Development of polysilane-supported palladium/alumina hybrid catalysts and their application to hydrogenation
Hidekazu
Oyamada
a,
Takeshi
Naito
a,
Shinpei
Miyamoto
a,
Ryo
Akiyama
ab,
Hiroyuki
Hagio
a and
Shū
Kobayashi
*ab
aDepartment of Chemistry, School of Science and Graduate School of Pharmaceutical Sciences, The University of Tokyo, The HFRE Division, ERATO, Japan Science and Technology Agency (JST), Hongo, Bunkyo-ku, Tokyo, 113-0033, Japan
bScience of Process Laboratories, ERATO, Japan Science and Technology Agency (JST), Matoba, Kawagoe, Saitama 350-1101, Japan. E-mail: shu_kobayashi@chem.s.u-tokyo.ac.jp; Fax: +81 3 5684 0634; Tel: +81 3 5841 4790
First published on 23rd November 2007
Abstract
Novel immobilized Pd catalysts, polysilane-supported palladium/alumina hybrid catalysts, have been developed. The catalysts showed high catalytic activity for hydrogenation, and could be used in an organic solvent or under solvent-free conditions.
Palladium is one of the most important hydrogenation catalysts used in both laboratories and industry. While immobilized palladium (Pd) catalysts such as Pd/C and Pd/SiO2, etc. have been often employed, leaching of the palladium from the supports, moderate yields of recovery, contamination of the reaction products with palladium, and poisoning of the catalyst by heteroatoms such as sulfur and nitrogen, etc. are sometimes serious problems especially in the manufacture of pharmaceuticals.1 To address these issues, new methods for the immobilization of homogeneous Pd catalysts onto inorganic or organic supports have been widely investigated.2 However, tedious procedures are often needed for the preparation of such heterogeneous catalysts, and lower activity/selectivity and leaching of catalysts from the supports remain serious problems.
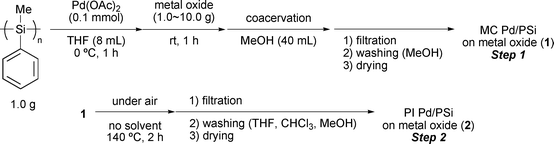
Previously, we have developed new methods for immobilizing metal catalysts onto polymers, microencapsulation3 and polymer incarcerated (PI) protocols,4 which are based on physical envelopment by the polymers, and electronic interaction between π electrons of the benzene rings of the polystyrene-based polymers and vacant orbitals of the metals. Next, we focused on polysilanes as a support for heterogeneous catalysts. Polysilanes have been widely studied due to their interesting electronic properties such as high hole mobility, photoconductivity, and nonlinear optical properties caused by σ-conjugation of the silicon backbone.5–7 In addition, polysilane is used as a starting material for ceramics and synthetic methods for mass production are established.8 Recently, we have synthesized immobilized Pd and Pt catalysts based on poly(methylphenyl)silane for the first time.9 Polysilane-supported Pd catalysts (Pd/PSi) have been successfully used in hydrogenation. The reactions proceeded in high yields, and the catalysts could be recovered and reused almost quantitatively by simple filtration. Polysilane-supported Pt (Pt/PSi) catalysts were prepared by a similar method and showed high activity in hydrosilylation. However, a small amount of Pd leaching was confirmed by ICP analysis when nitrogen-containing compounds such as nitrobenzene were used. Furthermore, these catalysts had a somewhat limited applicability profile, since the carrier polysilane dissolved in some organic solvents. In order to address these issues, we planned to apply the PI method to polysilane-supported catalysts. In this paper, we describe the synthesis of highly active PI Pd/PSi on aluminium oxide and its application to hydrogenation.†
We first examined several metal oxides and the ratio to Pd/PSi for the preparation of hybrid catalysts (Table 1). In a typical procedure, poly(methylphenyl)silane (Mw = 3.2 × 104) and Pd(OAc)2 were combined in THF at 0 °C for 1 h, and a metal oxide was added to this mixture. After stirring for a further 1 h, MeOH was added to form microencapsulated Pd (MC Pd/PSi) on the metal oxide. The resulting MC Pd/PSi (1) was heated at 140 °C for 2 h, washed sequentially with THF, CHCl3, and MeOH, and dried under reduced pressure to afford PI Pd/PSi on the metal oxide 2. We tested hybrid catalysts 2 on the hydrogenation of ethyl cinnamate (Scheme 1). When SiO2 was used, recovery of palladium was low (Table 1, entry 2). On the other hand, Al2O3, TiO2, and ZrO2 gave promising results (entries 3, 6 and 9). It was found that higher ratios of the metal oxides to Pd/PSi were important for good recovery of palladium and high activity for the hydrogenation (entries 4, 5, 8, 10, and 11).
