Consequently, the total concentration of metal in the soil cannot be directly related to toxicity. Indeed, the higher the sorption capability of the soil matrix, the lower is the bioavailability, and thus the toxicity of metals.3
Although adsorbed metal ions are not responsible for direct toxicity, many environmental risks are however related to heavy metal retention process in SOM. In particular, heavy metal accumulation is demonstrated to cause a reduction of soil biological activity, decreasing the decomposition of present organic substances.4 Metal retention by SOM is affected by many physical and chemical parameters, such as pH, organic matter composition, concentration of competitive cations, water content and ionic strength.1
Because of the presence of many conjugated functional groups, HSs have a remarkable ability to absorb light, leading to potential photodecomposition and becoming potential photosensitisers. Indeed, the photochemical properties of humic substances have been largely studied.5 The solar light induced production of oxidant species such as singlet oxygen, superoxide ion, hydroxyl and peroxy radicals by irradiation of HSs in natural waters has been described in literature.6 Primarily absorption of light by SOM has been proved to be involved in the photo-degradation of organic pollutants such as pesticides7 and disinfectants.8 On the contrary, the effect of solar light on the complexation of metals by HSs has received only little attention in literature.9 Since the toxicity of heavy metals depends on their speciation form and in particular on the possibility of existing as free cation in the environment, a study of the photochemical behaviour of metal–HSs complexes appears desirable. Typically, photo-degradation of coordination compounds can be followed by spectrophotometric measurement. However, because of their complex nature, HSs give a continuous absorption spectrum without characteristic peaks, making the measurement impracticable. An alternative approach can be represented by using simpler molecules as models for humic acids. Flavonoids can be taken as suitable models, as they are precursors of SOM and show functional groups that can be found also in humic acids. Flavonoids are a class of natural pigments found in the vegetal kingdom,10 playing important roles in biology, because of their antioxidant activity.11
3-Hydroxyflavone (3HF) is one of the most studied flavonoids (Fig. 1). Its photophysics12–14 and photochemistry15,16 have been largely studied during the last decades. Indeed, 3HF is taken as a model system in physical chemistry to investigate intramolecular photo-induced proton transfer reactions.17–18 Because of its photochemical and photophysical properties 3HF has also been used as a laser dye,19 as a scintillator,20 as a wavelength shifter,21 and as a reactant in photo-stimulated organic synthesis.22 In addition, several 3HF derivatives are widely used as fluorescent molecular sensor in analytical and biological fields.23
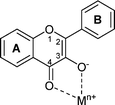 | ||
| Fig. 1 Binding sites of 3-hydroxyflavone. | ||
3HF shows great affinity with a wide range of metal ions and the complexation usually occurs at the 3-hydroxy-4-keto binding site24 (Fig. 1). In recent years our research group has investigated the mechanism of interaction of this molecule with some metals such as Zn(II),25Pb(II),26Al(III);27 the structure and stoichiometry of these complexes have been obtained thanks to an approach combining spectroscopic measurements and theoretical calculations.
In this paper, with the same approach we have investigated the photostability of these complexes. Interestingly, we have found that the nature of the metal cation can strongly modulate the photochemistry of the complex. These results are particularly interesting as the same effect can also exist in humic acids, insofar as 3HF is a precursor of SOM and flavonoid compounds are found in humic substances.28,29
2. Experimental and calculations
Experimental
3-Hydroxyflavone was purchased from Aldrich, and re-crystallised from cyclohexane before use. Anhydrous aluminium chloride, anhydrous zinc(II) chloride, lead(II) chloride, lead(II) nitrate and spectroscopic grade methanol were used without purification.Irradiation were performed using a 1000 W xenon lamp (Oriel) equipped with a monochromator 1/4 (resolution 0.15 nm). The irradiation wavelength has been adapted to the absorption band in the long wavelength range of each compound.
3HF photoreaction in dimethylsulfoxide (DMSO) was performed by using nitrogen-purged solution in quartz tubes and a multi-lamp reactor fitted with six 15 W phosphor-coated lamps (maximum of emission 310 nm).
UV-Vis spectra were recorded using a Varian Cary-1 spectrophotometer with cells of 1 cm path length. Fluorescence spectra were recorded on a Jobin-Yvon Fluoromax 3 fluorescence spectrophotometer. Quantum yields of 3HF and its complexes were determined with respect to eosin Y (Φ = 0.67 in alkaline ethanol).
HPLC analyses were performed using a Thermo Electron instrument, equipped with a P1000XR pump, a C18 5 µm hypersyl ODS column and a UV-Vis/diode detector. A mixture MeCN–water 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 was used as the eluent phase.
2 was used as the eluent phase.
Initially, the photo-degradation of 3-hydroxyflavone and its complexes was followed by spectrophotometric analysis; for this aim solutions containing a constant concentration of 8.4 × 10–5 M of 3HF in methanol were used. Analyses on metallic complexes were performed on an analogous concentration. In order to characterise the products deriving by the photochemical tests, irradiated solutions (2 mL) of free 3HF were analyzed directly by HPLC, while solutions of its complexes were first diluted with water (2 mL) and then extracted with dichloromethane (3 × 2 mL). Organic layers were then reunited, evaporated, the resulting residue dissolved in acetonitrile (2 mL) and then injected in HPLC.
NMR spectra were recorded on a Bruker 300 MHz spectrometer. The attributions were made on the basis of 1H and 13C NMR; chemical shifts are reported in ppm downfield from TMS.
FT-IR spectra were recorded on a Thermo Electron Magna-860 spectrometer. The concentration was 10–2 M for all samples.
FT-Raman spectra were recorded with a Bruker IFS 66 V spectrometer. Radiation of 1064 nm from a Nd:YAG laser was used for the excitation with a laser power of 1040 mW. The spectral resolution was set to 4 or 2 cm–1, as specified. Spectra results from averaging of 1500–6000 scans, according to the sample and the spectral resolution. The concentration was 10–2 M for all samples.
Computational methods
The DFT calculations have been performed with the GAUSSIAN 03 quantum chemical package.30Geometry optimizations of 3HF and its complexes were obtained by using the three-parameter hybrid functional B3LYP.31 For lead, we adopted the Los Alamos double-ξ (Lanl2dz) and the 6-31G(d,p) basis set for the other atoms including Al and Zn. For free and complexed forms, harmonic vibrational frequency calculations have been performed to ensure that the optimized structures correspond to energy minima. Computed vibrational frequencies were scaled by a factor 0.97.
The low-lying excited states were treated within the adiabatic approximation of time dependent density functional theory (DFT-RPA) with the B3LYP hybrid functional.32 Vertical excitation energies were computed for the first 30 singlet excited states, in order to reproduce the UV-Vis spectra of the photoproduct. Solvent effects on calculated UV-Vis spectra were introduced by the SCRF method, via the Polarized Continuum Model33 (PCM) implemented in the Gaussian program. In results, only transitions presenting high oscillator strengths (≥0.05) have been considered.
