Silica nanoparticles with a hybrid organic–inorganic shell
Marie-Alexandra
Neouze
*a,
Miroslava
Malenovska
a,
Ulrich
Schubert
a,
Vadim
Kotlyar
b,
Ella
Kuperschmidt
b,
Anna
Peled
b and
Jean-Paul
Lellouche
b
aInstitute of Materials Chemistry, Vienna University of Technology, Getreidemarkt 9/165, 1060 Vienna, Austria. E-mail: mneouze@mail.zserv.tuwien.ac.at
bDepartment of Chemistry, Bar-Ilan University, Ramat-Gan 52900, Israel
First published on 19th October 2007
Abstract
Non-agglomerated nanoparticles of an average diameter of 200 nm with a hybrid organic–inorganic shell were prepared by reacting the COOH groups of poly(N-dicarbazolyl-lysine)-covered silica nanoparticles with zirconium tert-butoxide, Zr(OtBu)4, followed by sol–gel processing.
Introduction
Various strategies are being developed to prepare nanoparticles of increasingly complex structures and morphologies, such as multi-shell, Janus, or hollow particles. Illustrative examples are given in ref. 1–9. A prominent role is played by inorganic particles covered by organic species. Bi- or multi-layer hybrid organic–inorganic particles with polymeric shells can be prepared by different approaches, such as reverse micelle systems,10 heterophase polymerizations,4 or surface-initiated atom transfer radical polymerization processes.11–13 In that case, organic shells are generally formed around the inorganic core of the nanoparticle. An inverse approach may also be used, i.e. growing inorganic nanoparticles inside dendrimeric nanoreactor voids for particle containment and passivation.14–17The great majority of known hybrid inorganic–organic nanoparticles contain only one kind of inorganic matter, e.g.titania, silica or gold, and there are only a few reports on hybrid nanoparticles possessing two different inorganic constituents. For example, titania nanoparticles were generated by sol–gel processing in hyperbranched polymers substituted by pendant (CH2)3Si(OEt)3groups. The Si(OEt)3 groups served as mediator functionalities between the titania nanoparticles and the polymeric shell and, consequently, do not constitute an intermediate inorganic shell.18 SiO2/polystyrene/TiO2 multilayer core–shell hybrid microspheres were obtained by mixing positively charged SiO2/polystyrene hybrid particles with Ti(OtBu)4 followed by sol–gel reaction.19Polystyrene is an inert polymer that does not interact with the metal alkoxide precursor. Thus, polymers equipped with pendant functional groups capable of coordinating metal alkoxides should be beneficial for the formation of an anchored secondary inorganic structure. A similar approach was previously used for the generation of a gold metal layer on bilayer gold/silica nanoparticles. The outer gold layer was obtained by interaction of a gold hydroxide aqueous solution with amine groups of (3-aminopropyl)trimethoxysilane grafted onto the silica layer followed by reduction.20 A three-layer Au/silica/polyisoprene nanocomposite was prepared by atom transfer radical polymerization from the surface of Au@SiO2nanoparticles.13 A different sequence of the layers was obtained by reacting dodecanethiol-stabilized gold nanoparticles with octadecyldimethyl(3-trimethoxysilylpropyl)ammonium chloride. Hydrophobic interactions between the two long alkyl chains of both the adsorbed thiol and the ammonium compound resulted in a self-assembled structure where the alkoxysilyl groups were located on the outer surface of the hybrid nanoparticles. The alkoxysilyl groups were converted in a silica shell around the organic-covered gold nanoparticles by hydrolytic polycondensation.21
In the present work, we describe the preparation of a morphologically different three-component nanoparticle system composed of a zirconia adlayer on (S)-N-dicarbazolyl-lysine (polyDCL)-coated silica nanoparticles. The functional polyDCL contains the necessary COOH groups for coordinating Zr(OR)x groups. Following sol–gel processing, the resulting spherical hybrid inorganic–organic–inorganic nanosized particles of a 200 nm average diameter are built on two inorganic components, an inorganic SiO2 core and an outer mixed hybrid zirconia/polyDCL adlayer.
Experimental
1. Measurements
The apparatus was an ALV/CGS-3 compact goniometer system, equipped with ALV/LSE-5003 light scattering electronics and multiple τ digital correlator, and a 632.8 nm JDSU laser 1145P.