 | ||
| Scheme 1 Hydrogenation of ethyl cinnamate | ||
| Entry | Preparation of PI Pd/PSi on metal oxide | Hydrogenationa | |||||
|---|---|---|---|---|---|---|---|
| Metal oxide (g) | Yield of 1 (g) | Yield of 2/1 (w/w) | Loading/µmol g−1 | Recovery of Pd (%)b | Conversion (%)c | Leaching (%)d | |
| a Reaction conditions: see Scheme 1. b Recovery over two steps. c Determined by GC analysis. d Determined by ICP analysis. e MC Pd/PSi was used. f Not determined. | |||||||
| 1 | None | 0.72 | — | 13.4 | 96 | 94 | NDf |
| 2 | SiO2 (1.0) | 1.81 | 0.56 | 49.8 | 51 | 88 | 0.27 |
| 3 | Al2O3 (1.0) | 1.63 | 0.77 | 72.3 | 91 | 55 | 0.23 |
| 4 | (5.0) | 5.62 | 0.95 | 15.1 | 81 | 99.8 | <0.28 |
| 5 | (10.0) | 10.57 | 0.96 | 8.3 | 85 | 99.8 | <0.28 |
| 6 | TiO2 (1.0) | 1.63 | 0.79 | 60.1 | 78 | 74 | <0.23 |
| 7 | (5.0) | 5.61 | 0.99 | 11.7 | 65 | 76 | <0.28 |
| 8 | (10.0) | 10.56 | 0.97 | 7.3 | 74 | >99.9 | <0.28 |
| 9 | ZrO2 (1.0) | 1.60 | 0.80 | 63.7 | 81 | 46 | <0.23 |
| 10 | (5.0) | 5.61 | 0.93 | 16.2 | 90 | 99.3 | <0.28 |
| 11 | (10.0) | 10.48 | 0.91 | 8.2 | 79 | >99.9 | <0.30 |
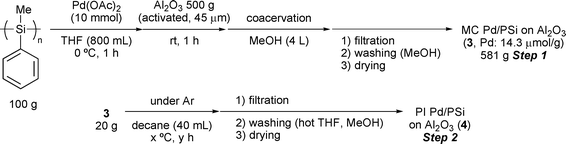
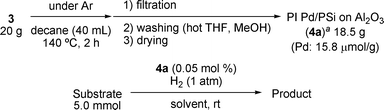
Among the metal oxides tested, alumina was revealed to be superior to other metal oxides in terms of yield and quality of the immobilized catalysts (entries 4 and 5). With these data in hand, we further studied preparation conditions of the catalyst using alumina. In a large-scale preparation of PI Pd/PSi, it was found that the heating at 140 °C under neat conditions (cross-linking step) gave low reproducibility, presumably due to irregularity of heating and influence of oxygen. After further investigations, good reproducibility was obtained when decane was used as a solvent under an argon atmosphere. Next, several conditions for the cross-linking step were examined (Table 2). It was found that whilst palladium catalysts 4 that had been prepared at 120 °C and 140 °C for 2 hours gave quantitative conversion in the hydrogenation reaction, those prepared over a longer period of time gave lower conversions in the subsequent hydrogenation (entries 2 and 4). On the other hand, using a preparation temperature of 160 °C even over the shortened time of 2 hours only afforded a catalyst whose activity was comparatively low (entry 5).