3. Results
Structural characterization of 3HF and its complexes
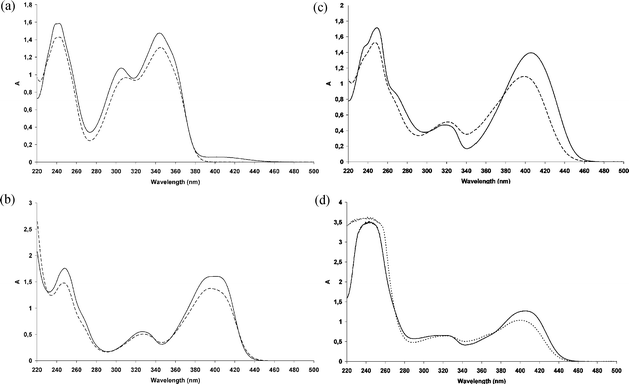 | ||
Fig. 2 (a) Absorption spectra of 3HF (1) in neat methanol (![[thick line, graph caption]](https://www.rsc.org/images/entities/char_e117.gif) ) and in MeOH 50%aq ( ) and in MeOH 50%aq (![[dash dash, graph caption]](https://www.rsc.org/images/entities/char_e091.gif) ); (b) absorption spectra of Al(3HF)2+ in neat methanol ( ); (b) absorption spectra of Al(3HF)2+ in neat methanol (![[thick line, graph caption]](https://www.rsc.org/images/entities/char_e117.gif) ) and in MeOH 50%aq ( ) and in MeOH 50%aq (![[dash dash, graph caption]](https://www.rsc.org/images/entities/char_e091.gif) ); (c) absorption spectra of Zn(3HF)+ in neat methanol ( ); (c) absorption spectra of Zn(3HF)+ in neat methanol (![[thick line, graph caption]](https://www.rsc.org/images/entities/char_e117.gif) ) and in MeOH 50%aq ( ) and in MeOH 50%aq (![[dash dash, graph caption]](https://www.rsc.org/images/entities/char_e091.gif) ); (d) absorption spectra of Pb(3HF)+ in neat methanol ( ); (d) absorption spectra of Pb(3HF)+ in neat methanol (![[thick line, graph caption]](https://www.rsc.org/images/entities/char_e117.gif) ) and in MeOH 50%aq (⋯). ) and in MeOH 50%aq (⋯). | ||
The complexation of Al(III), Zn(II) and Pb(II) by 3HF has been largely studied by our group.25–27 Chelation of metal occurs at the level of the α-hydroxy-carbonyl group with a complete deprotonation of the hydroxyl. The complex of 3HF with aluminium (Al(3HF)2+, hereafter referred to also as 2) is characterised by a strong and broad absorption band at ∼402 nm (Fig. 2(b)), and the complexed species exhibits a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 stoichiometry. In MeOH 50%aq the spectrum shows a blue-shift of the band to 398 nm. The chelating power of Zn(II) is less efficient than Al(III); however, using an excess of ZnCl2, a large absorption band of new species, Zn(3HF)+ (hereafter referred to also as 3) situated at 408 nm appears (Fig. 2(c)). When complexation is carried out in MeOH 50%aq, the absorption band of 3HF complex with Zn2+ is slightly blue-shifted to 400 nm.
2 stoichiometry. In MeOH 50%aq the spectrum shows a blue-shift of the band to 398 nm. The chelating power of Zn(II) is less efficient than Al(III); however, using an excess of ZnCl2, a large absorption band of new species, Zn(3HF)+ (hereafter referred to also as 3) situated at 408 nm appears (Fig. 2(c)). When complexation is carried out in MeOH 50%aq, the absorption band of 3HF complex with Zn2+ is slightly blue-shifted to 400 nm.
On the other hand, the chelating power of 3HF with respect to Pb(II) is estimated to be lower26 than that observed for Al(III) and Zn(II). In all cases, the resulting species Pb(3HF)+ (hereafter referred to also as 4) exhibits a strong absorption band at 405 nm. In MeOH 50%aq the spectrum shows an appreciable blue shift (399 nm) of its absorption maximum (Fig. 2(d)).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, as previously described in literature.19 On the other hand, excitation at 405 nm (corresponding to the weak absorption peak observed in the previous paragraph), gives an emission spectrum centred at 485 nm (Fig. 3(a)), as reported previously.34
1, as previously described in literature.19 On the other hand, excitation at 405 nm (corresponding to the weak absorption peak observed in the previous paragraph), gives an emission spectrum centred at 485 nm (Fig. 3(a)), as reported previously.34
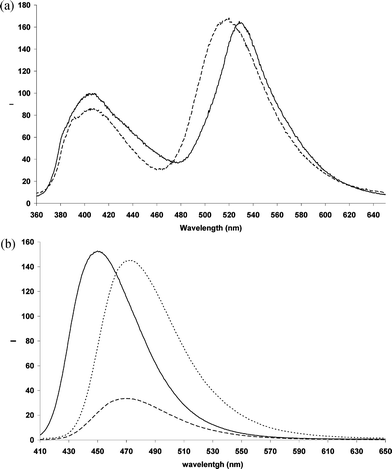 | ||
Fig. 3 (a) Fluorescence emission of 3HF in neat methanol (![[thick line, graph caption]](https://www.rsc.org/images/entities/char_e117.gif) ) and MeOH 50%aq ( ) and MeOH 50%aq (![[dash dash, graph caption]](https://www.rsc.org/images/entities/char_e091.gif) ). (b) Fluorescence spectra of complexes Al(3HF)2+ ( ). (b) Fluorescence spectra of complexes Al(3HF)2+ (![[thick line, graph caption]](https://www.rsc.org/images/entities/char_e117.gif) ), Zn(3HF)+ ( ), Zn(3HF)+ (![[dash dash, graph caption]](https://www.rsc.org/images/entities/char_e091.gif) ), Pb(3HF)+ (⋯). Important: as far as the intensities are concerned, spectra are not directly comparable within the same figure as they have been adjusted (by multiplication for a “normalization” factor) in order to keep all spectra in the same figure. ), Pb(3HF)+ (⋯). Important: as far as the intensities are concerned, spectra are not directly comparable within the same figure as they have been adjusted (by multiplication for a “normalization” factor) in order to keep all spectra in the same figure. | ||
In MeOH 50%aq, the fluorescence spectrum (upon irradiation at 345 nm) looks different. Whereas the green emission at 406 nm remains unchanged (only its intensity is slightly decreased) a quite strong blue-shift (518 nm) of the long wavelength emission maximum is observed.
In MeOH, all examined metal complexes exhibit an intense fluorescence whose peak is blue-shifted with respect to the highest emission of free 3HF, varying from 451 nm for Al(3HF)2+ to 473 nm for Zn(3HF)+ (Table 1 and Fig. 3(b)).
| Compound | λ em/nm | Φ em × 102 |
|---|---|---|
| 3HF | 406 (406), 531 (518) | 0.89 (0.81), 1.66 (2.19) |
| Al(3HF)2 | 450 (447) | 58.0 (44.6) |
| Zn(3HF)+ | 473 (475) | 37.6 (14.5) |
| Pb(3HF)+ | 470 (470) | 11.9 (13.5) |
The wavelength of maximum emission of the complexes does not show an appreciable change when the experiment is performed in MeOH 50%aq (not shown). Quantum yield fluorescence of complexes 2 and 3 is found to be lower in MeOH 50%aq than in neat methanol (Table 1). In particular, emission of Zn(3HF)+ is almost three times lower. Conversely, Pb(3HF)+ shows a slightly increased fluorescence yield in MeOH 50%aq.
It is important to highlight that the fluorescence quantum yields are higher for all complexes (0.11 to 0.58) than for the uncoordinated 3HF.
