Remarkable incorporation of the first sulfur containing indole derivative: another piece in the biosynthetic puzzle of crucifer phytoalexins†,‡
M. Soledade C.
Pedras
* and
Denis P. O.
Okinyo
Department of Chemistry, University of Saskatchewan, Saskatoon, SK S7N 5C9, Canada. E-mail: s.pedras@usask.ca; Fax: 1 306 966 4730; Tel: 1 306 966 4772
First published on 27th November 2007
Abstract
The first sulfur labelled compound, [2H4,34S]indolyl-3-acetothiohydroxamic acid, is incorporated into the phytoalexins cyclobrassinin and spirobrassinin and the indole glucosinolate glucobrassicin, indicating that both biosynthetic pathways are closely related.
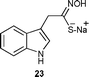
Plants have a complex range of defence responses that contribute to basal and specific disease resistance to many microbial pathogens. Phytoalexins are chemical defences produced de novo by plants to ward off pathogens and other stresses, including intense heat and UV irradiation.1 Because crucifers are widely cultivated crops and include valuable oilseeds, vegetables, and condiments, their resistance to pests and diseases is an ongoing concern. Considering the negative impact of pesticides and fungicides on the environment, it is crucial to devise safer strategies to protect cropping systems. Evidently, safer and sustainable strategies must derive from a better molecular understanding of plant defences and their metabolic pathways. Toward this end, the biosynthetic pathways of crucifer phytoalexins and related metabolites are of great importance, albeit an ongoing challenge.2 Specifically, the variety of phytoalexin structures3,4 and some unique metabolic reactions5 make mapping out these pathways very difficult. For example, the similarity between the chemical structures of brassinins 5 and 6 and indolyl glucosinolates 3 and 4 led to an earlier consensus that glucobrassicin (3) was a precursor of brassinin (5). Thus, it was rather surprising to find that glucobrassicin was not incorporated into brassinin.6 This lack of incorporation suggested to us that if not glucobrassicin (3), one of its precursors would be the branch point between the brassinin and glucobrassicin pathways. Subsequently, indolyl-3-acetaldoxime (1) was shown to be a precursor of brassinin (5), methoxybrassinin (6) and related phytoalexins.6
The biosynthetic pathway of crucifer phytoalexins and that of indolyl glucosinolates starts with the conversion of tryptophan to indolyl-3-acetaldoxime (1) and appears to diverge into various branches, three of which are pertinent to consider here: (i) indolyl glucosinolates (e.g.3, 4),7 (ii) brassinins (e.g.5, 6)6,8 and (iii) camalexin (7, Scheme 1).9 The biosynthesis of glucosinolates, due to their own importance, has been investigated for more than four decades.10 Hence, most of the genes and intermediates of the pathway to glucobrassicin (3) are known. On the other hand, no genes of the brassinin branch have been reported.2 However, camalexin (7) is produced in Arabidopsis thaliana, a wild species where a strong effort in functional genomics is concentrated. Thus, some intermediates and genes of the camalexin branch are known.9
 | ||
| Scheme 1 Biosynthetic precursors of (i) indolyl glucosinolates 3 and 4, (ii) phytoalexins brassinin (5), methoxybrassinin (6), and (iii) camalexin (7). | ||
Earlier work showed that the sulfur of glucobrassicin (3) was derived from cysteine. Furthermore, it was suggested that the C–S lyase that cleaves S-cysteinyl thiohydroximate is tightly coupled to the S-donating enzyme, and that the product of the C–S lyase is a thiohydroximic acid.10 As well, Monde et al.11 reported that the sulfur of the thiocarbonyl group of brassinin (5) was derived from cysteine and the SMe from methionine. Altogether the current information suggests to us that there should be a sulfur containing metabolite common to both brassinin (5) and glucobrassicin (3). Based on this reasoning, we synthesised 2H and 34S isotopically labelled indolyl-3-acetothiohydroxamic acid (10a) and administered it to UV-irradiated rutabaga tubers (Brassica napus L. ssp. rapifera). Remarkably, incorporation of this labelled compound into cyclobrassinin (14), spirobrassinin (15) and glucobrassicin (3) was established for the first time. Here we describe details of this biosynthetic investigation as well as the synthesis of the first doubly labelled indolyl thiohydroxamic acid. A detailed biosynthetic pathway for brassinin (5) is proposed that integrates all current knowledge.