| Entry | Preparation of PI Pd/PSi on Al2O3 | Hydrogenationa | ||||||
|---|---|---|---|---|---|---|---|---|
| x/°C | y/h | Yield of 4/3 (w/w) | Loading/µmol g−1 | Recovery of Pd (%)b | Conversion (%)c | Leaching (%)d | ||
| 1 h | 2 h | |||||||
| a Reaction conditions: ethyl cinnamate (1 mmol), Pd catalyst 4 (0.1 mol%), EtOH (1 mL) and H2 (1 atm), rt, 2 h. b Recovery of two steps. c Determined by GC analysis. d Determined by ICP analysis. | ||||||||
| 1 | 120 | 2 | 0.91 | 15.2 | 80 | 94 | Quant. | <0.74 |
| 2 | 120 | 4 | 0.91 | 12.7 | 67 | 74 | 93 | 1.06 |
| 3 | 140 | 2 | 0.93 | 15.8 | 85 | 73 | Quant. | <0.74 |
| 4 | 140 | 4 | 0.93 | 16.3 | 89 | 59 | 94 | <0.74 |
| 5 | 160 | 2 | 0.95 | 15.3 | 84 | 63 | 82 | <0.74 |
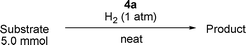
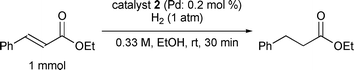
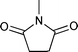
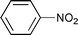
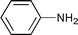
The substrate scope of the hydrogenation was then examined using 0.05 mol% PI Pd/PSi on alumina (4a) in organic solvents such as ethanol and dichloromethane under hydrogen gas at atmospheric pressure and room temperature (Table 3). Gratifyingly it was found that under these conditions ethyl cinnamate (entry 1) and 2-cyclohexen-1-one (entry 2) were reduced smoothly in EtOH to afford the desired products in high yields without leaching of Pd. Chalcone also reacted smoothly to give the corresponding ketone and alcohol in 91% and 6% yields, respectively (entry 3). While N-methylmaleimide, maleic acid, and a diene were reduced smoothly to afford N-methylsuccinimide, succinic acid, and alkane in high yields, respectively, a small amount of Pd leaching was observed (entries 4, 5 and 6). Furthermore, in the case of using catalyst 4a, nitrobenzene was reduced smoothly to afford aniline quantitatively. It is particularly noteworthy that the leaching of Pd in this instance was lower than that observed when using the MC Pd/PSi catalyst (entries 7 and 8).
| Entry | Substrate | Solvent | Conc./mol L−1 | Time/h | Product | Yield (%)b | Leaching (%)c |
|---|---|---|---|---|---|---|---|
| a Pd: 0.142 wt%, Al: 47.7 wt%, Si: 2.28 wt% (determined by ICP analysis). b Determined by 1H NMR analysis. c Determined by ICP analysis. d Conversion, determined by GC analysis. e 0.50 mol% of MC Pd/PSi without Al2O3 was used. See ref. 9 | |||||||
| 1 |
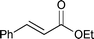
|
EtOH | 0.33 | 1 |
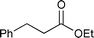
|
>99.9d | <0.22 |
| 2 |
|
EtOH | 1.0 | 4 |
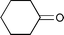
|
Quant. | <0.30 |
| 3 |
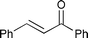
|
EtOH | 1.0 | 24 |
|
91 (6) | 0.99 |
| 4 |
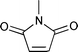
|
EtOH | 0.50 | 1 |
|
97 | 0.48 |
| 5 |

|
EtOH | 1.0 | 3.5 |

|
Quant. | 0.94 |
| 6 |

|
CH2Cl2 | 0.50 | 24 |

|
98 | 3.6 |
| 7 |
|
EtOH | 1.0 | 8 |
|
>99.9d | 0.34 |
| 8e | Hexane | 0.33 | 1.5 | >99.9d | 2.28 | ||
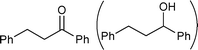
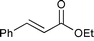
We also evaluated catalyst 4a in the hydrogenation of ethyl cinnamate, N-methylmaleimide, and chalcone using 0.05 or 0.20 mol% of Pd under solvent-free conditions (Table 4).10 We had previously found that when using MC Pd/PSi with a liquid chemical such as ethyl cinnamate as substrate, the catalyst was dissolved in the substrate and the palladium metal tended to leach from the support. Pleasingly however, it was discovered that in the case of 4a, the reaction could be conducted without leaching of Pd (entries 1 and 2). Solid chemicals such as N-methylmaleimide and chalcone could be used as substrates directly and good chemical yields were obtained (entries 3, 4, 5 and 6). Particularly, in the case of chalcone , it is noted that the leaching of Pd was lower than that observed when using ethanol as a solvent (entry 6, cf.Table 3, entry 3).