For 3HF in neat MeOH we observe (starting from high wavenumbers) a strong band at 1622 cm–1. We have assigned this band to a mechanical coupling between the C2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C3 and C
C3 and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O vibrators (Fig. 4), in agreement with previous assignment from our research group.25 At 1616 cm–1 another strong band is observed; as it is very close to the previous one, only in the spectrum recorded at 2 cm–1 resolution (Fig. 4) the distinction between these two bands becomes evident. We have assigned this band to a 8a vibrational mode (Wilson notation37,38) of the A benzene ring. Indeed, as it will be shown in the following paragraph, such band does not shift appreciably in the metal complexes, meaning that contributions arising from C
O vibrators (Fig. 4), in agreement with previous assignment from our research group.25 At 1616 cm–1 another strong band is observed; as it is very close to the previous one, only in the spectrum recorded at 2 cm–1 resolution (Fig. 4) the distinction between these two bands becomes evident. We have assigned this band to a 8a vibrational mode (Wilson notation37,38) of the A benzene ring. Indeed, as it will be shown in the following paragraph, such band does not shift appreciably in the metal complexes, meaning that contributions arising from C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching and/or O–H bending are negligible. Also this is in agreement with previous assignment from our group.26 However, this is at odds with the assignment reported by Wang et al.36 where this band was ascribed to a mixed C
O stretching and/or O–H bending are negligible. Also this is in agreement with previous assignment from our group.26 However, this is at odds with the assignment reported by Wang et al.36 where this band was ascribed to a mixed C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch, OH bend, C2
O stretch, OH bend, C2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C3 stretch mode. This interpretation is consistent neither with our DFT calculation nor with the presence of such band in all three metal complexes (see below).
C3 stretch mode. This interpretation is consistent neither with our DFT calculation nor with the presence of such band in all three metal complexes (see below).
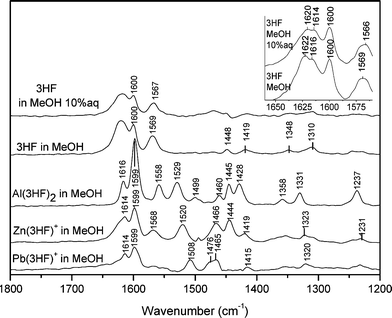 | ||
| Fig. 4 From top to the bottom: FT-Raman spectra of free 3HF in MeOH 50%aq, free 3HF in MeOH, Al(3HF)2+ in MeOH, Zn(3HF)+ in MeOH, Pb(3HF)+ in MeOH. Spectral resolution: 4 cm–1. In the top right part of the figure, FT-Raman spectra at higher resolution (2 cm–1) for 3HF in MeOH and MeOH 10%aq are reported. | ||
In addition two lines are observed at 1600 and 1569 cm–1 that can be assigned respectively to the 8a vibrational mode of the B ring and to the 8b vibrational mode of the A ring coupled to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch and C2
O stretch and C2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C3 stretch mode. Again, this interpretation agrees with ref. 26 and is in partial contrast with the description provided in Wang et al.36
C3 stretch mode. Again, this interpretation agrees with ref. 26 and is in partial contrast with the description provided in Wang et al.36
We have also investigated the influence of the presence of water in solution on the 3HF Raman spectrum. Because of its low solubility in a methanol–water 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture and of the intrinsic limitation in the sensitivity of our FT-Raman instrument, a methanol–water 9
1 mixture and of the intrinsic limitation in the sensitivity of our FT-Raman instrument, a methanol–water 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture was used. Interestingly, a 2 cm–1 downshift of the 1622 and 1616 cm–1 bands has been observed. The 1569 band also downshifts to 1566 cm–1 (compare spectra recorded at 2 cm–1 resolution in Fig. 4). Smaller changes are also visible at lower wavenumbers (not shown).
1 mixture was used. Interestingly, a 2 cm–1 downshift of the 1622 and 1616 cm–1 bands has been observed. The 1569 band also downshifts to 1566 cm–1 (compare spectra recorded at 2 cm–1 resolution in Fig. 4). Smaller changes are also visible at lower wavenumbers (not shown).
Metal chelation with 3HF induces strong changes in the structural parameters of the ligand and consequently modifies the vibrational spectrum. Moreover changes in the mechanical couplings modify significantly the vibrational frequencies. It should be noticed that the metal chelation is accompanied by a disappearance of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond and leads to two C–O coupled vibrators. The asymmetric CO stretching is observed at 1529, 1520 and 1508 cm–1 in Al(3HF)2+, Zn(3HF)+ and Pb(3HF)+, respectively (Fig. 4), in good agreement with the scaled calculated values (1523, 1509 and 1507 cm–1, respectively). For all complexes, the C2
O bond and leads to two C–O coupled vibrators. The asymmetric CO stretching is observed at 1529, 1520 and 1508 cm–1 in Al(3HF)2+, Zn(3HF)+ and Pb(3HF)+, respectively (Fig. 4), in good agreement with the scaled calculated values (1523, 1509 and 1507 cm–1, respectively). For all complexes, the C2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C3 stretching is calculated largely distributed on several vibrational modes in the high frequency range and no band in the Raman spectra could be assigned to this specific mode. In opposition the two lines observed in the high frequencies are calculated perfectly localised and could be assigned to the 8a mode. The band at 1616 in Al(3HF)2+ and 1614 in Zn(3HF)+ and Pb(3HF)+spectra are calculated at 1610, 1612 and 1612 cm–1, respectively and correspond to the 8a mode of the A ring. The line observed at 1599 cm–1 for all complexes could be assigned to the 8a mode of the B ring and is calculated at 1600, 1601, 1601 cm–1 for the three complexes, respectively. Finally the band observed at 1558, 1568 cm–1 and calculated at 1568 and 1571 cm–1 in Al(3HF)2+and Zn(3HF)+, respectively, could be assigned to a mixed mode involving the 8b of the A and B rings, slightly coupled with the C2
C3 stretching is calculated largely distributed on several vibrational modes in the high frequency range and no band in the Raman spectra could be assigned to this specific mode. In opposition the two lines observed in the high frequencies are calculated perfectly localised and could be assigned to the 8a mode. The band at 1616 in Al(3HF)2+ and 1614 in Zn(3HF)+ and Pb(3HF)+spectra are calculated at 1610, 1612 and 1612 cm–1, respectively and correspond to the 8a mode of the A ring. The line observed at 1599 cm–1 for all complexes could be assigned to the 8a mode of the B ring and is calculated at 1600, 1601, 1601 cm–1 for the three complexes, respectively. Finally the band observed at 1558, 1568 cm–1 and calculated at 1568 and 1571 cm–1 in Al(3HF)2+and Zn(3HF)+, respectively, could be assigned to a mixed mode involving the 8b of the A and B rings, slightly coupled with the C2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C3 stretching.
C3 stretching.
Photo-degradation of 3HF and metal complexes
The photo-reactivity of free 3HF and its complexes was studied by irradiation at absorption maxima of each species. Because of the low solubility of 3HF and its derivatives in water, we initially decided to irradiate the methanol solution. The photo-degradation of the examined species in aerated methanol is showed in Fig. 5.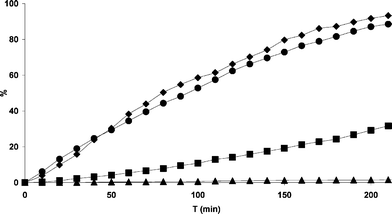 | ||
| Fig. 5 Photo-degradation of 3HF and its complexes in aerated methanol: ✦ free 3HF (1); ▲ Al(3HF)2 (2); ● Zn(3HF)+ (3); ■ Pb(3HF)+ (4). | ||
The presence of Al(III) stabilizes 3HF and leads to a very photostable complex. Whereas the presence of Pb(II) hamper the photo-decomposition of the ligand, no appreciable slowing effect is observed for Zn(3HF)+. The stabilizing effect is therefore Al(3HF)2+ ≫ Pb(3HF)+ > Zn(3HF)+ ∼ free 3HF.