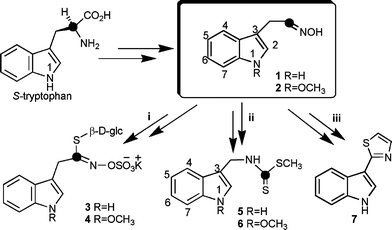
Because indolyl-3-acetothiohydroxamic acid (10) had not been characterised,§ we synthesised first non-labelled material to determine its chemical stability in aqueous methanol solutions to use in plant feeding experiments. Thus, non-labelled 10 was prepared from indole-3-carboxaldehyde (8) in three steps. Indole-3-carboxaldehyde (8) was transformed to 3-(2′-nitrovinyl)-indole upon treatment with ammonium acetate in nitromethane.12 Subsequent sodium borohydride reduction yielded 3-(2′-nitroethyl)-indole (9) which upon treatment with potassium hydride,13 followed by hexamethyldisilathiane14 yielded 10 in ca. 51% overall yield (Scheme 2). When sodium hydride was used instead of potassium hydride, only indolyl-3-acetonitrile (18) and indolyl-3-acetaldoxime (1) were obtained in a 1 : 1 ratio. The 13C NMR data of indolyl-3-acetothiohydroxamic acid (10) indicated that structure 10 is the most likely tautomer present in solution, since the chemical shift of C-1 is 189.0 ppm. This conclusion is in agreement with current data.15 In general, thiohydroximates (e.g.3 or 4 or the sodium salt of 11) have a lower δC for C-1 (ca. 162–172 ppm).¶16
 | ||
| Scheme 2 Synthesis of indolyl-3-acetothiohydroxamic acid (10). Reagents and conditions: (i) CH3NO2, NH4OAc, 128–130 °C (98%); (ii) NaBH4, i-PrOH, SiO2, CHCl3 (71%); (iii) KH, (Me3Si)2S, THF (74%). | ||
Withindolyl-3-acetothiohydroxamic acid (10) in hand, its chemical stability in aqueous solution was determined by HPLC analysis; 10 decomposed on standing (solution of water–methanol, 95 : 5, v/v) to indolyl-3-acetonitrile (18, ca. 50% in 7 h) with extrusion of sulfur. However, since the product of indolyl-3-acetothiohydroxamic acid (10) decomposition did not contain sulfur, any sulfur containing products obtained from incorporation of [4′,5′,6′,7′-2H4]indolyl-3-[34S]acetothiohydroxamic acid (10a) would inevitably derive from 10a itself. Nonetheless, to obtain a comprehensive map of these metabolic pathways, additional control experiments were carried out using tetradeuterated indolyl-3-acetonitrile (18a) as well.
The synthesis of [4′,5′,6′,7′-2H4]indolyl-3-[34S]acetothiohydroxamic acid (10a) started with [4,5,6,7-2H4]indole-3-carboxaldehyde (8a)17 and followed the steps shown in Scheme 2, but using (Me3Si)234S (prepared from 34S/Na and chlorotrimethylsilane),18 as described in the ESI.‡ The synthesis of [4′,5′,6′,7′-2H4]indolyl-3-acetonitrile was carried out as previously described.17
Next, solutions of [4′,5′,6′,7′-2H4]indolyl-3-[34S]acetothiohydroxamic acid (10a) and indolyl-3-acetothiohydroxamic acid (10) were separately added to UV-elicited rutabaga tubers, the tissues were incubated, extracted, and the extracts were fractionated to a non-polar fraction, containing phytoalexins, and a polar fraction, containing indolyl glucosinolates, as described in the ESI.‡ HPLC analysis of the fractions using photodiode array and mass detectors and comparison of the spectra of the components with those available in our metabolite library showed the presence of the phytoalexins cyclobrassinin (14), spirobrassinin (15), rutalexin (16) and brassicanate A (17) in the non-polar fraction, and the glucosinolates glucobrassicin (3), 1-methoxyglucobrassicin (4), and 4-methoxyglucobrassicin (22) in the polar fraction. LC-HRMS-ESI analysis of each fraction indicated the levels of deuterium and sulfur incorporation in each metabolite , as shown in Table 1. The [M + 4]± or [M + 6]± ion peaks were not detected in fractions of tubers incubated with non-labelled indolyl-3-acetothiohydroxamic acid (10).
