Cation–anion double hydrolysis derived mesoporous γ-Al2O3 as an environmentally friendly and efficient aldol reaction catalyst†
Peng
Bai
ab,
Pingping
Wu
a,
Guofeng
Zhao
b,
Zifeng
Yan
b and
X. S.
Zhao
*a
aDepartment of Chemical and Biomolecular Engineering, National University of Singapore, 4 Engineering drive 4, Singapore 117576. E-mail: chezxs@nus.edu.sg; Fax: +65-67791936; Tel: +65-65164727.
bState Key Laboratory for Heavy Oil Processing, PetroChina Key Laboratory of Catalysis, China University of Petroleum, Dongying 257061, China. E-mail: zfyancat@hdpu.edu.cn; Fax: +86-546-8391971; Tel: +86-546-8391527.
First published on 29th October 2007
Abstract
A mesoporous γ-Al2O3 without amorphous domains was synthesized using a modified cation–anion double hydrolysis method and was shown to display a remarkably high catalytic activity in the aldol reaction.
Mesoporous aluminas are gaining increasing research attention because of their improved structural properties compared with conventional aluminas. Apart from the work of Zhang et al.,1 most of the mesoporous aluminas reported in the literature have amorphous pore walls,2 resulting in low hydrothermal stability and limited applications. We have previously shown that a cation–anion double hydrolysis (CADH) method is very effective in synthesizing mesoporous γ-Al2O3.3 Upon mixing a cationic aluminium species with an anionic one, the hydrolysis rates of both ions are mutually promoted. As a result, the equilibria of both hydrolysis reactions are broken up and the hydrolysis reaction is enhanced to a complete extent. The resultant alumina materials consist of a γ-Al2O3 crystalline phase with a mesoporous structure characterized by a high surface area and narrow pore size distribution. Compared with the previous methods for the synthesis of mesoporous aluminas, the CADH strategy has several advantages. Firstly, cheaper inorganic aluminium sources, such as Al(NO3)3 and NaAlO2 can be used instead of expensive and toxic aluminium alkoxides. Secondly, by mixing a stoichiometric amount of Al3+ and AlO2−, the hydrolysis reactions proceed rapidly to a complete extent, thus avoiding complicated pH adjustment1,4 and shortening the synthesis time.5 These advantages are highly favorable in terms of industrial applications of mesoporous aluminas.
The aldol reaction is one of the most useful and powerful carbon–carbon bond construction reactions, which includes the reaction between aldehydes and ketones.6 Liquid strong acids like sulfuric acid and strong alkali catalysts like sodium hydroxide are the active catalysts for the reaction.7 However, these catalysts are highly corrosive. In addition, after reaction, such catalysts have to be neutralized, generating a huge amount of salts, which must be disposed of as solid wastes. Thus, an environmentally friendly and efficient catalyst for this reaction is strongly desirable. Mg–Al hydrotalcite and basic magnesium–lanthanum mixed oxide are alternative catalysts,8 and more catalyst systems need to be developed.
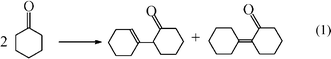
In this work, we further modified the CADH method following the procedures established by Zhang et al.1 It was observed that the structural properties have been greatly improved. The aldol reaction of cyclohexanone is used as a probe reaction to evaluate the catalytic activity of the synthesized mesoporous alumina samples. As shown in eqn (1), this reaction produces two kinetic isomers, namely 2-(1-cyclohexenyl) cyclohexanone (CHC) and 2-cyclohexylidene cyclohexanone (CDC) with CHC as the main product (hereafter, ‘dimer’ is used to represent the two isomers),9 which are the intermediates for o-phenylphenol (OPP), an important chemical widely used as a dye intermediate, photographic agent, additive in the rubber industry, bactericide of low toxicity, surface activator, and thermal stabilizer.10Catalytic measurements showed that the alumina samples thus synthesized displayed a remarkably high catalytic activity in the aldol reaction of cyclohexanone.