| Entry | Substrate | 4a/mol% | Temp. | Time/h | Product | Yield (%)a | Leaching (%)b |
|---|---|---|---|---|---|---|---|
| a Determined by 1H NMR analysis. b Determined by ICP analysis. c Conversion, determined by GC analysis. d 25 mmol scale. e Determined by GC analysis | |||||||
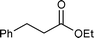
| 1 |
|
0.05 | RT | 8 |
|
>99.9c | <0.22 |
| 2d | 0.05 | 50 °C | 16 | Quant.e | <0.10 | ||
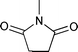
| 3 |
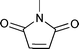
|
0.05 | 50 °C | 1 |
|
58 | — |
| 4 | 0.20 | RT | 1 | 79 | — | ||
| 5 |
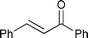
|
0.05 | 50 °C | 24 |
|
80 (Trace) | — |
| 6 | 0.20 | 50 °C | 24 | 95 (5) | 0.12 | ||
In order to probe the structure of the catalyst, we analyzed PI catalyst 4 (Table 2, entry 3) by 29Si CPMAS NMR (Fig. 1). In the preparation of MC Pd/PSi on alumina (3), while a portion of the Si–Si bonds were oxidized to form O–Si–O bonds, almost the same signals (chart c) as for those of poly(methylphenylsilane) (chart a) were observed. On the other hand, PI Pd/PSi on alumina [catalyst 4 (Table 2, entry 3)] gave interesting spectra (chart d). 29Si NMR analysis revealed four signals at about −100, −40, −33, and −25 ppm, corresponding to Si–O–Al, Si–Si, O–Si–O, and Si–O–Si bonds, respectively. It was assumed that polysilane was fixed on activated alumina by formation of linkages with aluminium through oxygen. In addition, since alumina has a lot of nanopores on the surface, it is expected that the polysilane-supported palladium clusters are included in these nanopores and stabilized. However, at the current time the precise structure of catalyst 4 is still unclear, and further investigation and information are needed.
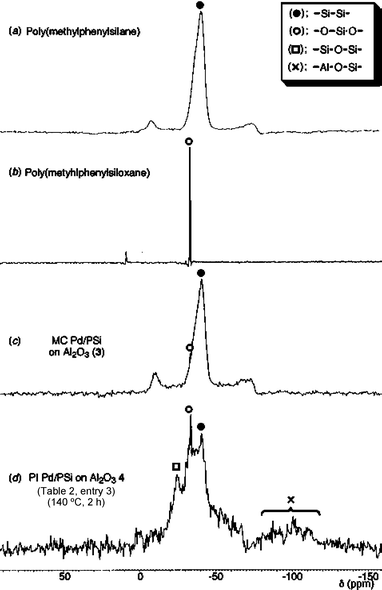 | ||
| Fig. 1 Comparison of 29Si CPMAS NMR spectra | ||
In summary, we have developed novel polysilane-supported palladium/alumina hybrid catalysts. These catalysts showed high catalytic activity in hydrogenation and could be used in an organic solvent or under solvent-free conditions. Further investigations to prepare other immobilized metal catalysts as well as their applications to other reactions are now in progress.11
Notes and references
- (a) D. L. Trimm, Design of Industrial Catalysts, Elsevier, Amsterdam, 1980 Search PubMed; (b) P. N. Rylander, Hydrogenation Methods, Academic Press, New York, 1985 Search PubMed; (c) C. N. Satterfield, Heterogeneous Catalysis in Industrial Practice, McGraw-Hill, New York, 2nd edn, 1991 Search PubMed.
- (a) C. A. McNamara, M. J. Dixon and M. Bradley, Chem. Rev., 2002, 102, 3275–3300 CrossRef CAS; (b) Polymeric Materials in Organic Synthesis and Catalysis, ed. M. R. Buchmeiser, Wiley-VCH, Weinheim, Germany, 2003 Search PubMed.
