Brightly shining nanoparticles: lipophilic perylene bisimides in aqueous phase†
Heinz
Langhals
*
Department of Chemistry, LMU University of Munich, Butenandtstr. 13, D-81377, Munich, Germany. E-mail: Langhals@lrz.uni-muenchen.de; Fax: +49 89-2180-77700; Tel: +49 89-2180-77699
First published on 6th December 2007
Abstract
The dispersion of lipophilic perylene bisimides down to nanoparticle dimensions opens the aqueous phase to these highly fluorescent, water insoluble materials.
Perylene dyes11, perylene tetracarboxylic bisimides, characterised by their high stability, are used as high performance pigments.2 The attachment of solubility increasing groups such as long-chain secondary alkyl groups3 (“swallow-tail groups”) to the nitrogen atoms of 1 results in readily soluble, highly fluorescent dyes for lipophilic solvents with fluorescence quantum yields 4 close to 100%.
However, the application of such dyes in aqueous solution turned out to be appreciably more difficult. Strongly hydrophilic groups such as sulfonic acid groups,5 polyether groups,6 and pyridyl substituents,7 basically induce water solubility of 1; however, these materials exhibit a strong tendency to aggregate in the highly hydrophilic medium. Moreover, such dyes exhibit a high aptitude for the collection of heavy metal ions6 and this interferes with the preparation of the dyes in high purity.
A dispersion8 of a special derivative of 1 with a labile crystal lattice was only successful for low concentrations of particles and a spontaneous dispersion of a crown ether9 derivative of 1 is limited to this special dye. A direct application of the lipophilic, water-insoluble materials would bring about an appreciable improvement.
We have applied a dispersing detergent of maleinated linseed oil10 as an alternative; commercial name11 Bomol-4N. The lipophilic dyes 1 were dissolved in the dispersant and spread in more than a hundred times the quantity of water; for the preparation of nanoparticles by the application of detergents see ref. 12. Water-insoluble dyes were therefore required that are both lipophilic and readily soluble in the dispersant.
High solubility of 1 was attained by the attachment of long-chain secondary alkyl groups to the nitrogen atoms.3 Such dye preparations 1a form strongly yellow fluorescent liquids, remaining unchanged with storage, where the dispersion of the dye is so fine that it is not retained by a D5 glass filter, not even in traces; see Fig. 1. The porosity13 of D5 glass filers is 1.0–1.7 µm and particles can be retained that are smaller by a factor of three14 or even more. The complete passing of particles leaving the glass filter colourless indicates a size of the order of nanometres. The dispersion of the dye must have progressed far beyond the size of the previously reported8,9 particles because the latter could be completely collected by a D4 glass filter with pores of 9–15 µm size.
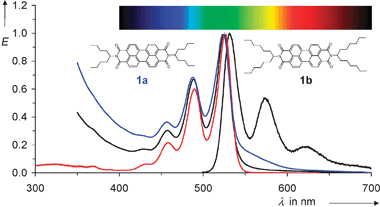 | ||
| Fig. 1 UV/Vis absorption and fluorescence spectra of 1. Left: absorption spectra from bottom to top: 1b in chloroform (red), 1b nanoparticles in water (black), 1a nanoparticle in water (blue). Right: fluorescence spectrum of 1b nanoparticles in water (black). | ||
The small size of the particles was verified by dynamic light scattering (DLS) measurements where a maximum of the distribution was found at 60 nm; see Fig. 2. The comparably narrow distribution is as remarkable as the result that no particles were found larger than 600 nm. Thus, complete dispersion down to nano-dimensions proceeded. Information about the direct environment of the nano-dispersed dye molecules was obtained by UV/Vis spectroscopy; the chromophore of 1 is very weakly, but characteristically, solvatochromic.15 Surprisingly, the UV/Vis spectra of nano-dispersed 1b in water are identical within 2 nm with the spectra in homogeneous solution in the lipophilic chloroform; see Fig. 1. The unaltered spectral shape indicates isolated chromophores in the nanoparticles where exciton interaction16 with neighbouring chromophores can be excluded. Such a type of nano-dispersion is very different from previously described microcrystalline particles with interacting chromophores; see for example ref. 17. Moreover, rather high concentrations of dyes in water can be obtained and are only limited by the solubility of the dye in the dispersant and the ratio between the dispersant and water. Flocculation does not occur, even not for the highest attainable dye concentrations. Appreciably coloured aqueous phases were obtained from 1a despite the rather short swallow-tail substituent. Even more deeply coloured phases can be obtained with the longer and more efficiently solubilizing substituents in 1b. The absorption of the dispersed dyes is overlaid by the absorption and light-scattering of the dispersant and the baseline of the spectrum deviates to higher values for shorter wavelengths; see Fig. 1. This deviation is strongest for 1a because its rather low solubility requires more dispersant than the more soluble dye 1b where matrix effects are essentially concentrated in the UV. Thus, 1b can be applied in the visible in the same way as a homogeneously dissolved dye.