| Labelled product | Incorporation (% of 2H and 34S)a |
|---|---|
| a The % of 2H and 34S incorporation was established by HRMS-ESI according to the following equation: % = {[M + n]±/([M]±+[M + n]±)} ×100 (n = 4 or 6). b Positive ion mode. c Negative ion mode. | |
| 34S-[4′,5′,6′,7′-2H4]Cyclobrassinin (14a)b | 7% |
| 34S-[4′,5′,6′,7′-2H4]Spirobrassinin (15a)b | 7% |
| [4′,5′,6′,7′-2H4]Rutalexin (16a)c | 10% |
| [4′,5′,6′,7′-2H4]Brassicanate A (17a)c | 2% |
| 34S-[4′,5′,6′,7′-2H4]Glucobrassicin (3a)c | 2% |
Additional experiments were carried out using [4′,5′,6′,7′-2H4]indolyl-3-acetonitrile (18a) and indolyl-3-acetonitrile (18), as reported above for acetothiohydroxamic acids 10a and 10. LC-HRMS-ESI analysis of each fraction showed the same components as above and indicated that none of the phytoalexins or indolyl glucosinolates contained deuterium. Interestingly, indolyl-3-acetonitrile (18) was completely metabolised within 24 h to indolyl-3-acetic acid (19), which in turn was metabolised to indole-3-carboxylic acid (20) and methyl indole-3-carboxylate (21).
Remarkably, as summarised in Table 1, LC-HRMS data indicated that cyclobrassinin (14a), spirobrassinin (15a) and glucobrassicin (3a) incorporated intact [4′,5′,6′,7′-2H4]indolyl-3-[34S]acetothiohydroxamic acid (10a) since only the [M + 6]± peak was detected. By contrast, the phytoalexins rutalexin (16a) and brassicanate A (17a) showed incorporation of only four deuteria (only [M + 4]± peak) and no sulfur. Because rutalexin (16) is biosynthesised from cyclobrassinin (14),19 these results indicate that the sulfur 34 of cyclobrassinin (14) was exchanged with unlabelled sulfur. Thus, it is likely that another sulfur donor (possibly also cysteine) was used in this conversion. A similar explanation could be used for brassicanate A (17a), since it appears to be derived from brassinin (5).19 The fact that among the three indolyl glucosinolates only glucobrassicin (3a) showed incorporation of four deuteria and sulfur 34 is consistent with our previous findings8 indicating that oxidation at position N-1 of indole occurs immediately after oxime formation, i.e. upstream of the step yielding thiohydroxamic acid 10.
As expected, [4′,5′,6′,7′-2H4]indolyl-3-acetonitrile (18a) was not incorporated into any of the phytoalexins or glucosinolates present in elicited tissues (Table 2). This result is of interest since indolyl-3-acetonitrile (18) appears to be an intermediate in the biosynthesis of camalexin (7).20 Nonetheless, since camalexin (7) is not produced in rutabaga we could not confirm that result.
| Labelled product | Incorporation (% of 2H)a |
|---|---|
| a The % of 2H incorporation was established by HRMS-ESI according to the following equation: % = {[M + 4]±/([M]++[M + 4]±)} ×100. b Negative ion mode. | |
| [4′,5′,6′,7′-2H4]Indole-3-carboxylic acid (20a)b | ≥98% |
| Methyl [4′,5′,6′,7′-2H4]indole-3-carboxylate (21a)b | ≥98% |
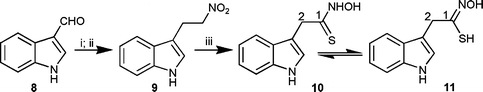
Based on the results described above and their integration with reported data,2,10 another intermediate (10) common to the pathways of brassinin (5) and glucobrassicin (3) can be proposed. Firstly, as depicted in Scheme 3, oxidation of indolyl-3-acetaldoxime (1) to the corresponding nitrile oxide 12 provides a putative intermediate suitable for nucleophilic attack by an alkylthiol (e.g.cysteine).10 The resulting intermediate could be converted through a C–S lyase to the thiohydroxamic acid 10, possibly the last intermediate common to both pathways. Indolyl-3-methylisothiocyanate (13) could then be thiomethylated to yield brassinin (5).
