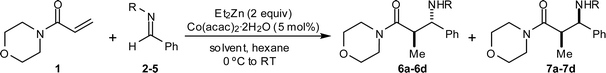
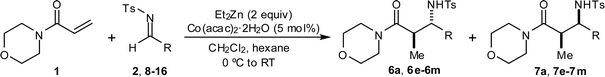
Cobalt-catalyzed reductive Mannich reactions of 4-acryloylmorpholine with N-tosyl aldimines †‡
Oscar
Prieto
and
Hon Wai
Lam
*
School of Chemistry, University of Edinburgh, Joseph Black Building, King’s Buildings, West Mains Road, Edinburgh, EH9 3JJ, UK. E-mail: h.lam@ed.ac.uk; Fax: +44 (0)131 650 6453; Tel: +44 (0)131 650 4831
First published on 19th November 2007
Abstract
Using cobalt catalysis, diethylzinc promotes the conjugate reduction of 4-acryloylmorpholine to produce the corresponding ethylzinc enolate, which reacts with N-tosyl aldimines to afford β-aminoamides.
Chiral β-amino acids and their derivatives are important compounds due to their presence in natural products and biologically active compounds, as building blocks for the assembly of β-peptides, and as precursors to β-lactams.1 One of the most powerful methods for accessing β-aminocarbonyl compounds is the Mannich reaction,2 and recent years have witnessed many advances in the development of new procedural variants,2b and catalytic asymmetric variants3 using chiral metal-based catalysts4 or organocatalysts.5 These reactions typically require either the use of preformed enolates such as silyl enol ethers , or acid/base-mediated enolization of aldehydes , ketones , or relatively acidic ester equivalents such as malonate esters and glycineimine esters . An alternative strategy for enolization that has received more limited attention in Mannich reactions is the use of α,β-unsaturated carbonyl compounds as “latent enolates ”.6 Here, conjugate reduction of an α,β-unsaturated carbonyl compound with a hydride source generates an enolate that is then trapped with a suitable imine . Reductive Mannich reactions have been described in racemic form by Isayama,7a and the groups of Morken,7b Matsuda,7c Krische,7d and Nishiyama.7e In addition, Córdova and Zhao reported a sequential organocatalytic asymmetric conjugate reduction–Mannich reaction of β,β-disubstituted α,β-unsaturated aldehydes .8
In recent studies, we disclosed that ethylzinc enolates may be generated via conjugate reduction by treatment of α,β-unsaturated amides with diethylzinc and a substoichiometric quantity of a cobalt salt,9 and that these enolates could be trapped in situ with ketones in both intramolecular9 and intermolecular10 aldol reactions. In this communication, we show that zinc enolates generated in this fashion also undergo intermolecular Mannich reactions with N-sulfonyl aldimines .
Our preliminary experiments commenced with reaction of commercially available 4-acryloylmorpholine (1) with a range of benzaldehyde-derived imines to identify the optimum nitrogen protecting group (Table 1). Using N-tosyl imine 2 and THF as solvent, the desired Mannich product was obtained as a 68 : 32 ratio of anti : syn diastereoisomers, which were isolated in 47% and 14% yields respectively (entry 1).11 Switching the solvent to CH2Cl2 afforded improved results (entry 2). Although N-(2-thienyl)sulfonyl imine 34c provided a slight improvement in the diastereoselectivity, the isolated yield of the major anti isomer 6b was modest (entry 3). N-Diphenylphosphinoyl imine 4 and N-tert-butoxycarbonyl imine 5 provided only complex mixtures, with minimal quantities of Mannich products being detected (entries 4–5).