The possible influence of external parameters, i.e. presence of water and influence of O2, has also been investigated. To study the influence of water on the photo-reactivity of 3HF and its complexes, we had to take into account the low solubility of these substrates in neat water. Therefore, we utilized a mixture water–methanol 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 as solvent (Fig. 6(a)).
1 as solvent (Fig. 6(a)).
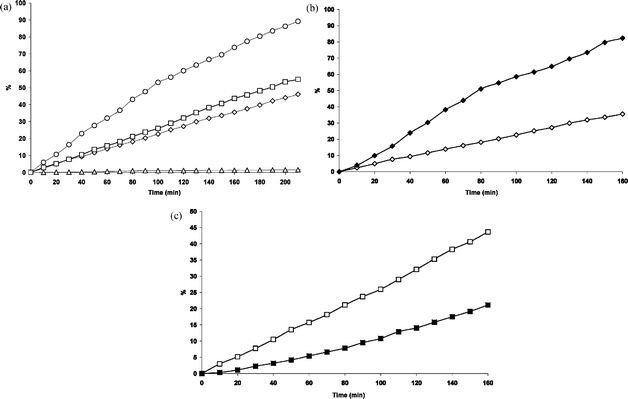 | ||
| Fig. 6 (a) Photo-degradation of 3HF and its complexes in MeOH 50%aq: ◇ free 3HF (1); △ Al(3HF)2 (2); ○ Zn(3HF)+ (3); □ Pb(3HF)+ (4). (b) Comparison of photo-degradation of 1 in: ◆ neat MeOH; ◇ MeOH 50%aq. (c) Comparison of photo-degradation of 4 in: ■ neat MeOH; □ MeOH 50%aq. | ||
As shown in Fig. 6(b), photo-degradation of 3HF in MeOH–water is slower than in neat methanol. On the contrary the influence of water on the complexes appears quite different and depends on the nature of the metal; in fact, while Zn(II) and Al(III) complexes results are weakly influenced by water, Pb(3HF)+ appears more photo-degradable in MeOH 50%aq than in pure MeOH (Fig. 6(c)).
To investigate the possible influence of O2, the photo-degradation of species 1, 3, 4 has been studied in deaerated methanol. We found that O2 can influence 3HF and its metal complexes in opposite ways. As shown in Fig. 7(a), the presence of O2 slightly inhibits 3HF degradation. On the contrary, O2 exercises a weak, positive influence on the photo-degradation of metal complexes, in particular Zn(3HF)+, as shown in Fig. 7(b).
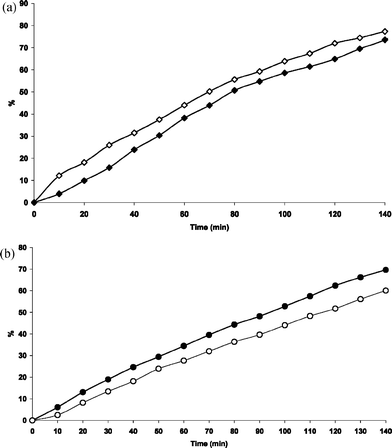 | ||
| Fig. 7 (a) Comparison of the photodegradation of 3HF in deaerated (◇) and aerated (◆) MeOH. (b) Comparison of the photodegradation of Zn(3HF)+ (3) in deaerated (○) and aerated (●) MeOH. | ||
Irradiated solutions were studied by HPLC analysis (diode array detector), revealing the presence of a major photoproduct having two strong absorption bands situated at 290 and 246 nm (it should be recalled that the eluent phase used is a acetonitrile–water mixture 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20). The same compound was found to be formed after irradiation of free 3HF, Zn(3HF)+, and Pb(3HF)+.
20). The same compound was found to be formed after irradiation of free 3HF, Zn(3HF)+, and Pb(3HF)+.
Photo-degradation of 3HF in methanol is described in the literature.15 The main photoproduct is reported to be 3-hydroxy-3-phenyl-1,2-indandione (hereafter referred to also as 5). The same reaction has been reported also in other polar solvents.39,40 Performing a preparative reaction on our methanol complex solutions, would have resulted in very low yields, because of the limited solubility of the complexes, and consequently would have required consumption of high amounts of reactants, solvents, and time. An alternative approach is represented by photo-synthesis (and characterization) of 5 in optimised conditions and then compare its properties with those found for the photoproducts originating from our substrates. Indeed, 5 has been isolated by irradiation of 3 mg mL–1 (1.3 × 10–2 M) solution of 1 in deaerated DMSO (oil, 90% yield). Indeed, 3HF has a huge solubility in this solvent, so that the most appropriate concentration can be chosen in order to maximise the reaction yield.
Characterization of 5 led to the following data: 1HNMR (δ, CDCl3): 3.95 (bs, 1H), 7.20–7.25 (m, 2H), 7.30–7.35 (m, 3H), 7.60–7.65 (t, 1H, J = 7 Hz), 7.75–7.80 (d, 1H, J = 9 Hz), 7.85–7.90 (t, 1H, J = 7 Hz), 7.95–8.00 (d, 1H, J = 8 Hz). 13CNMR (δ, CDCl3): 75.3, 123.9 (CH), 124.5 (2 CH), 126.1 (CH), 128.5 (CH), 128.7 (2 CH), 130.2 (CH), 137.0, 138.8 (CH), 139.5, 151.6, 187.2, 200.8. IR (ν, neat): 3395, 3065, 3031, 29873, 1769, 1733, 1602, 1495, 1468, 1448, 1419, 1373, 1293, 1208, 1060, 1021 cm–1. UV-Vis (methanol): 297, 250 and 210 nm. UV-Vis (acetonitrile): 292, 246 and 206 nm.
A comparison of chromatograms of irradiated solutions of 1, 3 and 4 with an authentic sample of 5 was performed, revealing that the photo-degradation product is characterised by the same retention time as 5. In addition, the absorption spectra of the photoproduct recorded on the HPLC-diode array apparatus were identical to the spectrum of isolated 5 in acetonitrile.
The formation of 5 has been confirmed by real-time monitoring of the reaction of 1, 3, 4 in methanol. First, we recorded time-resolved UV spectra of the examined solutions after different irradiation periods. The final spectra (peaks at ∼297 and 250 nm) look very similar in all cases, the small differences being due to absorption of residual reactant. A comparison of degradation spectra of 1 and 3 in MeOH 50%aq again shows the decrease of absorption bands of two substrates and the synchronous formation of two new absorption bands, centred at 251 and 295 nm (Fig. 8). The same trends are observed for the Pb(II) complex.