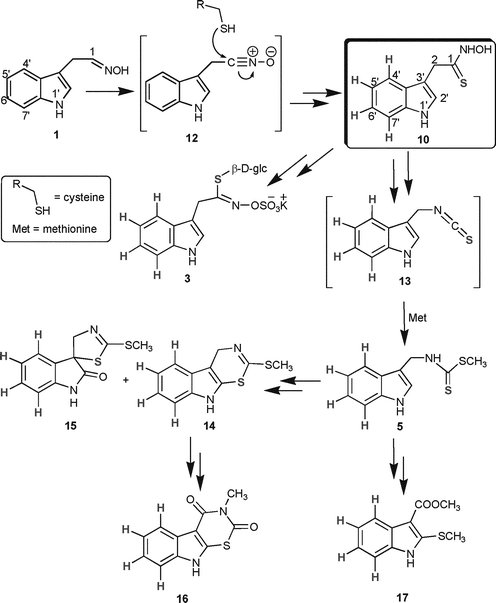 | ||
| Scheme 3 Proposed biosynthetic pathway of glucobrassicin (3) and phytoalexins brassinin (5), cyclobrassinin (14), spirobrassinin (15), rutalexin (16) and brassicanate A (17); compounds 12 and 13 are proposed intermediates. | ||
[4′,5′,6′,7′-2H4]Indolyl-3-acetonitrile (18a) appeared to be the main precursor of indolyl-3-acetic acid (19a), indole-3-carboxylic acid (20a) and methyl indole-3-carboxylate (21a) under the experimental conditions described in the ESI,‡ since these three metabolites were isolated fully tetradeuterated (about ≥98%, Table 2, Scheme 4). In agreement with these findings compounds 19–21 were not detected in control experiments.
![Incorporation of [4′,5′,6′,7′-2H4]indolyl-3-acetonitrile (18a) into indolyl-3-acetic acid (19a), indole-3-carboxylic acid (20a) and methyl indole-3-carboxylate (21a).](/image/article/2008/OB/b714743k/b714743k-s4.gif) | ||
| Scheme 4 Incorporation of [4′,5′,6′,7′-2H4]indolyl-3-acetonitrile (18a) into indolyl-3-acetic acid (19a), indole-3-carboxylic acid (20a) and methyl indole-3-carboxylate (21a). | ||
As well, our findings have implications on the pathway of indole glucosinolates. The results described above reinforce our previous data,6 which indicated that glucosinolates 4 and 22 derive from oxidation of indolyl-3-acetaldoxime (1) and not from glucosinolate 3. This is likely because glucosinolates 4 and 22 did not incorporate the thiohydroxamic acid 10a. Previously, it was proposed that these indole glucosinolates (4 and 22) resulted from enzymatic methoxylation of 3.21 In addition, our results suggest that brassinin (5) is perhaps only a couple of steps from the thiohydroxamic acid 10 (side-chain rearrangement and thiomethylation).
Eventually, with a better understanding of the biosynthetic intermediates of crucifer phytoalexins and their corresponding enzymes and genes, it is expected that plants may be bred to produce a wider variety of chemical defences and display higher levels of resistance to stress.
Selected spectroscopic data
Notes and references
- Phytoalexins, ed. J. A. Bailey and J. W. Mansfield, Blackie & Son, Glasgow, UK, 1982, pp. 1–334 Search PubMed.
- For reviews on cruciferous phytoalexins: (a) M. S. C. Pedras, F. I. Okanga, I. L. Zaharia and A. Q. Khan, Phytochemistry, 2000, 53, 161–176 CrossRef CAS; (b) M. S. C. Pedras, M. Jha and P. W. K. Ahiahonu, Curr. Org. Chem., 2003, 7, 1635–1647 CrossRef CAS; (c) M. S. C. Pedras, Q. A. Zheng and V. K. Sarma-Mamillapalle, Nat. Prod. Commun., 2007, 2, 319–330.