Fig. 1 shows the XRD patterns of the double hydrolysis derived alumina (DHA) samples (see ESI for sample names†) synthesized at different temperatures. It is seen that all samples display a similar XRD pattern. The diffraction peaks can be indexed to the γ-Al2O3 phase (JCPDS card no. 10–0425). In comparison with our previously reported alumina samples (see Fig. S1, ESI†),3 the XRD peaks of the DHA samples prepared in this work are better resolved and more intense. Besides the strongest (400) and (440) reflections of the γ-Al2O3 phase as observed in Fig. S1, some weak reflections, such as (220), (311), (222) and (511), are also observed in this work, indicating improved crystallinity of the DHA samples. In addition, it can be seen that with increasing the crystallization temperature, the intensity of the XRD peaks increases and the weak reflections of the γ-Al2O3 phase become more discernible and better resolved, evidencing the increased crystallinity of γ-Al2O3.
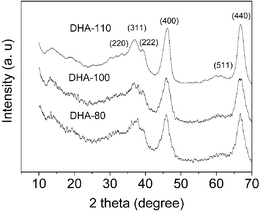 | ||
| Fig. 1 XRD patterns of DHA samples synthesized at different crystallization temperatures. | ||
To further examine the crystalline phase of the DHA samples, 27Al MAS NMR spectra were recorded and are shown in Fig. 2. It is seen that all DHA samples have two well-resolved NMR signals at about 65 and 5 ppm, attributed to tetrahedrally and octahedrally coordinated aluminium species, respectively.11 The ratio of tetrahedral Al to octahedral Al is about 1 : 3, characteristic of the γ-Al2O3 phase,12 further confirming that the DHA samples consist of a γ-Al2O3 phase. It should be noted that the MAS NMR signal at about 35 ppm, attributed to the penta-coordinated Al species in amorphous domains,13 is not observed in the DHA samples. This observation is different from that of the mesoporous γ-Al2O3 materials reported previously.13,14 The absence of this NMR signal in the alumina samples prepared in this work verifies that the DHA samples are a pure γ-Al2O3 phase without amorphous domains.
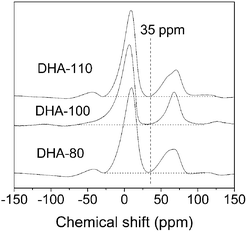 | ||
| Fig. 2 27Al MAS NMR spectra of DHA samples. | ||
Fig. 3 (top) shows the N2 sorption isotherms of the DHA samples, all of which exhibit a type IV isotherm, characteristic of mesoporous materials. The type H-2 hysteresis loops in the isotherms suggest “ink-bottle-like” pores may be present in the samples. With increasing crystallization temperature, the mesopore size of the DHA samples increased and then decreased as can be seen from Fig. 3 (bottom). The pore structure parameters presented in Table S1† show that sample DHA-100 has the largest pore diameter (about 7.1 nm) and the highest pore volume (about 0.81 cm3 g−1), whereas sample DHA-80 has the largest BET surface area (about 471 m2 g−1). To the best of our knowledge, this is the highest surface area of pure γ-Al2O3 without amorphous domains so far reported.1,13,15 The TEM image in Fig. S2 (top)† shows the DHA samples have a “worm-like” mesopore motif and the selected area electron diffraction pattern (SAED) in Fig. S2 (bottom) exhibits a polycrystalline reflection ring pattern, corresponding to the strongest (400), (440) and (311) reflections of γ-Al2O3.