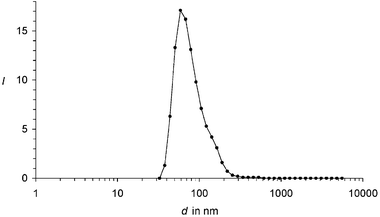 | ||
| Fig. 2 Particle distribution of 1b nanoparticles in water: Intensity I of counts versus the size d of particles in nm. | ||
The pronounced fluorescence of the aqueous phases of 1 is remarkable. The fluorescence spectra of the aqueous phases of 1 resemble the spectra in chloroform; see Fig. 1. The fluorescence quantum yields of 1a and 1b are close to 100% as in solution in chloroform.4 The high fluorescence quantum yields are additionally indicators for isolated chromophores in the nanoparticles because interaction of chromophores such as in aggregates causes a weakening of fluorescence.18
Completely novel applications become possible for strongly lipophilic dyes which were otherwise inhibited by the insolubility in polar media. In particular, the high heat capacity of water allows applications with high radiation densities. On the other hand, the aqueous phase is of central importance for any use of dyes in biochemistry.
The dispersion of dyes demonstrated with perylene derivatives can be extended to other classes of dyes. Lipophilic and readily soluble dyes are therefore required. For example, indigo was substituted in the positions 5,5′ (2)19 and 6,6′ (3)20 with tert-butyl groups in order to increase the solubility.21 Saturated solutions in the dispersant were prepared with these dyes and spread in water as was described for 1. More dispersant than for 1 is necessary for 2 and 3, respectively, because of the lower solubility. Analogously to 1, coloured aqueous media were obtained with UV/Vis spectra resembling the spectra in lipophilic solvents; see Fig. 3. The nearly identical spectra of 2 in the dispersion and in lipophilic solvents are indicative of isolated chromophores in the particles. On the other hand, the spectra of 3 are slightly differently structured and this deviation may be taken as an indication of some aggregation of chromophores in the particles.
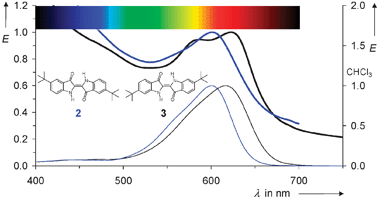 | ||
| Fig. 3 UV/Vis absorption spectra of 2 (blue) and 3 (black). Upper, thick lines: nano-dispersions in water; lower, thin lines: reference spectra in chloroform. | ||
The very similar results with the completely different chromophores 1, 2, and 3 indicate a broad application of the dispersion of dyes to form nanoparticles. The aqueous phase is of central importance for biochemical systems; thus, the novel concept opens up such applications for lipophilic dyes.
We thank Professor Dr. T. Bein for the particle measurements and Dr. H. Mottl from ISP Switzerland AG for a gift of Bomol-4N.
Experimental
The perylene dyes 1 were prepared according to literature procedures.3 Saturated solutions of these dyes in Bomol-4N10,11 were prepared, left in contact with the solid material for 16 h, decanted, poured into 100 times the quantity of distilled water or even more and filtered through a D5 glass filter. The indigo derivatives 2 and 3 were prepared according to the literature19,20 and dispersed completely analogously to 1. The dispersions were diluted with distilled water to obtain optical densities between 0.5 and 1.0 in 1 cm cuvettes and the UV/Vis spectra were recorded with a Cary 5000 spectrometer from Varian. The fluorescence spectra were recorded with an SP3000 fluorescence spectrometer from Perkin Elmer and quantum corrected.4 Fluorescence quantum yields were determined analogously to refs 4 and 22 with 1b as the standard. The particle distribution was measured with a DLS counter: Malvern Nano-ZS ZEN 3600.References
- (a) H. Langhals, Helv. Chim. Acta, 2005, 88, 1309–1343 CrossRef CAS; (b) H. Langhals, Heterocycles, 1995, 40, 477–500 CrossRef CAS.
- (a) H. Zollinger, Color Chemistry, Synthesis, Properties, and Applications of Organic Dyes and Pigments, Wiley-VCH, Zürich, 3rd edn, 2003, ISBN 3-906390-23-3 Search PubMed; (b) W. Herbst and K. Hunger, Industrielle Organische Pigmente, Herstellung, Eigenschaften, Anwendung, VCH Verlag, Weinheim, 2nd edn, 1995, ISBN 3-527-28744-2 Search PubMed; (c) J. Fabian and H. Hartmann, Light Absorption of Organic Colorants, Springer Verlag, Berlin, 1980, ISBN 3-540-09914-X Search PubMed; (d) H. R. Schweizer, Künstliche Organische Farbstoffe und ihre Zwischenprodukte, Springer-Verlag, Berlin, 1st edn, 1964, LCCC-Nr. 63-23138, pp. 385 Search PubMed.
- (a) S. Demmig and H. Langhals, Chem. Ber., 1988, 121, 225–230 CrossRef CAS; (b) H. Langhals, S. Demmig and T. Potrawa, J. Prakt. Chem., 1991, 333, 733–748 CrossRef CAS.
- H. Langhals, J. Karolin and L. B.-Å. Johansson, J. Chem. Soc., Faraday Trans., 1998, 94, 2919–2922 RSC.