- M. S. C. Pedras, Q. A. Zheng and M. G. Sarwar, Org. Biomol. Chem., 2007, 5, 1167–1169 RSC.
- M. S. C. Pedras, M. Suchy and P. W. K. Ahiahonu, Org. Biomol. Chem., 2006, 4, 691–697 RSC.
- M. S. C. Pedras and M. Hossain, Org. Biomol. Chem., 2006, 4, 2581–2590 RSC.
- M. S. C. Pedras, S. Montaut, Y. Xu, A. Q. Khan and A. Loukaci, Chem. Commun., 2001, 1572–1573 RSC.
- M. D. Mikkelsen, P. Naur and B. A. Halkier, Plant J., 2004, 37, 770–777 CrossRef CAS.
- M. S. C. Pedras and S. Montaut, Chem. Commun., 2004, 452–453 RSC.
- E. Glawischnig, B. G. Hansen, C. E. Olsen and B. A. Halkier, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 8245–8250 CrossRef CAS.
- For a recent review on glucosinolates see: B. A. Halkier and J. Gershenzon, Ann. Rev. Plant Biol., 2006, 57, 303–333 Search PubMed.
- K. Monde, M. Takasugi and T. Ohnishi, J. Am. Chem. Soc., 1994, 116, 6650–6657 CrossRef CAS.
- L. Canoira, J. G. Rodriguez, J. B. Subirats, J.-A. Escario, I. Jimenez and A. R. Martinez-Fernandez, J. Med. Chem., 1989, 24, 39–42 CrossRef CAS.
- A. K. Sinhababu and R. T. Borchardt, Tetrahedron Lett., 1983, 24, 227–230 CrossRef CAS.
- J. R. Hwu and S. C. Tsay, Tetrahedron, 1990, 46, 7413–7428 CrossRef CAS.
- A. Chimiak, W. Przychodzen and J. Rachon, Heteroat. Chem., 2002, 13, 169–194 CrossRef CAS.
- D. W. Reed, L. Davin, J. C. Jain, V. Deluca, L. Nelson and E. W. Underhill, Arch. Biochem. Biophys., 1993, 305, 526–532 CrossRef CAS.
- M. S. C. Pedras and D. P. O. Okinyo, J. Labelled Compd. Radiopharm., 2006, 49, 33–45 CrossRef CAS.
- J.-H. So and P. Boudjouk, Synthesis, 1989, 4, 306–307 CrossRef.
- M. S. C. Pedras, S. Montaut and M. Suchy, J. Org. Chem., 2004, 69, 4471–4476 CrossRef CAS.
- M. Nafisi, S. Goregaoker, C. J. Botanga, E. Glawischnig, C. E. Olsen, B. A. Halkier and J. Glazebrook, Plant Cell, 2007, 19, 2039–2052 CrossRef CAS.
- M. D. Mikkelsen, B. L. Petersen, E. Glawischnig, A. B. Jensen, E. Andreasson and B. A. Halkier, Plant Physiol., 2003, 131, 298–308 CrossRef CAS.
Footnotes |
| † Support for the authors′ work was obtained from the Natural Sciences and Engineering Research Council of Canada (Discovery Research Grant to M.S.C.P.) and the University of Saskatchewan (graduate assistantship to D.P.O.O.). We acknowledge the technical assistance of K. Thoms and K. Brown, Department of Chemistry, in MS and NMR determinations, respectively. |
| ‡ Electronic supplementary information (ESI) available: Experimental section. See DOI: 10.1039/b714743k |
| § Sodium indolyl-3-thiohydroximate (23) was previously reported not to be stable in a buffer solution at pH 6.16 |
| ¶ All compounds gave satisfactory spectroscopic data; in each case the percentage of deuterated synthetic compound was ≥98%. Labelled compounds are numbered with an additional letter a (e.g.10a). |
| This journal is © The Royal Society of Chemistry 2008 |