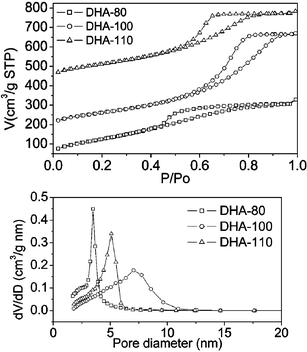 | ||
| Fig. 3 N2 adsorption isotherms (top) and pore size distributions (bottom) of DHA samples. | ||
The catalytic performance of the DHA samples prepared in this work is compared with that of commercial aluminas in Fig. 4 and Table S2.† It can be seen that all DHA samples exhibited a much higher catalytic activity than the commercial alumina samples. Among the commercial alumina samples, the basic alumina sample displayed the highest activity with a conversion of 50.8% after 2 h of reaction. Sample DHA-80 showed the highest activity among the DHA samples synthesized in this work with a catalytic conversion of 95.6%. In addition, the DHA samples gave a higher dimer yield than the commercial ones with the highest yield of 82.2%. An analysis of the data presented in Tables S1 and S2† immediately led to a conclusion that the catalyst activity is proportional to the BET surface area of the alumina samples. This indicates that the key factor determining the catalyst activity is the amount of the accessible active sites on the alumina surface. It should be noted that the selectivity for the mesoporous alumina is slightly lower than for the commercial alumina. However, it is reasonable, as the dimers can also react with cyclohexanone to form trimers. When the conversion is high, the concentration of dimers is also high, correspondingly the side reaction rate will increase and the selectivity of dimers will naturally decrease. However this problem can be solved by shortening the reaction time. For example, in the case of DHA-80, when the reaction time decreases to 30 min, the selectivity of dimers is as high as 95.6% together with relatively high conversion of cyclohexanone (64.5%).
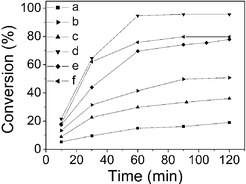 | ||
| Fig. 4 Conversion of cyclohexanonevs. time for DHA and commercial alumina catalysts. (a) C-Al2O3-a; (b) C-Al2O3-b; (c) C-Al2O3-n; (d) DHA-80; (e) DHA-100; (f) DHA-110. | ||
The commercial neutral and basic alumina samples have a similar BET surface area and their crystalline structure is similar to that of DHA samples as evidenced by the XRD and MAS NMR data (see Fig. S3–S4, ESI†). However, their catalytic activities are quite different. The basic alumina sample has a higher catalytic activity than the neutral one, suggesting that basic sites are the active sites for the aldol reaction of cyclohexanone, as has been reported previously.16
The temperature-programmed desorption of CO2 (CO2-TPD) technique was used to determine surface basicity and the CO2-TPD profiles of the samples are shown in Fig. 5. All commercial alumina samples exhibited a maximum at around 150 °C, which is attributed to the CO2 desorption from weak basic sites.17 The DHA samples, on the other hand, exhibited a peak at about 160 °C. These observations indicate that the DHA samples possess a slightly higher surface basicity than the commercial aluminas. In addition, the TPD curves of the DHA samples came to the baseline slowly from the maximum at temperatures of 309 °C for DHA-110, 340 °C for DHA-100, and 353 °C for DHA-80. In the case of the commercial alumina samples, their TPD curves came to the baseline earlier at temperatures of 258 °C for C-Al2O3-n, 278 °C for C-Al2O3-a, and 306 °C for C-Al2O3-b, respectively. Alumina surface has both acidic and basic sites.18 Thus, all alumina samples should have weak basic sites as verified by the data in Fig. 5. However the density and strength of the basic sites strongly depend on the preparation conditions, such as solution pH and ionic strength.18 The existence of the stronger basic sites on the DHA samples compared with the commercial alumina samples must be related to the unique double hydrolysis synthesis route. In our synthesis, after crystallization, the solution pH is commonly around 10, which suggests that the basic synthesis environment may bestow the obtained DHA samples with basic sites on the surface, and is thus responsible for the observed highly efficient catalytic performance.