- H. Langhals, Ger. Offen., DE 3703513, February 5, 1987 (Chem. Abstr., 1988, 109, P212376w) Search PubMed.
- H. Langhals, H. Jaschke, H. Bastani-Oskoui and M. Speckbacher, Eur. J. Org. Chem., 2005, 4313–4321 CrossRef CAS.
- (a) D. Matschulat, Diplomarbeit, Universität München, 2001 Search PubMed; (b) C. Kohl, T. Weil, Jianqiang Qu and K. Müllen, Chem.–Eur. J., 2004, 10, 5297–5310 CrossRef CAS; (c) Jianqiang Qu, C. Kohl, M. Pottek and K. Müllen, Angew. Chem., 2004, 116, 1554–1557 ( Angew. Chem., Int. Ed. , 2004 , 43 , 1528–1531 ) CrossRef.
- H. Langhals, Dyes for Fluorescent Immunoassays, in Immunochemical Detection of Pesticides and their Metabolites in the Water Cycle, ed. B. Hock, VCH, Weinheim, 1995, ISBN 3-527-27137-6 (Chem. Abstr., 1996, 124, 24966z) Search PubMed.
- H. Langhals, W. Jona, F. Einsiedl and S. Wohnlich, Adv. Mater., 1998, 10, 1022–1024 CrossRef CAS.
- (a) S. Sato, Y. Kato and A. Kawai, Kogyo Kagaku Zasshi, 1961, 64, 1013–17 ( Chem. Abstr. , 1962 , 57 , 9702 ) CAS; (b) Pittsburgh Plate Glass Co. (inv. F. S. Shahade, R. M. Christenson and M. Roger), US Patent 3293201 (7.3. 1962) (Chem. Abstr., 1967, 66, 38935) Search PubMed.
- ISP Switzerland AG, Lindenstrasse 10, 6340 Baar, Switzerland.
- (a) K. Holmberg, J. Colloid Interface Sci., 2004, 274, 355–264 CrossRef CAS; (b) R. H. Mueller, M. Radtke and S. A. Wissing, in Encyclopedia of Nanoscience and Nanotechnology, ed. H. S. Nalwa, American Scientific Publishers, Stevenson Ranch, CA, USA, 2004, vol. 10, pp. 43–56 (Chem. Abstr., 2004, 142, 120223) Search PubMed.
- (a) H. Kiel, Diplomarbeit, TU Ilmenau, 2003 Search PubMed; (b) Robu Glasfilter-Geräte GmbH, Hattet, Germany.
- N. Roussel, T. N. H. Nguyen and P. Coussot, Phys. Rev. Lett., 2007, 98, 114502-1–114502-4.
- H. Langhals, Description of Properties of Binary Solvent Mixtures, in Similarity Models in Organic Chemistry, Biochemistry and Related Fields, ed. R. I. Zalewski, T. M. Krygowski and J. Shorter, Elsevier, Amsterdam, 1991, pp. 283–342 Search PubMed.
- (a) A. S. Davydov, Zh. Eksp. Teor. Fiz., 1948, 18, 210–218 ( Chem. Abstr. , 1949 , 43 , 4575f ) Search PubMed; (b) A. S. Davydov, in Theory of Molecular Excitations (transl.), ed. H. Kasha and M. Oppenheimer, Jr, McGraw-Hill, New York, 1962 Search PubMed; (c) W. Kuhn, Trans. Faraday Soc., 1930, 26, 293 RSC; (d) M. Kasha, H. R. Rawls and M. A. El-Bayoumi, Pure Appl. Chem., 1965, 11, 371–392 CrossRef CAS.
- D. Horn and J. Rieger, Angew. Chem., 2001, 113, 4460–4492 ( Angew. Chem., Int. Ed. , 2001 , 40 , 4330–4361 ) CrossRef.
- H. Langhals, R. Ismael and O. Yürük, Tetrahedron, 2000, 56, 5435–5441 CrossRef CAS.
- (a) G. M. Oksengaendler and M. O. Lozinskii, Ukr. Khim. Zh., 1959, 25, 95–98 ( Chem. Abstr. , 1959 , 53 , 111741 ) Search PubMed; (b) D. Schulte-Frohlinde, H. Herrmann and G. M. Wyman, Z. Phys. Chem. (München), 1976, 101, 115–121 CAS; (c) H. Meier and W. Luettke, Liebigs Ann. Chem., 1981, 1303–1333 CrossRef CAS.
- (a) M. Battegay and P. Haeffely, Bull. Soc. Chim. Fr., 1924, 35, 981–988 CAS; (b) E. Wille and W. Luettke, Liebigs Ann. Chem., 1980, 2039–2054.
- H. Langhals, Ger. Offen., DE 3016764, April 30, 1980 (Chem. Abstr., 1982, 96, P70417x) Search PubMed.
- H. Langhals, Ger. Offen., DE 19847370.2, October 14, 1998 (Chem. Abstr., 2000, 132, 286129q) Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details. See DOI: 10.1039/b717795j |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2008 |