Enzymatic sensing with organic electrochemical transistors
Daniel A.
Bernards
,
Daniel J.
Macaya
,
Maria
Nikolou
,
John A.
DeFranco
,
Seiichi
Takamatsu
and
George G.
Malliaras
*
Cornell University, Ithaca, NY, USA. E-mail: ggm1@cornell.edu; Fax: 607 255 2365; Tel: 607 2551956
First published on 12th October 2007
Abstract
Since their development in the 1980's organic electrochemical transistors (OECTs) have attracted a great deal of interest for biosensor applications. Coupled with the current proliferation of organic semiconductor technologies, these devices have the potential to revolutionize healthcare by making point-of-care and home-based medical diagnostics widely available. Unfortunately, their mechanism of operation is poorly understood, and this hinders further development of this important technology. In this paper glucose sensors based on OECTs and the redox enzyme glucose oxidase are investigated. Through appropriate scaling of the transfer characteristics at various glucose concentrations, a universal curve describing device operation is shown to exist. This result elucidates the underlying device physics and establishes a connection between sensor response and analyte concentration. This improved understanding paves the way for rational optimization of enzymatic sensors based on organic electrochemical transistors.
1. Introduction
The field of organic electronics is in the midst of tremendous development, with organic semiconductors being considered for applications in electronic and optoelectronic devices, including light emitting diodes, photovoltaic cells, and thin-film transistors.1 Key advantages of these materials include tunability of their electronic properties via chemical synthesis and compatibility with roll-to-roll fabrication, which can yield ultra-low cost manufacturing. An emerging focus in the field involves the use of organic-based devices as transducers in chemical and biological sensors.2–6 In particular, organic thin-film transistors (OTFTs) are attracting a great deal of interest for sensor applications due to their simple electrical readout, inherent signal amplification, straightforward miniaturization, and facile incorporation into arrays and circuits. To date, OTFTs have been used to sense mechanical deformation and pressure, humidity and organic vapors, pH and ion concentrations, and a variety of biologically relevant analytes.2–7Within the family of organic semiconductor-based sensors, organic electrochemical transistors (OECTs—also known as conducting polymer transistors) have attracted particular interest.2,4 These devices were first developed in the 1980s by the group of Wrighton8–10 and rapidly found application in sensors.9–11 Unlike the majority of OTFTs, which are field-effect devices that rely on electrostatic gating, OECT operation relies on electrochemical doping/de-doping of an organic semiconductor film in contact with an electrolyte. This results in lower operating voltages, which make OECTs particularly suitable for biological and chemical detection in aqueous environments. The intimate contact between organic semiconductor and electrolyte in these devices makes them an excellent intermediary between the fields of biology and electronics: these devices have been used as ion-to-electron converters,12 have been integrated with cell membranes,13 and, more recently, have been used as ion pumps promoting cell growth.14 Their simple structure allows for fabrication using roll-to-roll techniques15 and lends itself to easy integration with microfluidic channels for lab-on-a-chip applications.16
2. Materials and methods
The commercially available PEDOT:PSS Baytron P was used as the active material for the fabrication of the OECTs. It was deposited on glass slides and patterned using the parylene technique described by DeFranco et al.17 Chemical vapor deposition was used to deposit 1.5 µm films of parylene on glass. Conventional lithography followed by an oxygen plasma etch was used to pattern the parylene film. Excess resist was removed with acetone. Prior to spin coating, the glass slides were treated with an oxygen plasma to improve film adhesion. A mixture of Baytron P and ethylene glycol (4 : 1 by volume) was spin cast at 1500 rpm onto the patterned support. Ethylene glycol was used to improve the conductivity of the PEDOT:PSS films. A small amount (∼5 µL mL–1) of dodecylbenzenesulfonic acid (DBSA) was added as a surfactant to improve film formation. The parylene film was then peeled from the