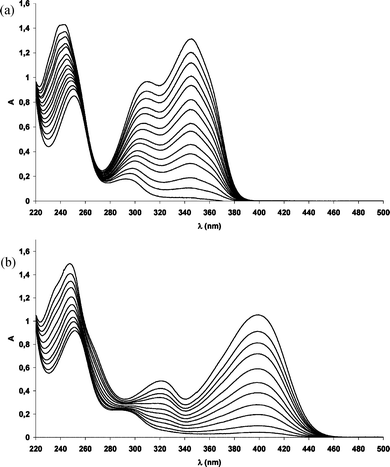 | ||
| Fig. 8 (a) Absorption spectra of 3HF (1) in MeOH 50%aq during irradiation. (b) Absorption spectra of Zn(3HF)+ (3) in MeOH 50%aq during irradiation (0–320 min irradiation). | ||
Furthermore, the electronic absorption spectrum of 3-hydroxy-3-phenyl-1,2-indandione has been calculated by TD-DFT, from an optimized structure, taking into account the solvent effect (methanol). Table 2 collects the wavelengths of the experimental and of the calculated transitions of 5. Only the contribution of the transitions having an oscillator strength higher than 0.05 are reported, and a great number of other transitions presenting much weaker probability could be added, notably in the 200–250 nm spectral range. The good agreement between the experimental wavelengths of 5 and the calculated wavelengths of the transitions of 3-hydroxy-3-phenyl-1,2-indandione validates the identification of the photo-product. The relatively low values calculated for the oscillator strengths explain the very weak absorption spectrum of 5 (Fig. 8). The HOMO–LUMO transition of 5 is calculated at 381 nm with oscillator strength very close to 0 in accordance with the fact that no absorption band is observed experimentally in this spectral range. The first band recorded at 297 nm is assigned to a transition mainly involving the HOMO–4 and the LUMO orbitals and corresponds to a charge transfer from the benzene ring A to the keto groups (Fig. 9). The other spectral features correspond to numerous transitions involving an important number of molecular orbitals.
| λ exptl/nm | λ calc/nm | f | M.O. contribution (%) |
|---|---|---|---|
| 297 | 300 | 0.21 | H–4 → L (64), H–3 → L (19) |
| 250 | 237 | 0.06 | H–2 → L+1(40), H–1 → L+1 (36) |
| 220 | 0.07 | H → L+3 (44), H–2 → L+3 (17), H → L+4 (17) | |
| 210 | 213 | 0.06 | H–2 → L+2 (30), H–1 → L+2 (20), H → L+4 (16) |
| 208 | 0.10 | H–4 → L+1 (21), H–1 → L+4 (19), H–2 → L+3 (18), H → L+4 (17) |
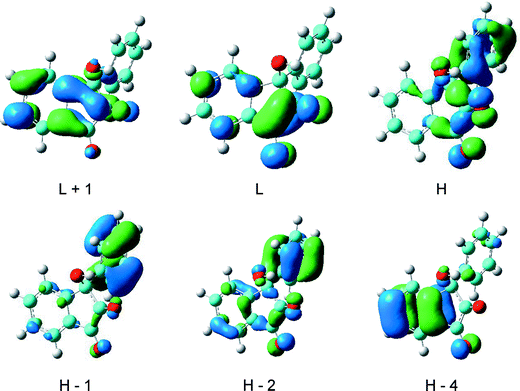 | ||
| Fig. 9 Main molecular orbitals involved in the electronic transitions of 3-hydroxy-3-phenyl-1,2-indandione (5). | ||
Subsequently, photo-degradation of 1, 3 and 4 in methanol was also followed by a time-resolved FT-IR analysis. In all cases, the simultaneous formation of two new signals at 1733 and 1766 cm–1, attributed to the carbonyl symmetric and asymmetric stretching modes of 5,39 was observed (data not shown). In the case of free 3HF, the growth of these bands was synchronous with the decrease of the band due to 3HF carbonyl stretching (1621 cm–1) showing 3HF consumption with time. For complexes 3–4, the reaction was followed looking at the band at 1614 cm–1 (present also in the Raman spectra and due to a 8a mode of the A ring); its decrease was found to be synchronous with the growth of the 1733 and 1766 cm–1 signals.
The fact that in all three cases the 3-hydroxy-3-phenyl-1,2-indandione carbonyl bands lie at the same frequencies (1766 and 1733 cm–1) indicates that these two bonds are not involved in a complexation process with the metal; in other words, photo-decomposition of the complexes 3–4 entails liberation of a free cation and the production of a non-complexed photorearrangement product.
To confirm this fact, we performed a titration of a methanol solution of 5 by PbCl2 and ZnCl2, following the process by UV-Vis spectroscopy. No spectral changes were observed, meaning that no complexation was occurring in solution. We also observed the same behaviour in the presence of AlCl3. All these results therefore show a very low or inexistent affinity of 3-hydroxy-3-phenyl-1,2-indandione with the three considered cations.
4. Discussion
Photophysical properties
The spectroscopic UV-Vis characterization of 3HF and its complexes in methanol described in this paper agrees with previous data in the literature. On the other hand, data obtained in MeOH 50%aq are original, and show some interesting features. First, the weak absorption band at ∼410 nm observed for 3HF in neat methanol disappears in the methanol–water mixture. A similar effect had already been noticed for lower concentration of water34 in alcoholic solution of 3HF. In addition, the absorption band at 304 nm assigned to the HOMO–1 → LUMO transition25 is red-shifted to 309 nm.Fluorescence of 3HF in neat methanol has been described by Woolfe and Thistlethwait18 in 1981. In this paper the short wavelength band (406 nm) has been attributed at the emission of mono-solvated 3HF in the normal form, while the emission at 531 nm appears related to its mono-solvated tautomeric form (Scheme 1(a)), generated by an Excited-State Proton-Transfer (ESPT) process.
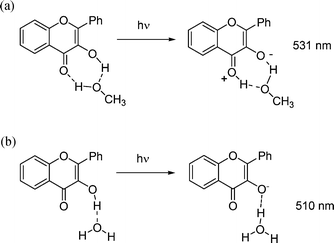 | ||
| Scheme 1 Proposed interaction of 3HF with methanol and water molecules, as proposed in ref. 14 and 18. | ||
In contrast, the long wavelength band of the emission spectra of 3HF in pure water (reported by McMorrow and Kasha14), is reported to exhibit a broad band located at 510 nm. This has been related to the photo-generated anion species (Scheme 1(b)). In our case the emission at 518 nm observed in MeOH 50%aq cannot be just related to the anion emission†. Another hypothesis could be related to different hydrogen bonding properties of the two media (water and methanol). Indeed, in a recent work, Mandal and Samanta34 studied the interaction between 3HF and a protic solvent, showing that methanol could form strong hydrogen bonds, behaving both as hydrogen donor and as hydrogen acceptor. Under these conditions acid base processes can take place, leading to the formation of the 3HF anion, characterised by absorption at ∼410 nm. Conversely, the hydrogen bond acceptor capability of water is not as good as that of methanol41 and proton transfer between 1 and the solvent cannot take place (at least in the ground state). On the other hand, we reasoned that, in water–MeOH medium, a new solvated form of 3HF could exist, resulting in the green emission centred at 518 nm. This is in agreement with the observation, from our FT-Raman results, that in the methanol–water 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture the presence of water modifies solvent–solute interactions. However, it should be pointed out that a small participation of photo-generated anion of 1 in the very large green emission band cannot be excluded.
1 mixture the presence of water modifies solvent–solute interactions. However, it should be pointed out that a small participation of photo-generated anion of 1 in the very large green emission band cannot be excluded.
Concerning the complexes, the UV-Vis spectrum of Al(3HF)2+ in MeOH 50%aq shows a 4 nm blue-shift compared to methanol solution. The same behaviour is observed for zinc and lead complexes, with blue-shifts of 7 and 6 nm, respectively (see Fig. 2). This also could be related to a different coordinative geometry of the solvent on the complexes. The fluorescence quantum yield of the complexes is much higher than that of free 3HF; in the case of Al(3HF)2+, Φ is more than 35 times higher. Indeed, flavonols such as 3HF42 or morin are known to form fluorescent chelates with a variety of metal ions; i.e.morin was used to titrate solution of various metal ions until the advent of atomic absorption spectroscopy (before the seventies43), and few years ago a method to detect flavonoids by chelation with aluminium was reported.44
We point out that the very high fluorescence quantum yield of Al(3HF)2+, together with its extreme photostability, could be exploited also for technological applications. Indeed, in our laboratory we are currently developing luminescent devices based on this complex (results not yet published).