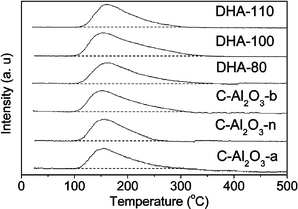 | ||
| Fig. 5 CO2-TPD profiles of the DHA and commercial alumina catalysts. | ||
In summary, pure mesoporous γ-Al2O3 materials synthesized using a modified cation–anion double hydrolysis method display an excellent catalytic performance in the aldol reaction of cyclohexanone. The observed high catalytic activity is attributed to both the high surface area and strong basic sites of the mesoporous alumina. Moreover, it is reasonable to envisage that the mesoporous alumina synthesized in this work has great potential in the aldol reaction of other organic reactants, namely aldehydes and ketones,6,19 or the cross-aldol reaction between them,20 which will produce numerous useful self-aldol or cross-adol compounds.
References
- Z. Zhang and T. J. Pinnavaia, J. Am. Chem. Soc., 2002, 124, 12294 CrossRef CAS; Z. Zhang, R. W. Hicks, T. R. Pauly and T. J. Pinnavaia, J. Am. Chem. Soc., 2002, 124, 1592 CrossRef CAS.
- J. Čejka, Appl. Catal., A, 2003, 254, 327 CrossRef CAS.
- P. Bai, W. Xing, Z. Zhang and Z. Yan, Mater. Lett., 2005, 59, 3128 CrossRef CAS.
- S. Valange, J. L. Guth, F. Kolenda, S. Lacombe and Z. Gabelica, Microporous Mesoporous Mater., 2000, 35–36, 597 CrossRef CAS; N. Yao, G. X. Xiong, Y. H. Zhang, M. Y. He and W. S. Yang, Catal. Today, 2001, 68, 97 CrossRef CAS.
- W. Deng, P. Bodart, M. Pruski and B. H. Shanks, Microporous Mesoporous Mater., 2002, 52, 169 CrossRef CAS; S. Cabrera, J. El Haskouri, J. Alamo, A. Beltrán, D. Beltrán, S. Mendioroz, M. D. Marcos and P. Amorós, Adv. Mater., 1999, 11, 379 CrossRef CAS.
- C. H. Healthcock, in Comprehensive Organic Synthesis, ed. B. M. Trost and I. Fleming, Pergamon, Oxford, 1991, vol. 2, pp. 181–238 Search PubMed.
- H. O. House, D. S. Crumrine, A. Y. Teranishi and H. D. Olmstead, J. Am. Chem. Soc., 1973, 95, 3310 CrossRef CAS; S. E. Denmark and W. Lee, Tetrahedron Lett., 1992, 33, 7729 CAS.
- K. K. Rao, M. Gravelle, J. S. Valente and F. Figueras, J. Catal., 1998, 173, 115 CrossRef CAS; M. L. Kantam, V. Balasubrahmanyam, K. B. S. Kumar, G. T. Venkanna and F. Figueras, Adv. Synth. Catal., 2007, 349, 1887 CrossRef CAS.
-
K. Ramm, W. Thielecke, M. Zander, G. Collin and K. Ruhl, US Pat., 3
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 880
880![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 930, 1975 Search PubMed;
J. F. M. Klein, P. A. M. J. Stifis and J. A. Thomas, US Pat., 4
930, 1975 Search PubMed;
J. F. M. Klein, P. A. M. J. Stifis and J. A. Thomas, US Pat., 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 052
052![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 458, 1977 Search PubMed;
J. F. M. Klein, P. A. M. J. Stifis and J. A. Thomas, US Pat., 4
458, 1977 Search PubMed;
J. F. M. Klein, P. A. M. J. Stifis and J. A. Thomas, US Pat., 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 171
171![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 326, 1979 Search PubMed;
P. J. H. Thomissen, US Pat., 4
326, 1979 Search PubMed;
P. J. H. Thomissen, US Pat., 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 484
484![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 005, 1984 Search PubMed; J. M. Aragón, J. M. R. Vegas and L. G. Jodra, Ind. End. Chem. Res., 1993, 32, 2555 Search PubMed; J. M. Aragón, J. M. R. Vegas and L. G. Jodra, Ind. End. Chem. Res., 1994, 33, 592 Search PubMed.