glass support, leaving 1 mm wide PEDOT:PSS stripes. Devices were first baked on a hotplate for 20 s at 140 °C and then baked under vacuum at 140 °C for 1 h. PEDOT:PSS films were then soaked in de-ionized water to remove any surface contamination. Sylgard 184, poly(dimethylsiloxane) (PDMS), was used to confine the electrolyte to a 4 mm × 10 mm area over the PEDOT:PSS stripes. Sylgard 184 was mixed at a 20 : 1 base to cross-linker ratio and cured at 60 °C for 1 h prior to being used. The active PEDOT:PSS device area was 1 mm × 10 mm. A platinum wire was used as the gate electrode. All measurements were made with Keithley 2400 SourceMeters. Devices were tested using a fixed Vd = –0.2 V with Vg = 0, 0.1, 0.2, and 0.4 V. A 0.15 mM phosphate buffered saline (PBS) was used as the electrolyte for all experiments, which had a pH of 6.8 as measured by pH meter. Different concentrations of glucose in PBS were achieved by mixing a 50 µL solution of PBS and glucose with 50 µL solution of PBS and glucose oxidase (500 units mL–1).3. Results and discussion
Fig. 1a shows the typical configuration of an OECT. The transistor consists of an organic semiconductor film and an electrolyte with a gate electrode immersed in it. A common organic semiconductor material used in contemporary OECTs is poly(3,4-ethylenedioxythiophene) doped with poly(styrenesulfonate) (PEDOT:PSS). This is a degenerately doped p-type organic semiconductor (also referred to as a conducting polymer), in which hole transport takes place in the PEDOT phase while the PSS phase supports the sulfonate acceptors. The overlap of the electrolyte with the organic semiconductor defines the transistor channel, where the electrolyte is contained by a poly(dimethylsiloxane) (PDMS) well. Source and drain electrodes make electrical contact to the organic semiconductor film and the gate electrode is immersed in the electrolyte. By convention, the source electrode is grounded, a small bias is applied on the drain electrode (Vd), and the source–drain current (Isd) that flows through the channel is measured. The latter is modulated by a voltage applied to the gate electrode (Vg). For the case of p-type materials such as PEDOT:PSS, application of a positive Vg causes positive ions from the electrolyte to enter the organic film and de-dope it, decreasing the source–drain current.18 Therefore, PEDOT:PSS OECTs operate in depletion mode (i.e. as gate voltage increases, source–drain current decreases). De-doping in these devices is reversible, and the source–drain current recovers when Vg is returned to zero. | ||
| Fig. 1 (a) Schematic of a typical organic electrochemical transistor (not to scale). The reaction of interest is shown at the gate electrode. (b) Reaction cycle involved in the detection of glucose using GOx. | ||
Soon after the demonstration of the first OECT, these devices found use as transducers in biological sensors—in particular, coupled with redox enzymes for the detection of metabolites.19–24Metabolite levels are an important diagnostic marker in healthcare since they often correspond to physiological irregularities that warrant treatment or further investigation. Enzymes are excellent recognition elements for metabolites since they specifically react with these and do not suffer from significant interference. The use of redox enzymes, in particular, can provide versatility in the output signal and enhance the range of sensing possibilities. For sensor applications, enzymes can be blended with or chemically linked to the organic semiconductor or gate electrode,19–22 or introduced free-floating into the electrolyte.23,24 When the appropriate analyte is present in the electrolyte, its interaction with the enzyme modulates the source–drain current. A calibration curve that correlates the relative change in Isd to analyte concentration can be generated and subsequently used to deduce unknown analyte concentrations.
Despite the large interest in the application of OECTs for enzymatic sensing and the potential of these devices to make a major contribution to healthcare, the operational mechanism for these devices is not understood in real detail. For example, it is not clear how sensor metrics, such as sensitivity, relate to materials’ properties and device parameters. This hinders further development and deployment of these devices. In this paper we describe experiments that elucidate the physics underlying OECT operation and connect device response to analyte concentration in OECT-based enzymatic sensors.