On the other hand, as reported above, while the fluorescence emission peaks of complexes 2–4 remain substantially unchanged in MeOH 50%aq solutions, quantum yields vary considerably, showing a clear effect of the solvent on the rate of competitive photophysical processes (e.g. internal conversion vs. radiative decaying).
Photochemical behaviour
The photochemistry of 3HF in neat methanol has been largely investigated, although the nature of the photochemical processes occurring is not completely understood. Yokoe et al.15 described the photolysis of 1 in aerated methanol, affording 3-hydroxy-3-phenyl-1,2-indandione (5) as the primary product. Furthermore, in the same paper the photo-reactivity of 5 to give ester derivatives as a secondary photoproduct has been also demonstrated. The same behaviour has been observed in other polar and protic media such as a mixture of isopropanol–benzene.39 The same photo-induced rearrangement has been observed for simpler molecules such as 2-hydroxy-γ-pyrones.45 On the contrary, more recently, Tommasini et al.40 reported that a photo-induced rearrangement process in methanol could be hindered by strong hydrogen bonding between solute and solvent. Unlike this latter report, we have observed only 5 as the major photoproduct and no secondary processes were observed during our experiments. This result is in agreement with Yokoe et al. observations.15Concerning the mechanism, Matsuura et al.39 reported that photo-rearrangement of 1 is due to a [σ2 + π2] cycloaddition of the substrate in its triplet state leading to the epoxy intermediate 5′ (see Scheme 2). Triplet state of 3HF has been characterised in literature by laser flash photolysis studies.46 The presence of oxygen in solution weakly hampers the photo-rearrangement of 3HF, and this behaviour is most probably due to the quenching operated by oxygen on 3HF in triplet state.
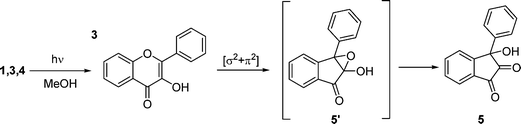 | ||
| Scheme 2 Proposed mechanism for the photolysis of species 1, 3, 4. | ||
Degradation of 1 has been also hampered by the presence of water, whereas no other modification of the photochemical behaviour of 1 has been observed. In the literature, it has been suggested that a triplet state of 3HF is generated during the excited-state proton transfer cycle.47 On the other hand, it is known than solvent–solute interactions play an important role on determining the quantum yield of the excited-state proton transfer process.48,12 As described by Mandal and Samanta,34water can act as a hydrogen bond donor in its interaction with carbonyl of 1. Therefore, a possible explanation is that in a MeOH–water mixture the ESPT between the hydroxyl group and complexed carbonyl is hampered, resulting in a lower quantum yield of triplet formation entailing, as a final consequence, a slower photo-degradation process.
Photo-degradation of complexes 3–4 also gives 5; UV absorption maxima of this do not change in the presence of the examined metals. On the other hand, if a complex between 5 and a cation was present, we would certainly observe a different absorption spectrum. In fact, as the absorption band observed at 297 nm involved a charge transfer from the benzene ring to the keto groups (see the Results section), a mono-or bi-dentate metal complex formation with 5 would have modified significantly the wavelength of the transition.
Also the infrared carbonyl bond stretching of 5 (that could play an important role for cation chelation) situated at 1733 and 1766 cm–1 do not present significant shift. These results suggest the absence of coordinative interactions between the carbonyls of 5 and the metals.
For the complexes, oxygen slightly favours the reactivity of complexes. A similar effect was reported by Tran et al. on the photo-stability of flavotionato metal complexes,49 although it should be noted in that case oxygen favoured degradation of both free ligand and Zinc complex.
On the other hand, in the presence of water, complexes 2 and 3 show the same photostability than in neat methanol, while 4 results less photostable, and this effect is probably due to a different coordination structure with the media.
Since species 1, 3, 4 exhibit the same kind of photoreactivity, we reasoned that the same possible intermediate (which, we recall, Matsuura et al. reported to be the triplet state of 3HF39) could be responsible for their reaction. In order to obtain3(3HF) by irradiation of complexes, a preliminary dissociation between metal and 3HF must take place in an excited state of the complexes. We recall that photo-decomposition experiments were performed by illuminating at the absorption band in the long wavelength range of each complex, so that the possibility that the reaction is given by the portion of free, uncomplexed 3HF present in solution can be ruled out.
High value for fluorescence quantum yield of complex 2 shows that for this complex radiative decaying emission is the predominant phenomenon (Φ = 0.58 in MeOH). Conversely, in the case of complexes 3 and 4 the fluorescence quantum yield is considerable (Φ = 0.38 and 0.12 in MeOH respectively) but is not the only phenomenon occurring during irradiation. So, competitive pathways originating from the first singlet excited state such as formation of 3HF in its triplet state could be allowed. In other words, in complex 2 the huge fluorescence quantum yield (together with other photophysical pathways such as, e.g. internal conversion) prevents competitive photo-reactions, whereas in complexes 4 the enhancement of the fluorescence compared to free 3HF entails just a decreasing in the photo-reactivity.
A very peculiar situation is found for complex 3 in MeOH, where the high fluorescence quantum yield do not imply a decrease in photo-reactivity compared to free 3HF (the reactivity of 1 and 3 are comparable). Even more stunning is the comparison between complexes 3 and 4, with the former showing simultaneously a higher fluorescence quantum yield (0.38 compared to 0.12) and a higher photo-reactivity. Such effect cannot be easily rationalized, and it is most probably related to other factors such as the structure of the complex in the excited state or solvent coordination‡.
Indeed, the key role of the solvent in the mechanism for the photo-reaction (especially of complex 4 and free 3HF) is underlined by the strong change in kinetics in MeOH 50%aq compared to pure MeOH. Particularly interesting is the fact that the presence of water act in opposite ways in the two cases (increasing effect for complex 4, decreasing effect for free 3HF). Further investigations are under way in our laboratories in order to clarify this issue.
In conclusion, we reported that metallic cations such as Zn2+, Pb2+ and Al3+ can influence the rate of photodecomposition of 3HF, although the same photoproduct was formed during irradiation of both free 3HF and its complexes (with the exception of the extremely photo-stable Al(3HF)2+ complex). The photochemistry of species 1, 3 and 4 is influenced by important environmental factor such as presence of water or oxygen. In addition, the primary product of photodecomposition shows no interaction with metals. Thus could represent an important observation in order to investigate the photoreactivity of chelated metals with SOM in the soil. In fact, we could suggest that, as far as the 3-hydroxyflavone moieties are concerned, photo-induced decomposition of SOM could be hampered by complexation with Pb(II) or even, in presence of Al(III) inhibited, concurring at accumulation of organic matter in the soil. On the other hand, when the decomposition occurs, free Zn2+ and Pb2+ cations (representing the most toxic of these pollutants) are generated, leading to important problem in term of pollution and phytotoxicology.
In the future, we plan to extend our investigation to other natural polyphenols and their metal complexes. This could help to understand the influence of light on metal retention by other functional groups such as catechol and COOH. In perspective, we plan to extend our investigation also to humic acids.
