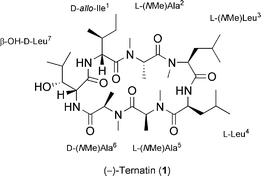
Synthesis and inhibitory effect on fat accumulation of (−)-ternatin derivatives modified in the β-OH-D-Leu7 moiety†
Kenichiro
Shimokawa
a,
Yoshiaki
Iwase
a,
Kaoru
Yamada
a and
Daisuke
Uemura
*ab
aDepartment of Chemistry, Graduate School of Science, Nagoya University, Furo-cho, Chikusa, Nagoya 464-8602, Japan. E-mail: uemura@chem3.chem.nagoya-u.ac.jp; Fax: 81-52-789-3654; Tel: 81-52-789-3654
bInstitute for Advanced Research, Nagoya University, Furo-cho, Chikusa, Nagoya 464-8602, Japan
First published on 22nd November 2007
Abstract
An efficient synthesis of (−)-ternatin derivatives directed toward their SAR at the β-OH-D-Leu7 moiety and their biological activities against 3T3-L1 murine adipocytes are described.
(−)-Ternatin (1) is a highly N-methylated cyclic heptapeptide that was isolated from the mushroom Coriolus versicolor during our continuing search for potential anti-obesity agents from natural resources such as mushrooms. In our previous paper, we described the isolation, structure elucidation and synthesis of 1, which potently inhibited fat accumulation against 3T3-L1 murine adipocytes.1 Additionally, we also reported a concise synthesis of 1 in solution and its in vivo biological activity.2 Treatment with 1 at 5 mg kg−1 per day was found to suppress the increase in body weight and fat accumulation in diet-induced obese mice.
To further evaluate 1 as a new lead compound for therapeutic development, we commenced on new research to clarify the detailed mode of action of 1 with regard to fat-accumulation inhibition in adipocytes. Moreover, a structure–activity relationship (SAR) study of 1 was first investigated in parallel, aimed at the recognition of the importance of side chain functionalities as well as a suitable site for advanced functionalization in its structure, e.g., biotinylation and introduction of a fluorescent unit.
Structurally, the existence of non-coded (D- and N-methylated) amino acids is a novel feature in 1. Of particular interest is the effect on biological activity of modifying the unusual β-OH-D-Leu [(2R,3R)-3-hydroxyleucine], which is a key constituent of 1. Based on an analysis of the X-ray crystal structure of (−)-ternatin (1),3 it is proposed that 1 adopts type II β-turn structure in the region of L-Leu4 to β-OH-D-Leu7 moieties with the assistance of a novel intramolecular H-bond network (Fig. 1A). In this network, the OH group in the β-OH-D-Leu7 moiety is supposed to contribute toward the stabilization of the macrocyclic conformation of 1 by forming a H-bond between the OH proton (HC) in that moiety and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in the Ile1 moiety, whereas two H-bonds of NH protons (HA, HB) act strongly on stabilizing β-turn structure. Presumably, these intramolecular H-bonds are key interactions in 1, since they build the β-turn structure, which is one of the major motifs of peptide and protein secondary structure playing a key role in many biological processes.4,5
O in the Ile1 moiety, whereas two H-bonds of NH protons (HA, HB) act strongly on stabilizing β-turn structure. Presumably, these intramolecular H-bonds are key interactions in 1, since they build the β-turn structure, which is one of the major motifs of peptide and protein secondary structure playing a key role in many biological processes.4,5
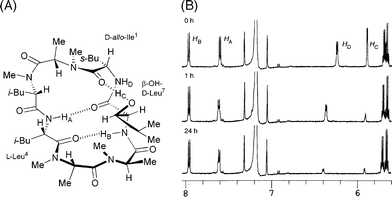 | ||
| Fig. 1 (A) Stereostructure of 1 fixed by intramolecular H-bond networks (indicated with dotted lines); (B) H–D exchange experiment evaluated in 1H NMR spectrum (600 MHz) in C6D6 with addition of D2O. | ||
In order to demonstrate the intramolecular H-bonds of compound 1 in solution, we evaluated hydrogen–deuterium (H–D) exchange properties of NH and OH protons (Fig. 1B). The experiment was conducted by adding 20 μL of D2O in a C6D6 solution. As a result, NH protons HA and HB remained over 24 h, expectedly. Meanwhile, a OH protonHC and a NH protonHD smoothly exchanged within 24 h. These results strongly suggested the existence of intramolecular H-bonds of two NH protons, HA and HB. However, the possibility of a H-bond of HC was unclear due to the flexible nature of the OH proton.