The fruit fly of enzymatic sensing involves the detection of glucose using glucose oxidase (GOx). The reaction cycle involved is show in Fig. 1b. GOx catalyzes the conversion of D-glucose to D-glucono-1,5-lactone and is reduced in the process. Reactivation of GOx from its reduced state produces hydrogen peroxide. Since the concentration of peroxide is directly related to the concentration of glucose, peroxide is often used to detect and measure the concentration of glucose. A Pt electrode immersed in the electrolyte can catalyze the decomposition of peroxide:
| H2O2 → 2H+ + O2 + 2e– | (1) |
Glucose can be sensed with a PEDOT:PSS OECT using a Pt gate electrode and an electrolyte (in this case phosphate buffered saline) with free-floating GOx. Introduction of glucose into the electrolyte results in the reactions described above and consequently affects the source–drain current. This is shown in Fig. 2a, where the transistor transfer characteristics are plotted for different glucose concentrations. All curves show a monotonic decrease of the source–drain current with increasing gate voltage due to de-doping of the organic semiconductor. With increasing glucose concentration, however, the magnitude of the modulation increases. The presence of glucose clearly affects electrical characteristics, but the mechanism responsible for these changes is not clear.
 | ||
| Fig. 2 (a) Source–drain current plotted as a function of applied gate voltage for a fixed drain voltage (Vd = –0.2 V) and various glucose concentrations. (b) Source–drain current plotted as a function of effective gate voltage, where the applied gate voltage is shifted by an offset voltage (Voffset) that depends on concentration. Voffset is chosen such that the measured current lies along a universal curve, where the extent of the shift is determined by glucose concentration. | ||
Essential to understanding the mechanism of operation is the observation that the data in Fig. 2a can be scaled to yield a universal curve. This is shown in Fig. 2b, where the gate voltage was scaled according to:
| Veffg = Vg + Voffset | (2) |
 | (3) |
 | ||
| Fig. 3 Dependence of the offset voltage on glucose concentration. The solid line is a fit to the data up to a glucose concentration of 1 mM. | ||
In order to understand the physical meaning of the offset voltage one needs to consider a comparison between OECTs and sensors based on conventional electrochemical detection. In conventional electrochemistry, the effects of the Nernst equation are manifested in changes of the potential at a working electrode (where the reaction occurs) relative to a reference electrode potential. In contrast, the potential of the gate electrode in an OECT is fixed. Consequently, the potential shift described by the Nernst equation is manifested by a shift of the electrolyte potential relative to that of the gate. This alternate but physically equivalent reference frame is shown in Fig. 4. For simplicity Vd was assumed to be small compared to Vg so that the entire channel is effectively grounded. In the absence of glucose, peroxide is not generated and the reaction shown in eqn (1) does not take place.
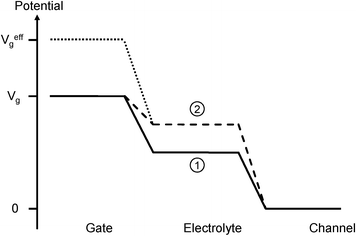 | ||
| Fig. 4 Potential diagram of the OECT. In the absence of glucose (solid line), the electrolyte potential (1) is determined by the relative capacitances at the gate and channel interfaces. In the presence of glucose (dashed line), the electrolyte potential (2) is increased according to the Nernst equation. The effective gate voltage (dotted line) is the gate voltage required to produce the same electrolyte potential (2) in the absence of Faradaic effects. The lines connecting gate, electrolyte, and channel potentials are meant as guides to the eye. | ||
Moreover, for gate voltages that are small enough to prevent electrolysis of water, there is no charge transfer between the electrolyte and the Pt electrode (non-Faradaic regime). The electrolyte potential in this case is determined by the capacitances associated with double layer formation at the gate and the channel (solid line in Fig. 4) and is equal to:
 | (4) |
 | (5) |
The current that flows in the channel of an OECT for a particular drain voltage depends only on the potential of the electrolyte, and the details at the gate electrode can be neglected. This was recently demonstrated in carbon nanotube electrochemical transistors,27 where a reference electrode was used to monitor the potential of the electrolyte. From eqn (5) it is clear that the addition of glucose increases the electrolyte potential and correspondingly decreases the source–drain current. It is convenient to define an effective gate voltage:
 | (6) |
The above analysis also clarifies the physical meaning of the offset voltage involved in the transformation shown in Fig. 2. Voffset is represented by the last two terms in eqn (6) and describes the Faradaic contribution to the effective gate voltage. It originates from the shift in the chemical potential described by the Nernst equation and is scaled by the capacitance ratio. The line in Fig. 3 is a fit to Voffset with γ = 4. Given that the capacitance of polymer electrodes is greater than that of metals per unit area28 and that the area of the gate electrode was smaller than that of the channel, a value for γ that is larger than one is reasonable. It should be noted that the capacitance associated with metals and polymers is mechanistically distinct: while metals such as Pt are impermeable to ionic charge, ions can penetrate polymers12 (although this is not always the case29), which gives rise to a unique origin for the capacitance in each.30 The potential drop between the electrolyte and the channel in Fig. 4 implies ion accumulation on the surface of the PEDOT:PSS. An effective capacitance can still be used for the case where ions completely penetrate the PEDOT:PSS.