Acknowledgements
The authors are grateful to Eng. M. Martel for technical assistance, Dr N. Idrissi for fruitful discussion, and to Prof. A. Albini, Dr D. Dondi and Prof. M. Fagnoni for fruitful discussion and careful reading of the manuscript.S. P. acknowledges financial support from Egide (fellowship French Embassy in Rome and Italian Ministry of Foreign Affairs) and “A. Della Riccia” Foundation, Florence, Italy.
References
- R. L. Wershaw and G. R. Aiken, in Humic substances in soils, sediments and water, Wiley Interscience, New York, 1995 Search PubMed.
- G. Sposito, in The chemistry of soils, Oxford Univeristy Press, New York, 1989, 277 Search PubMed.
- P. Doelman and L. Haanstra, Effect of lead on soil respiration and on the decomposition of organic matter, Soil Biol. Biochem., 1979, 11, 475–479 CrossRef CAS.
- E. Baath, Effects of heavy metals in soil on microbial processes and populations (a review), Water Air Soil Poll., 1989, 47, 335–379 CrossRef CAS.
- I. V. Sokolova and O. N. Tchaikovskaya, Fluorescence and photochemical properties of humic acids, Atmos. Oceanic. Opt., 2006, 19, 220–222 Search PubMed; D. E. Latch and R. McNeil, Microheterogeneity of Singlet Oxygen Distributions in Irradiated Humic Acid Solutions, Science, 2006, 311, 1743–1747 CrossRef CAS and references therein.
- W. L. Miller, Effects of UV radiation on aquatic humus : photochemical principles and experimental considerations, Ecol. Studies, 1998, 133, 125–143 Search PubMed; R. G. Zepp, G. L. Baughman and P. F. Schlotzhauer, Comparison of photochemical behaviour of various humic substances in water: I. Sunlight, induced reactions of aquatic pollutants photosensitized by humic substances, Chemosphere, 1981, 10, 109–117 CrossRef CAS; R. G. Zepp, G. L. Baughman and P. F. Schlotzhauer, Comparison of photochemical behavior of various humic substances in water: II. Photosensitized, oxygenations, Chemosphere, 1981, 10, 119–26 CrossRef CAS and references therein.
- D. Vialaton and C. Richard, Phototransformation of aromatic pollutants in solar light: Photolysis versus photosensitized reactions under natural water conditions, Aquat. Sci., 2002, 64, 207–215 CrossRef CAS.
- B. C. Faust and J. Hoignè, Sensitized photooxidation of phenols by fulvic acids and in natural waters, Environ. Sci. Technol., 1987, 21, 957–964 CrossRef CAS.
- J. Buschmann, S. Canonica and L. Sigg, Photoinduced oxidation of Antimony(III) in the presence of humic acids, Environ. Sci. Technol., 2005, 39, 5335–5341 CrossRef CAS.
- The flavonoids: advances in research since 1986, ed. J. B. Harborne, Chapman-Hall, London, 1994 Search PubMed.
- C. Rice-Evans, Flavonoids antioxidants, Curr. Med. Chem., 2001, 8, 797–807 CAS; P. J. Pietta, Flavonoids as antioxidants, J. Nat. Products, 2000, 63, 1035–1042 Search PubMed.
- A. S. Klymchenko, C. Kenfack, G. Duportail and Y. Mély, Effect of polar protic solvents on dual emissions of 3-hydroxychromones, J. Chem. Sci., 2007, 119, 83–89 Search PubMed and references therein.
- D. McMorrow and M. Kasha, Proton transfer spectroscopy of 3-hydroxychromones. Extreme sensitivity to hydrogen-bonding perturbations, J. Am. Chem. Soc., 1983, 105, 5133–5134 CrossRef CAS; D. McMorrow and M. Kasha, Proton-transfer spectroscopy on 3-hydroxyflavone in an isolated-site crystal matrix, Proc. Natl. Acad. Sci. USA, 1984, 81, 3375–3378 CrossRef CAS.
- D. McMorrow and M. Kasha, Intramolecular excited-state proton transfer in 3-hydroxyflavone. Hydrogen-bonding solvent perturbations, J. Phys. Chem., 1984, 88, 2235–2243 CrossRef CAS.
- I. Yokoe, K. Higugi, Y. Shirataki and M. Komatsu, Photochemistry of Flavonoids. III. Photorearrangement, of Flavonols, Chem. Pharm. Bull., 1981, 29, 894–898 CAS.
- S. L. Studer, W. E. Brewer, M. L. Martinez and P.-T. Chou, Time-resolved Study of the Photooxygenation of 3-Hydroxyflavone, J. Am. Chem. Soc., 1989, 111, 7643–7644 CrossRef CAS.
- B. J. Schwartz, L. A. Peteanu and C. B. Harris, Direct observation of fast proton transfer: femtosecond photophysics of 3-hydroxyflavone, J. Phys. Chem., 1992, 96, 3591–3598 CrossRef CAS and references therein.
- J. Woolfe and P. J. Thistlethwaite, Direct observation of Excited State Intramolecular Proton Transfer Kinetics in 3-Hydroxyflavone, J. Am. Chem. Soc., 1981, 103, 6916–6923 CrossRef CAS.
- P. Chou, D. McMorrow, T. J. Aartsma and M. Kasha, The proton-transfer laser: gain spectrum and amplification of spontaneous emissiom of 3-hydroxyflavone, J. Phys. Chem., 1984, 88, 4596–4599 CrossRef CAS.
- E. Biagtan, E. Goldberg, R. Stephens, E. Valeroso and J. Harmon, Gamma radiation dose rate effects on a polymer scintillator containing a large Stokes shift dye, 3-hydroxyflavone, Nucl. Instrum. Methods Phys. Res., Sect. B, 1996, 114, 88–90 CrossRef CAS.
- S. Carturan, A. Quaranta, G. Maggioni, M. Bonafini and G. Della, Mea, 3-Hydroxyflavone-based wavelength shifting system for near UV optical sensors, Sens. Actuators, A, 2004, 113, 288–292 CrossRef.
- B. Gerard, G. Jones II and J. A. Porco, Jr., A biomimetic approach to the rocaglamides employing photogeneration of oxidopyryliums derived from 3-hydroxyflavones, J. Am. Chem. Soc., 2004, 126, 13620–13621 CrossRef CAS; J. A. Porco, Jr., B. Gerald, G. Jones II, Synthesis of rocaglamide natural products via photochemical generation of oxydopyrylium species, PCT Int. Appl., WO2005-US10005-20050323, 2005 Search PubMed.
- V. V. Shynkar, A. S. Klymchenko, C. Kunzelmann, G. Duportail, C. D. Muller, A. P. Demchenko, J.-M. Freyssinet and Y. Mely, Fluorescent biomembrane probe for ratiometric detection of apoptosis, J. Am. Chem. Soc., 2007, 129, 2187–2193 CrossRef CAS and references therein.
- N. Binbuga, W. P. Henry and T. P. Schultz, Binding of hydroxychromones with Al3+ in methanol, Polyhedron, 2007, 26, 6–10 CrossRef CAS.
- J.-P. Cornard, L. Dangleterre and C. Lapouge, DFT and TD-DFT investigation and spectroscopic characterization of the molecular and electronic structure of Zn(II)-3-hydroxyflavone complex, Chem. Phys. Lett., 2006, 419, 304–308 CrossRef CAS.
- L. Dangleterre and J.-P. Cornard, Interation of lead(II) chloride with hydroxyflavones in methanol: A spectroscopic study, Polyhedron, 2005, 24, 1593–1598 CrossRef CAS; C. Lapouge and J. P. Cornard, time dependent density functional theory study of electronic absorption properties of lead(II) complexes with a series of hydroxyflavones, J. Phys. Chem. A, 2005, 109, 6752–6761 CrossRef CAS.