- (a) S. Kobayashi and R. Akiyama, Chem. Commun., 2003, 449–460 RSC; (b) T. Ishida, R. Akiyama and S. Kobayashi, Adv. Synth. Catal., 2003, 345, 576–579 CrossRef CAS; (c) T. Ishida, R. Akiyama and S. Kobayashi, Adv. Synth. Catal., 2005, 347, 1189–1192 CrossRef CAS.
- (a) R. Akiyama and S. Kobayashi, J. Am. Chem. Soc., 2003, 125, 3412–3413 CrossRef CAS; (b) K. Okamoto, R. Akiyama and S. Kobayashi, J. Org. Chem., 2004, 69, 2871–2873 CAS; (c) K. Okamoto, R. Akiyama and S. Kobayashi, Org. Lett., 2004, 6, 1987–1990 CAS; (d) R. Akiyama, T. Sagae, M. Sugiura and S. Kobayashi, J. Organomet. Chem., 2004, 689, 3806–3809 CrossRef CAS; (e) K. Okamoto, R. Akiyama, H. Yoshida, T. Yoshida and S. Kobayashi, J. Am. Chem. Soc., 2005, 127, 2125–2135 CrossRef CAS; (f) H. Hagio, M. Sugiura and S. Kobayashi, Synlett, 2005, 813–817 CAS; (g) S. Kobayashi, H. Miyamura, R. Akiyama and T. Ishida, J. Am. Chem. Soc., 2005, 127, 9251–9254 CrossRef CAS; (h) M. Takeuchi, R. Akiyama and S. Kobayashi, J. Am. Chem. Soc., 2005, 127, 13096–13097 CrossRef CAS; (i) R. Nishio, M. Sugiura and S. Kobayashi, Org. Lett., 2005, 7, 4831–4834 CrossRef CAS; (j) H. Miyamura, R. Akiyama, T. Ishida, R. Matsubara, M. Takeuchi and S. Kobayashi, Tetrahedron, 2005, 61, 12177–12185 CrossRef CAS; (k) R. Nishio, S. Wessely, M. Sugiura and S. Kobayashi, J. Comb. Chem., 2006, 8, 459–461 CrossRef CAS; (l) H. Hagio, M. Sugiura and S. Kobayashi, Org. Lett., 2006, 8, 375–378 CrossRef CAS; (m) R. Nishio, M. Sugiura and S. Kobayashi, Org. Biomol. Chem., 2006, 4, 992–995 RSC; (n) H. Miyazaki, H. Hagio and S. Kobayashi, Org. Biomol. Chem., 2006, 4, 2529–2531 RSC; (o) H. Miyamura, R. Matsubara, Y. Miyazaki and S. Kobayashi, Angew. Chem., Int. Ed., 2007, 46, 4151–4155 CrossRef CAS; (p) T. Matsumoto, M. Ueno, J. Kobayashi, H. Miyamura, Y. Mori and S. Kobayashi, Adv. Synth. Catal., 2007, 349, 531–534 CrossRef CAS.
- (a) R. D. Miller and J. Michl, Chem. Rev., 1989, 89, 1359–1410 CrossRef CAS; (b) R. G. Kepler, J. M. Zeigler, L. A. Harrah and S. R. Kurtz, Phys. Rev. B: Condens. Matter Mater. Phys., 1987, 35, 2818–2822 CrossRef CAS; (c) M. Abkowitz, F. E. Kneier, H.-J. Yuh, R. J. Weagley and M. Stolka, Solid State Commun., 1987, 62, 547–550 CrossRef CAS; (d) R. West, J. Organomet. Chem., 1986, 300, 327–346 CrossRef CAS; (e) H. Suzuki, H. Meyer, J. Simmerer, J. Yang and D. Haarer, Adv. Mater., 1993, 5, 743–746 CAS.
- (a) H. Suzuki, H. Meyer and S. Hoshino, J. Appl. Phys., 1995, 78, 2684–2690 CrossRef CAS; (b) S. Hoshino and H. Suzuki, Appl. Phys. Lett., 1996, 69, 224–226 CrossRef CAS.