According to the above, OECTs can be viewed as remote voltage sensors. Charge transfer reactions that alter the potential near the gate electrode can be detected by measuring the source–drain current in the organic semiconductor film. Despite its indirect nature, this mode of detection lends itself to uncomplicated, high-sensitivity transduction. Changes in the source–drain current can be easily measured with high accuracy and only require simple equipment.
Bernards and Malliaras have shown that the source–drain current in OECTs for the case of uniform de-doping is given by:18
 | (7) |
 | (8) |
Fig. 5 demonstrates the validity of the analysis presented in this paper. The normalized response (NR) of the source–drain current is plotted as a function of glucose concentration and gate voltage. Normalization was done relative to the zero concentration limit as:
 | (9) |
 | ||
| Fig. 5 Normalized response of the source–drain current as a function of applied gate voltage and glucose concentration. Filled circles are experimental data and the grid is a fit. | ||
The excellent agreement between theory and measurement in Fig. 5 illustrates that the analysis described above can quantitatively describe the operation of OECT-based enzymatic sensors. This paves the way for the rational design of better devices and helps explain some of the unique characteristics of OECTs. One example is the increase of sensitivity (slope of NR versusglucose concentration) with gate voltage (Fig. 5). It can be shown that this arises from a term in NR that is proportional to:
4. Conclusions
The behavior of OECT-based enzymatic sensors was investigated. PEDOT:PSS was used as the conducting polymer, and the redox enzyme glucose oxidase was introduced to the electrolyte of the OECT in order to detect glucose. Appropriate scaling of the transistor transfer characteristics at various glucose concentrations yielded a universal curve of the source–drain current versus effective gate voltage. This observation helped elucidate the physics of OECT-based enzymatic sensors. An effective gate voltage was used to account for Faradaic contributions to the potential at the gate electrode. A connection between source–drain current and analyte concentration was developed and resulted in an excellent fit to experimental data. This improved understanding paves the way for rational optimization of OECT-based enzymatic sensors.Acknowledgements
We thank Burak Ulgut and Héctor Abruña for suggestions and comments regarding this work. D.A.B. thanks the United States Department of Defense for a fellowship. This work was supported by the Center for Nanoscale Systems, and was partly performed in the Cornell NanoScale Science and Technology Facility, which is funded by the National Science Foundation and the New York State Office of Science, Technology & Academic Research.References
- G. Malliaras and R. Friend, Phys. Today, 2005, 58, 53 CAS.
- L. Wang, D. Fine, D. Sharma, L. Torsi and A. Dodabalapur, Anal. Bioanal. Chem., 2005, 384, 310 CrossRef.
- J. Locklin and Z. Bao, Anal. Bioanal. Chem., 2005, 384, 336 CrossRef.
- J. T. Mabeck and G. G. Malliaras, Anal. Bioanal. Chem., 2005, 384, 343 CrossRef.
- C. Bartic and G. Borghs, Anal. Bioanal. Chem., 2005, 384, 354 CrossRef.
- M. Bouvet, Anal. Bioanal. Chem., 2005, 384, 366 CrossRef.
- T. Someya, T. Sekitani, S. Iba, Y. Kato, H. Kawaguchi and T. Sakurai, Proc. Natl. Acad. Sci. U. S. A., 2004, 100, 9966 CrossRef.