|
|
|||||||
|---|---|---|---|---|---|---|---|
| Entry | R | Imine | Solvent | Products | Anti : synb | Yield (%)c | |
| Anti (6) | Syn (7) | ||||||
| a Reactions were conducted using 1.1 mmol of 1 and 1.0 mmol of imine for 5 h. b Determined by 1H NMR analysis of the unpurified reaction mixtures. c Isolated yields. d A complex mixture was obtained. | |||||||
| 1 | Ts | 2 | THF | 6a/7a | 68 : 32 | 47 | 14 |
| 2 | Ts | 2 | CH2Cl2 | 6a/7a | 80 : 20 | 72 | 11 |
| 3 | SO2(2-thienyl) | 3 | CH2Cl2 | 6b/7b | 83 : 17 | 52 | 10 |
| 4 | P(O)Ph2 | 4 | CH2Cl2 | 6c/7c | n/a | 0d | 0d |
| 5 | Boc | 5 | CH2Cl2 | 6d/7d | n/a | 0d | 0d |
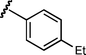
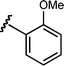
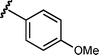
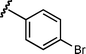
Based upon these results, we examined the scope of the reductive Mannich reaction of 1 (Table 2). A range of N-tosyl imines were found to undergo reductive coupling with 1 in moderate to good yields and with up to 88 : 12 dr. Imines prepared from benzaldehyde derivatives containing electron-donating substituents such as methyl (entries 2–3), ethyl (entry 4), and methoxy (entries 5–6) groups provided better results than those containing electron-withdrawing groups (entries 7–8). The process is not restricted to imines derived from substituted benzaldehydes. Imines 15 and 16, containing 1-naphthyl and 2-furyl groups respectively, were also tolerated in the reaction (entries 9–10). At the current level of development, we have not yet been able to induce β-substituted α,β-unsaturated morpholine amides or N-tosylimines derived from aliphatic aldehydes to undergo the reductive Mannich reaction with satisfactory yields.12
|
|
||||||
|---|---|---|---|---|---|---|
| Entry | R | Imine | Products | Anti : synb | Yield (%)c | |
| Anti (6) | Syn (7) | |||||
| a Reactions were conducted using 1.1 mmol of 1 and 1.0 mmol of imine for 5 h. b Determined by 1H NMR analysis of the unpurified reaction mixtures. c Isolated yields. d The syn-Mannich product could not be isolated in pure form. e A complex mixture was obtained. | ||||||
| 1 | Ph | 2 | 6a/7a | 80 : 20 | 72 | 11 |
| 2 |
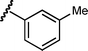
|
8 | 6e/7e | 78 : 22 | 65 | 9 |
| 3 |
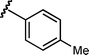
|
9 | 6f/7f | 88 : 12 | 67 | 11 |
| 4 |
|
10 | 6g/7g | 86 : 14 | 78 | —d |
| 5 |
|
11 | 6h/7h | 87 : 13 | 49 | 7 |
| 6 |
|
12 | 6i/7i | 87 : 13 | 54 | 8 |
| 7 |
|
13 | 6j/7j | 83 : 17 | 44 | —d |
| 8 |
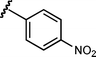
|
14 | 6k/7k | n/a | 0e | 0e |
| 9 |
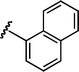
|
15 | 6l/7l | 82 : 18 | 49 | 11 |
| 10 |
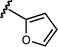
|
16 | 6m/7m | 64 : 36 | 36 | 17 |
In conclusion, we have developed a new variant of the reductive Mannich reaction, involving the coupling of commercially available 4-acryloylmorpholine (1) with a range of aromatic N-tosyl aldimines using diethylzinc as the stoichiometric reductant, and an inexpensive cobalt salt as the precatalyst. Furnishing acyclic anti-β-aminoamides as the major isomers, these reactions complement existing syn-selective reductive Mannich reactions7a,d and Morken’s methodology that provides β-lactams as the products.7b Compared with existing anti-selective reductive Mannich reactions that give acyclic products using β-unsubstituted α,β-unsaturated carbonyls as pronucleophiles,7c,e the present method delivers products with comparable or higher levels of diastereoselectivity. Expansion of the substrate scope of these reactions, and the development of asymmetric variants will be the subjects of future work in this area.
This work was supported by the EPSRC. We thank Professor Simon Parsons, Russell D. L. Johnstone, and Alessandro Prescimone at the School of Chemistry, University of Edinburgh for assistance with X-ray crystallography. We thank the EPSRC National Mass Spectrometry Service Centre at the University of Wales, Swansea for providing high resolution mass spectra.
Notes and references
- (a) Enantioselective Synthesis of β-Amino Acids, ed. E. Juarist, Wiley-VCH, New York, 1997 Search PubMed; (b) M. Liu and M. P. Sibi, Tetrahedron, 2002, 58, 7991–8035 CrossRef CAS; (c) S. Abele and D. Seebach, Eur. J. Org. Chem., 2000, 1–15 CrossRef CAS; (d) S. H. Gellman, Acc. Chem. Res., 1998, 31, 173–180 CrossRef CAS.
- (a) E. F. Kleinmann, in Comprehensive Organic Synthesis, ed. B. M. Trost and I. Fleming, Pergamon Press, New York, 1991, vol. 2, ch. 4.1 Search PubMed; (b) M. Arend, B. Westermann and N. Risch, Angew. Chem., Int. Ed., 1998, 37, 1044–1070 CrossRef.
- For reviews, see: (a) S. Kobayashi and H. Ishitani, Chem. Rev., 1999, 99, 1069–1094 CrossRef CAS; (b) A. E. Taggi, A. M. Hafez and T. Lectka, Acc. Chem. Res., 2003, 36, 10–19 CrossRef CAS; (c) A. Córdova, Acc. Chem. Res., 2004, 37, 102–112 CrossRef CAS; (d) M. M. B. Marques, Angew. Chem., Int. Ed., 2006, 45, 348–352 CrossRef CAS.