005, 1984 Search PubMed; J. M. Aragón, J. M. R. Vegas and L. G. Jodra, Ind. End. Chem. Res., 1993, 32, 2555 Search PubMed; J. M. Aragón, J. M. R. Vegas and L. G. Jodra, Ind. End. Chem. Res., 1994, 33, 592 Search PubMed. -
H. Goto, N. Shibamoto and S. Tanaka, US Pat., 4
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 088
088![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 230, 1978 Search PubMed;
J. Imamura, US Pat., 4
230, 1978 Search PubMed;
J. Imamura, US Pat., 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 080
080![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 390, 1978 Search PubMed;
I. R. King and A. M. Hildon, US Pat., 4
390, 1978 Search PubMed;
I. R. King and A. M. Hildon, US Pat., 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 002
002![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 693, 1977 Search PubMed;
C. S. Elliott, US Pat., 3
693, 1977 Search PubMed;
C. S. Elliott, US Pat., 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 980
980![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 716, 1976 Search PubMed;
H. Goto, N. Shibamoto and S. Tanaka, US Pat., 4
716, 1976 Search PubMed;
H. Goto, N. Shibamoto and S. Tanaka, US Pat., 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 060
060![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 559, 1977 Search PubMed.
559, 1977 Search PubMed. - P. Bai, F. Su, P. Wu, L. Wang, F. Y. Lee, L. Lv, Z. F. Yan and X. S. Zhao, Langmuir, 2007, 23, 4599 CrossRef CAS.
- C. S. John, N. C. M. Alma and G. R. Hays, Appl. Catal., 1983, 6, 341 CrossRef CAS; K. Sohlberg, S. T. Pantelides and S. J. Pennycook, J. Am. Chem. Soc., 2001, 123, 26 CrossRef CAS.
- H. Park, S. H. Yang, Y. S. Jun, W. H. Hong and J. K. Kang, Chem. Mater., 2007, 19, 535 CrossRef CAS.
- Q. Liu, A. Wang, X. Wang and T. Zhang, Microporous Mesoporous Mater., 2006, 92, 10 CrossRef CAS.
- H. Y. Zhu, J. D. Riches and J. C. Barry, Chem. Mater., 2002, 14, 2086 CrossRef CAS.
- Z. Vit, L. Nordek and J. Malek, Collect. Czech. Chem. Commun., 1982, 47, 2235 CAS.
- P. Berteau, M. Kellens and B. Delmon, J. Chem. Soc., Faraday Trans., 1991, 87, 1425 RSC.
- G. A. Parks and P. L. de Bruyn, J. Phys. Chem., 1962, 66, 967 CrossRef CAS; J. A. Yopps and D. W. Fuerstenau, J. Colloid Sci., 1964, 19, 61 CrossRef CAS; X. Yang, Z. Sun, D. Wang and W. Forsling, J. Colloid Interface Sci., 2007, 308, 395 CrossRef CAS.
- K. M. Cergol, P. Turner and M. J. Coster, Tetrahedron Lett., 2005, 46, 1505 CrossRef CAS.
- G. W. Wang, Z. Zhang and Y. W. Dong, Org. Process Res. Dev., 2004, 8, 18 Search PubMed; A. A. Esmaeili, M. S. Tabas, M. A. Nasseri and F. Kazemi, Monatsh. Chem., 2005, 136, 571 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Details of experimental procedures and characterizations, TEM image and SAED pattern, table of structural properties of DHA and C-Al2O3 samples, table of catalytic results, XRD patterns and 27Al MAS NMR spectra of C-Al2O3 samples. See DOI: 10.1039/b713283b |
| This journal is © The Royal Society of Chemistry 2008 |