- S. Hayase, Prog. Polym. Sci., 2003, 28, 359–381 CrossRef CAS.
- D. Seyferth, T. G. Wood, H. J. Tracy and J. L. Robison, J. Am. Ceram. Soc., 1992, 75, 1300–1302 CAS.
- H. Oyamada, R. Akiyama, H. Hagio, T. Naito and S. Kobayashi, Chem. Commun., 2006, 4297–4299 RSC.
- (a) K. Tanaka and F. Toda, Solvent-free Organic Synthesis, Wiley-VCH, Weinheim, 2003 Search PubMed; (b) Supported Reagents and Catalysts in Chemistry, ed. B. K. Hodnett, A. P. Kybett, J. H. Clark and K. Smith, The Royal Society of Chemistry, Cambridge, 1998 Search PubMed; (c) Solid Supports and Catalyst in Organic Synthesis, ed. K. Smith, Ellis Horwood Limited, New York, 1992 Search PubMed.
- The reusability of the catalyst has not been determined and will be also the subject of future study.
Footnote |
| † Synthesis of poly(methylphenylsilane): Methylphenyldichlorosilane (382 g, 2.00 mol) was added dropwise to a suspension of sodium (96.6 g, 4.20 mol) in THF (1 L) over 30 min with vigorous stirring at reflux temperature. After stirring for further 3.5 h, the mixture was cooled in an ice bath and diluted with toluene (500 mL). 3 N HCl (500 mL) was added dropwise to the mixture, the organic layer was separated, and water was added to the aqueous layer to dissolve NaCl. The aqueous layer was extracted with toluene and the combined organic layers were washed with H2O, 5% aqueous solution of NaHCO3, H2O and brine. The organic layer was dried over Na2SO4 and concentrated to ca. 600 mL in vacuo. MeOH (750 mL) was added to the solution, the precipitate was collected by filtration and washed with MeOH. The crude polysilane was dissolved in toluene (370 mL) and precipitated by adding iPrOH. The precipitate was collected, washed with toluene–iPrOH (1 : 5) and dried under reduced pressure at 55 °C to afford poly(methylphenylsilane) (135 g, 56% yield). Mw = 3.21 × 104, Mn = 1.12 × 104, Mw/Mn = 2.87. General procedure for the preparation of PI Pd/PSi on Al2O3 (4) (Table 2): Poly(methylphenylsilane) (100 g) was dissolved in THF (800 mL) and cooled to 0 °C. To this solution, palladium(II) acetate (2.25 g, 10.0 mmol) was added and the mixture was stirred for 1 h at this temperature. To the dark brown polymer solution was added alumina (500 g), and the mixture was allowed to warm to room temperature. After 2 h, MeOH (4 L) was slowly added for coacervation and the resulting precipitate was collected by filtration, washed with MeOH several times and dried under reduced pressure at 55 °C to afford the microencapsulated palladium catalyst (MC Pd/PSi on Al2O33, 581 g, Pd = 14.3 mmol g−1, 83% of Pd was loaded). The Pd loading was determined by ICP analysis. MC catalyst 3 (20 g) was suspended in decane (40 mL) at room temperature, and heated to 120–160 °C for 2–4 h. The resulting solid was collected by filtration, washed with hot THF and MeOH several times and dried under reduced pressure at 55 °C. The polymer incarcerated palladium catalysts (PI Pd/PSi on Al2O34) were obtained. The loading of Pd was determined by ICP analysis. Hydrogenation catalyzed by PI Pd/PSi on Al2O3 (4a) (Table 3): A typical experimental procedure is described for the hydrogenation of ethyl cinnamate. Ethyl cinnamate (5 mmol) and PI Pd/PSi on Al2O3 (4a, 0.05 mol%) were combined in ethanol (15 mL). The mixture was stirred for 1 h at room temperature under H2 atmosphere (1 atm). The yield and conversion were determined by GC analysis with reference to naphthalene or 1H NMR analysis with reference to 1,2,3,4-tetramethylbenzene (durene) as an internal standard. The leaching of Pd from the support was determined by ICP analysis. |
| This journal is © The Royal Society of Chemistry 2008 |