- For selected recent examples, see: (a) H. Murimoto, G. Lu, N. Aoyama, S. Matsunaga and M. Shibasaki, J. Am. Chem. Soc., 2007, 129, 9588–9589 CrossRef; (b) Y. Suto, M. Kanai and M. Shibasaki, J. Am. Chem. Soc., 2007, 129, 500–501 CrossRef CAS; (c) A. S. González, R. G. Arrayás and J. C. Carretero, Org. Lett., 2006, 8, 2977–2980 CrossRef CAS; (d) T. Hamada, K. Manabe and S. Kobayashi, Chem.–Eur. J., 2006, 12, 1205–1215 CrossRef CAS; (e) B. M. Trost, J. Jaratjaroonphong and V. Reutrakul, J. Am. Chem. Soc., 2006, 128, 2778–2779 CrossRef CAS; (f) S. Harada, S. Handa, S. Matsunaga and M. Shibasaki, Angew. Chem., Int. Ed., 2005, 44, 4365–4368 CrossRef CAS; (g) T. Yoshida, H. Morimoto, N. Kumagai, S. Matsunaga and M. Shibasaki, Angew. Chem., Int. Ed., 2005, 44, 3470–3474 CrossRef CAS; (h) M. Marigo, A. Kjaersgaard, K. Juhl, N. Gathergood and K. A. Jørgensen, Chem.–Eur. J., 2003, 9, 2359–2367 CrossRef CAS.
- For selected recent examples, see: (a) Q.-X. Guo, L. Hua, C. Guo, S.-W. Luo, Y. Gi and L.-Z. Gong, J. Am. Chem. Soc., 2007, 129, 3790–3791 CrossRef CAS; (b) J. Y. Yang, M. Stadler and B. List, Angew. Chem., Int. Ed., 2007, 46, 609–611 CrossRef CAS; (c) S. S. V. Ramasastry, H. Zhang, F. Tanaka and C. F. Barbas, III, J. Am. Chem. Soc., 2007, 129, 288–289 CrossRef CAS; (d) F. Fini, L. Bernadi, R. P. Herrera, D. Petterson, A. Ricci and V. Sgarzani, Adv. Synth. Catal., 2006, 348, 2043–2046 CrossRef CAS; (e) J. Song, Y. Wang and L. Deng, J. Am. Chem. Soc., 2006, 128, 6048–6049 CrossRef CAS; (f) A. L. Tilman, J. Ye and D. J. Dixon, Chem. Commun., 2006, 1191–1193 RSC; (g) S. Mitsumori, H. Zhang, P. H.-Y. Cheong, K. N. Houk, F. Tanaka and C. F. Barbas, III, J. Am. Chem. Soc., 2006, 128, 1040–1041 CrossRef CAS; (h) S. Lou, B. M. Taoka, A. Ting and S. E. Schaus, J. Am. Chem. Soc., 2005, 125, 11256–11257 CrossRef.
- R. R. Huddleston and M. J. Krische, Synlett, 2003, 12–21 CAS.
- (a) S. Isayama, Yuki Gosei Kagaku Kyokaishi, 1992, 50, 190–201 Search PubMed; (b) J. A. Townes, M. A. Evans, J. Queffelec, S. J. Taylor and J. A. Morken, Org. Lett., 2002, 4, 2537–2540 CrossRef CAS; (c) T. Muraoka, S.-i. Kamiya, I. Matsuda and K. Itoh, Chem. Commun., 2002, 1284–1285 RSC; (d) S. A. Garner and M. J. Krische, J. Org. Chem., 2007, 72, 5843–5846 CrossRef CAS; (e) H. Nishiyama, J. Ishikawa and T. Shiomi, Tetrahedron Lett., 2007, 48, 7841–7844 CrossRef CAS.
- G.-L. Zhao and A. Córdova, Tetrahedron Lett., 2006, 47, 7417–7421 CrossRef CAS.
- H. W. Lam, P. M. Joensuu, G. J. Murray, E. A. F. Fordyce, O. Prieto and T. Luebbers, Org. Lett., 2006, 8, 3729–3732 CrossRef CAS.
- R. J. R. Lumby, P. M. Joensuu and H. W. Lam, Org. Lett., 2007, 9, 4367–4370 CrossRef CAS.
- The relative stereochemistries of the major diastereomers obtained from these reactions were assigned as anti on the basis of the X-ray crystal structures of products 6b, 6h and 6i. See ESI† for further details.
- Reactions conducted using CoCl2–Cy2PPh (see ref. 9 and 10) in place of Co(acac)2·2H2O offered no improvement.
Footnotes |
| † Electronic supplementary information (ESI) available: General information, preparation of imines , cobalt-catalyzed reductive Mannich reactions, stereochemical determinations and NMR spectra of new compounds. See DOI: 10.1039/b715839d |
| ‡ CCDC reference numbers 658714–658716. For crystallographic data in CIF or other electronic format see DOI: 10.1039/b715839d |
| This journal is © The Royal Society of Chemistry 2008 |