- A.-C. Boudet, J.-P. Cornard and J.-C. Merlin, Conformational and spectroscopic investigation of 3-hydroxyflavone-aluminium chelates, Spectrochim. Acta, Part A, 2000, 56, 829–839 CrossRef CAS.
- D. S. Smith and J. R. Kramer, Multisite metal binding to fulvic acid determined using multiresponse fluorescence, Anal. Chim. Acta, 2000, 416, 211–220 CrossRef CAS; D. S. Smith and J. R. Kramer, Fluorescence analysis for multi-site aluminum binding to natural organic matter, Environ. Inter., 1999, 25, 295–306 Search PubMed.
- C. B. Coulson, R. I. Davies and D. A. Lewis, Polyphenols in plant, humus and soil. I. Polyphenols, of leaves litter and superficial humus from mull and more sites, J. Soil Sci., 1960, 11, 20–29 Search PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, J. A. Montgomery, Jr., T. Vreven, K. N. Kudin, J. C. Burant, J. M. Millam, S. S. Iyengar, J. Tomasi, V. Barone, B. Mennucci, M. Cossi, G. Scalmani, N. Rega, G. A. Petersson, H. Nakatsuji, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, M. Klene, X. Li, J. E. Knox, H. P. Hratchian, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. Ochterski, P. Y. Ayala, K. Morokuma, G. A. Voth, P. Salvador, J. J. Dannenberg, V. G. Zakrzewski, S. Dapprich, A. D. Daniels, M. C. Strain, O. Farkas, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. V. Ortiz, Q. Cui, A. G. Baboul, S. Clifford, J. Cioslowski, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. L. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A. Nanayakkara, M. Challacombe, P. M. W. Gill, B. G. Johnson, W. Chen, M. W. Wong, C. Gonzalez and J. A. Pople, GAUSSIAN 03 (Revision B.04), Gaussian, Inc., Wallingford, CT, 2003 Search PubMed.
- A. D. Becke, Density-functional thermochemistry. III. The, role of exact exchange, J. Chem. Phys., 1993, 98, 5648–5652 CrossRef CAS; C. Lee, W. Yang and R. G. Parr, Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density, Phys. Rev. B, 1988, 37, 785–789 CrossRef CAS.
- R. Bauernschmitt and R. Ahlrichs, Treatment of electronic excitations within the adiabatic approximation of time dependent density functional theory, Chem. Phys. Lett., 1996, 256, 454–464 CrossRef CAS.
- M. Cossi, G. Scalmani, N. Rega and V. Barone, New developments in the polarizable continuum model for quantum mechanical and classical calculations on molecules in solution, J. Chem. Phys., 2002, 117, 43–54 CrossRef CAS.
- P. K. Mandal and A. Samanta, Evidence of ground-state proton-transfer reaction of 3-hydroxyflavone in neutral alcoholic solvents, J. Phys. Chem A, 2003, 107, 6334–6339 CrossRef CAS.
- J.-P. Cornard, L. Vrielynck, J.-C. Merlin and J.-C. Wallet, Structural and vibrational study of 3-hydroxyflavone and 3-methoxyflavone, Spectrochim. Acta, Part A, 1995, 51, 913–923 CrossRef.
- M. Wang, T. Teslova, F. Xu, T. Spataru, J. R. Lombardi and R. L. Birke, Raman and surface enhanced Raman scattering of 3-Hydroxyflavone, J. Phys. Chem. C, 2007, 111, 3038–3043 CrossRef CAS.
- E. B. Wilson, The Normal Modes and Frequencies of Vibration of the Regular Plane Hexagon Model of the Benzene Molecule, Phys. Rev., 1934, 45, 706–714 CrossRef CAS.
- G. Varsanyi, in Assignment for vibrational spectra of seven hundreds benzene derivatives, ed. L. Lang, Hilger, Budapest, 1974 Search PubMed.
- T. Matsuura, T. Takemoto and R. Nakashima, Photoinduced reactions-LXXI. Photorearrengements, of 3-hydroxyflavones to 3-aryl-3-Hydroxy-1,2-indandione, Tetrahedron, 1973, 29, 3337–3340 CrossRef CAS and references therein.
- R. Ficarra, P. Ficarra, S. Tommasini, S. Campagna and G. Guglielmo, Photochemistry of flavonoids. Solvent effect on photochemical of 3-hydroxyflavone, Boll. Chim. Farm., 1994, 665–669 Search PubMed; S. Tommasini, M. L. Calabroprime;, P. Donato, D. Raneri, G. Guglielmo, P. Ficarra and R. Ficarra, J. Pharm. Biomed. Anal., 2004, 35, 389–397 CrossRef CAS.
- C. Reichardt, in Solvents and solvent effects in organic chemistry, VCH, Weinheim, Germany, 1988, p. 378, ch. 7 Search PubMed.
- F. L. Urbach and A. Timnick, Spectrophotometric and spectrofluorimetric study of Flavonol-Aluminum chelates in absolute ethyl alcohol, Anal. Chem., 1968, 40, 1269–1272 CrossRef CAS.
- M. Katyal, Flavones as analytical reagents - A review, Talanta, 1968, 15, 98–106.
- P. C. H. Hollman, J. M. P. van Trijp and M. N. C. P. Buysman, Fluorescence detection of flavonols in HPLC by postcolumn chemation with Aluminum, Anal. Chem., 1996, 68, 3511–3515 CrossRef CAS.
- M. Shozaki and T. Hiraoka, Photochemistry of β-hydroxy-γ-pyrone. A new synthesis of 3-methylcyclopent-2-en-2-ol-1-one from maltol, Tetrahedron Lett., 1972, 13, 4655–4658 CrossRef.
- K. Tokumura, M. Kurauchi, N. Yagata and M. Itoh, Phototautomerization of 3-hydroxyflavone in the lowest triplet state, Chem. Phys. Lett., 1996, 258, 495–500 CrossRef CAS; M. Itoh, Y. Tanimoto and K. Tokumura, Transient absorption study of the intramolecular excited state and ground-state proton transfer in 3-hydroxyflavone and 3-hydroxychromone, J. Am. Chem. Soc., 1983, 105, 3339–3340 CrossRef CAS.
- W. E. Brewer, S. L. Studer, M. Standiford and P.-T. Chou, Dynamics of the triplet state and reverse proton transfer of 3-hydroxyflavone, J. Phys. Chem., 1989, 93, 6088–6094 CrossRef CAS.
- A. J. Strandjord and P. F. Barbara, Proton-transfer kinetics of 3-hydroxyflavone: solvent effects, J. Phys. Chem., 1985, 89, 2355–2361 CrossRef CAS.
- B. L. Tran and S. M. Cohen, Flavothionato metal complexes: implications for the use of Hydroxyflavothiones as green pesticides, Chem. Commun., 2006, 203–205 RSC.
Footnotes |
| † Indeed, the spectrum of the anion in this medium (obtained by addition of NaOH) is characterized by an emission centred at 505 nm (data not shown). Indeed, we have also investigated the emission of 3HF anion in pure water trying to reproduce the data reported in ref. 14. Aqueous solutions in the pH range 12–14 were investigated, showing a broad emission centred at 504 nm instead of 515 nm as reported before14 (data not shown). |
| ‡ In this framework, it is also important to mention that the structure of the complex in its fundamental state is also strongly dependent on the solvent (J.P. Cornard, L. Dangleterre, data not published). |
| This journal is © The Royal Society of Chemistry and Owner Societies 2008 |