To confirm directly whether the β-OH-D-Leu7 moiety is important for the bioactivity of 1, chemical modification at this position was first investigated. We describe here the first and efficient synthesis of ternatin derivatives and the SAR at the β-OH-D-Leu7 moiety with regard to the inhibitory activity of fat accumulation against 3T3-L1 adipocytes.
For this purpose, we designed two types of derivatives, a non-OH series [D-Ala7-1 (1a), D-Leu7-1 (1b), D-Ser(OBn)7-1 (1c)] and a OH series [D-Thr7-1 (1d) and D-Ser7-1 (1e)], as final targets. The latter compounds were used to realize whether β-OH-D-Leu7 could be replaced with normal β-hydroxy-α-amino acids such as serine and threonine.
Synthesis of the derivatives 1a–e was performed in solution by exploiting our efficient synthetic route to 1, which is amenable to large-scale synthesis for extensive biological evaluations. Beginning with four D-amino acid methyl esters 2a–d, we assembled tripeptides 5a–d in the C to N direction (Scheme 1). Couplings of 2a–d with Boc-NMe-D-Ala-OH provided dipeptides 3a–d. The Boc deprotections followed by second HATU-mediated condensations with Boc-NMe-L-Ala-OH gave tripeptides 4a–d.
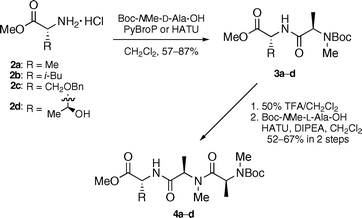 | ||
| Scheme 1 Synthesis of the carboxylic acid fragments 4a–d. | ||
The final conversions to the desired 1a–d were elaborated by key fragment couplings and cyclization reactions (Scheme 2). The alanine methyl esters of 4a–d were cleaved by treatment with LiOH to yield the carboxylic acid fragments 5a–d as key intermediates. Attempts to couple 5a–d with amine 7, prepared by the Boc deprotection of tetrapeptide 6, proceeded smoothly to provide the linear peptides 8a–d in 65–100% yields from 5a–d. The leucine methyl esters were then saponified, and the resulting carboxylic acids were subjected to Boc deprotection. Finally, the key macrolactamizations of the linear peptides were accomplished in the presence of HATU, HOAt and DIPEA under dilute conditions to generate the cyclized products 1a–d in successful yields (56–62% yields from 8a–d). Furthermore, modification of compound 1c was performed. The Bn protecting group of 1c was removed by hydrogenolysis to give 1e in 74% yield.
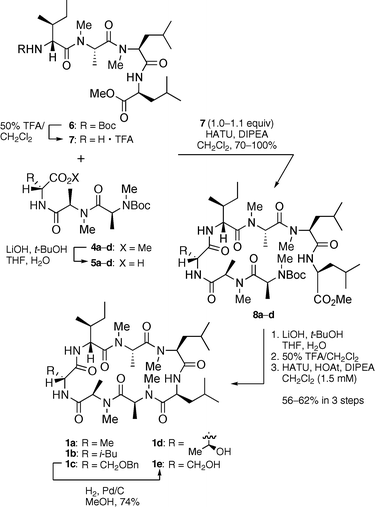 | ||
| Scheme 2 Synthesis of ternatin derivatives 1a–e. | ||
The bioactivities of synthetic derivatives were then evaluated with regard to their inhibitory effect on fat accumulation and cytotoxicity against 3T3-L1 murine adipocytes. Table 1 summarizes the biological activities of derivatives 1a–e with two controls, (−)-ternatin (1) and (−)-noradrenaline (+)-bitartrate salt, respectively. Based on the results, all synthetic derivatives were found to exhibit an inhibitory effect. The potency of inhibition tended to decrease in the order 1b > 1d > 1c > 1a > 1e. Interestingly, the simplified D-Leu7-derivative 1b displayed 8-fold lower activity compared to compound 1 which differs only in the presence of the hydroxy group in the β-position of the Leu7 moiety. In contrast, other derivatives showed less activity (120–520 fold) than 1, though they still remained active. It is interesting to note that synthetic derivatives possessed almost the same IC50 values of fat-accumulation inhibition independent of the presence of the hydroxy group in their structures [a non-OH series (1a and 1c) vs. a OH series (1d and 1e)].