- H. S. White, G. P. Kittlesen and M. S. Wrighton, J. Am. Chem. Soc., 1984, 106, 5375 CrossRef.
- E. W. Paul, A. J. Ricco and M. S. Wrighton, J. Phys. Chem., 1985, 89, 1441 CrossRef CAS.
- J. W. Thackeray, H. S. White and M. S. Wrighton, J. Phys. Chem., 1985, 89, 5133 CrossRef CAS.
- J. W. Thackeray and M. S. Wrighton, J. Phys. Chem., 1986, 90, 6674 CrossRef CAS.
- D. Nilsson, M. Chen, T. Kugler, T. Remonen, M. Armgarth and M. Berggren, Adv. Mater., 2002, 14, 51 CrossRef CAS.
- D. A. Bernards, G. G. Malliaras, G. E. S. Toombes and S. M. Gruner, Appl. Phys. Lett., 2006, 89, 053505 CrossRef.
- J. Isaksson, P. Kjäll, D. Nilsson, N. Robinson, M. Berggren and A. Richter-Dahlfors, Nat. Mater., 2007, 6, 673 CrossRef CAS.
- M. Berggren, D. Nilsson and N. D. Robinson, Nat. Mater., 2007, 6, 3 CrossRef CAS.
- J. T. Mabeck, J. A. DeFranco, D. A. Bernards, G. G. Malliaras, S. Hocde and C. J. Chase, Appl. Phys. Lett., 2005, 87, 013503 CrossRef.
- J. A. DeFranco, B. S. Schmidt, M. Lipson and G. G. Malliaras, Org. Electron., 2006, 7, 22 Search PubMed.
- D. A. Bernards and G. G. Malliaras, Adv. Funct. Mater. DOI:10.1002/adfm.200601239.
- T. Matsue, M. Nishizawa, T. Sawaguchi and I. Uchida, J. Chem. Soc., Chem. Commun., 1991,(15), 1029 RSC.
- M. Nishizawa, T. Matsue and I. Uchida, Anal. Chem., 1992, 64, 2642 CrossRef CAS.
- D. T. Hoa, T. N. S. Kumar, N. S. Punekar, R. S. Srinivas, R. Lal and A. Q. Contractor, Anal. Chem., 1992, 64, 2645 CrossRef CAS.
- A. Q. Contractor, T. N. S. Kumar, R. Narayanan, S. Sukeerthi, R. Lal and R. S. Srinivas, Electrochim. Acta, 1994, 39, 1321 CrossRef CAS.
- Z. T. Zhu, J. T. Mabeck, C. C. Zhu, N. C. Cady, C. A. Batt and G. G. Malliaras, Chem. Commun., 2004,(13), 1556 RSC.
- D. J. Macaya, M. Nikolou, S. Takamatsu, J. T. Mabeck, R. M. Owens and G. G. Malliaras, Sens. Actuators, B, 2007, 123, 374 CrossRef.
- S. M. Sze, Physics of Semiconductor Devices, Wiley, New York, 1981 Search PubMed.
- A. J. Bard and L. R. Faulkner, Electrochemical Methods, John Wiley and Sons, New York, 1981 Search PubMed.
- L. Larrimore, S. Nad, X. Zhou, H. Abruna and P. L. McEuen, Nano Lett., 2006, 6, 1329 CrossRef CAS.
- J. D. Stenger-Smith, C. K. Webber, N. Anderson, A. P. Chafin, K. Zong and J. R. Reynolds, J. Electrochem. Soc., 2002, 149, A973 CrossRef CAS.
- M. J. Panzer and C. D. Frisbie, J. Am. Chem. Soc., 2007, 129, 6599 CrossRef CAS.
- J. Wang and A. J. Bard, J. Am. Chem. Soc., 2001, 123, 498 CrossRef CAS.
Footnote |
| † In this case, saturation of Voffset is due to insufficient sampling time—steady state operation is increasingly difficult to obtain as depletion increases. |
| This journal is © The Royal Society of Chemistry 2008 |