| Compound | Fat-accumulation inhibitory effect: IC50/μM | Cell viability b: IC50/μM |
|---|---|---|
| a Values are means of quadruplicate determinations. b Cell viability was calculated independently to exclude undesired fat-accumulation inhibition arising from the toxicity of tested compounds. At the concentration of 50% fat-accumulation inhibition (IC50), no cell toxicity was observed for any of the compounds. | ||
| [D-Ala7]ternatin (1a) | 14 ± 4 | >150 |
| [D-Leu7]ternatin (1b) | 0.22 ± 0.02 | 3.3 ± 0.2 |
| [D-Ser(OBn)7]ternatin (1c) | 5.9 ± 1 | 89 ± 10 |
| [D-Thr7]ternatin (1d) | 3.1 ± 0.4 | 51 ± 9 |
| [D-Ser7]ternatin (1e) | 12 ± 3 | >150 |
| (−)-Ternatin (1) | 0.027 ± 0.003 | 0.28 ± 0.03 |
| (−)-Noradrenaline (+)-bitartrate salt | 260 ± 6 | >300 |
With regard to the amino acid in the 7-position in 1, the results indicated that the specific side chain, i.e., isobutyl group, is strictly required for potent bioactivity. On the other hand, the presence of the hydroxy group in the β-position of the amino acid residue is not absolutely important. Therefore, it may be possible to install new functional groups at this position, since all derivatives still possess considerable activity.
At 10–15 fold higher concentrations compared to those of IC50 values of the fat-accumulation inhibitory effect, cytotoxicity was observed for all derivatives. This may suggest that fat-accumulation inhibition is the direct cause of toxicity at higher concentration. In addition, the fat-accumulation inhibitory effect of compounds may be caused by the prevention of adipogenesis. Further investigation on the mechanism of inhibition is urgently needed.
In conclusion, we have constructed a series of ternatin derivatives modified in the β-OH-D-Leu7 moiety based on our convergent synthetic route. Actually, unusual β-OH-leucine is a novel class of amino acid that is commonly found in pharmacologically important cyclic peptides such as lysobactin,6 YM-254890,7 GE3,8 and cyclosporin A9,10 (as MeBmt; (4R)-4-[(E)-butenyl]-4,N-dimethyl-L-threonine). On the other hand, we found that the naturally occurring β-OH-D-Leu7 in 1 is not absolutely required but is important for the potent inhibitory effect on fat accumulation against 3T3-L1 adipocytes. Further studies on this bioactive molecule are ongoing.
This study was supported in part by Grants-in-Aid for Scientific Research for Creative Scientific Research (16GS0206) and the 21st century COE program (Establishment of COE on Materials Science) from the Ministry of Education, Culture, Sports, Science and Technology, Japan. We are indebted to Ono Pharmaceutical Co., Ltd. and Banyu Pharmaceutical Co., Ltd. for their financial support.
Notes and references
- (a) K. Shimokawa, I. Mashima, A. Asai, K. Yamada, M. Kita and D. Uemura, Tetrahedron Lett., 2006, 47, 4445 CrossRef CAS; (b) K. Shimokawa, I. Mashima, A. Asai, T. Ohno, K. Yamada, M. Kita and D. Uemura, Chem. Asian J. DOI:10.1002/asia.200700243.
- K. Shimokawa, K. Yamada, M. Kita and D. Uemura, Bioorg. Med. Chem. Lett., 2007, 17, 4447 CrossRef CAS.
- R. Miller, N. M. Galitsky, W. L. Duax, D. A. Langs, V. Z. Pletnev and V. T. Ivanov, Int. J. Pept. Protein Res., 1993, 42, 539 CAS.
- H. Kessler, Angew. Chem., Int. Ed. Engl., 1982, 21, 512 CrossRef.
- A. J. Souers and J. A. Ellman, Tetrahedron, 2001, 57, 7431 CrossRef CAS.
- B. A. Tymiak, T. J. McCormick and S. E. Unger, J. Org. Chem., 1989, 54, 1149 CrossRef CAS.
- M. Taniguchi, K. Suzumura, K. Nagai, T. Kawasaki, T. Saito, J. Takasaki, K. Suzuki, S. Fujita and S. Tsukamoto, Tetrahedron, 2003, 59, 4533 CrossRef CAS.
- (a) Y. Sakai, T. Yoshida, T. Tsujita, K. Ochiai, T. Agatsuma, Y. Saitoh, F. Tanaka, T. Akiyama, S. Akinaga and T. Mizukami, J. Antibiot., 1997, 50, 659 CAS; (b) T. Agatsuma, Y. Sakai, T. Mizukami and Y. Saitoh, J. Antibiot., 1997, 50, 704 CAS.
- A. Rüegger, M. Kuhn, H. Lichti, H. R. Loosli, R. Huguenin, C. Quiquerez and A. von Wartburg, Helv. Chim. Acta, 1976, 59, 1075 CrossRef CAS.
- D. H. Rich, M. K. Dhaon, B. Dunlap and S. P. F. Miller, J. Med. Chem., 1986, 29, 978 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details. See DOI: 10.1039/b714710d |
| This journal is © The Royal Society of Chemistry 2008 |