2. Synthesis
![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000 rpm, 10 min at 4 °C). The solid precipitate was washed five times using a sequential redispersion–centrifugation cycle using a 1 : 1 v/v CH3COCH3–H2O washing mixture (10 mL). Resulting SiO2/polyDCL-based composite nanoparticles were dried in high vacuum (10−4 mm Hg, 8 h) before full characterization using a combination of analytical (elemental analysis, TGA/DSC, spectroscopic (FT-IR)) and microscopic (SEM and TEM) methods.
000 rpm, 10 min at 4 °C). The solid precipitate was washed five times using a sequential redispersion–centrifugation cycle using a 1 : 1 v/v CH3COCH3–H2O washing mixture (10 mL). Resulting SiO2/polyDCL-based composite nanoparticles were dried in high vacuum (10−4 mm Hg, 8 h) before full characterization using a combination of analytical (elemental analysis, TGA/DSC, spectroscopic (FT-IR)) and microscopic (SEM and TEM) methods.
Hybrid SiO2/polyDCL-based nanoparticles contained 87.5 wt% of a polymeric polycarboxylated polyDCL shell for an average 100 nm diameter.
Results and discussion
The starting silica/polyDCL nanosized composites are new composites based on both silica and the conducting polymer polyDCL (Scheme 1). They were prepared using an ultrasound-assisted chemical oxidation of the monocarboxylated (S)-N-dicarbazolyl-lysine monomer (DCL) in the presence of 50 nm-sized SiO2nanoparticles (1 : 1 v/v acetone–H2O, (NH4)2S2O8 oxidant, 1 h, 55–60 °C).22 The resulting hybrid SiO2/polyDCL-based nanoparticles were composed of 87.5 wt% of an insoluble polycarboxylated polyDCL shell with an average 80–150 nm particle diameter, centered on 100 nm (Fig. 1). All particle size and size distribution curves were obtained by averaging ten dynamic light scattering (DLS) measurements. | ||
| Fig. 1 DLS graph of the size and size distribution of the starting SiO2/polyDCL nanoparticles (dispersion in ethanol). | ||
 | ||
| Scheme 1 Synthesis strategy for the preparation of silica/polyDCL/zirconia nanoparticles. | ||
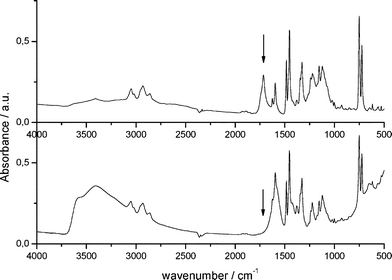
The FT-IR spectrum of the starting SiO2/polyDCL nanoparticles (Fig. 2, top) shows peaks relating to the different bonds and functional groups that characterize polyDCL. The band around 3490 cm−1 corresponds to polymer O–H groups, while both 3050 and 2940 cm−1 vibration bands relate to N–H and C–H groups respectively. C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O vibrations are located at 1720 cm−1 (indicated with an arrow on Fig. 2, top), while the peak at 1600 cm−1 indicates the presence of C
O vibrations are located at 1720 cm−1 (indicated with an arrow on Fig. 2, top), while the peak at 1600 cm−1 indicates the presence of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds. Vibrations corresponding to the silica matrix can be found at 1460 and 1330 cm−1. The presence of the strong band at 1720 cm−1 proves the presence of the carboxylic acid functionality within the polyDCL shell, which is strongly adsorbed onto the SiO2 particle surface.
C double bonds. Vibrations corresponding to the silica matrix can be found at 1460 and 1330 cm−1. The presence of the strong band at 1720 cm−1 proves the presence of the carboxylic acid functionality within the polyDCL shell, which is strongly adsorbed onto the SiO2 particle surface.
 | ||
| Fig. 2 FT-IR spectra of SiO2/polyDCL (top), and SiO2/polyDCL/ZrO2 (bottom) nanoparticles. | ||
Reaction of metal alkoxides with carboxylic acids is a well-known method to obtain organically modified precursors for sol–gel processing.23 Although the initial reaction of Zr(OR)4 with carboxylic acids often results in the formation of carboxylate-capped oxo/hydroxo clusters,24 sol–gel processing provides carboxylate-substituted zirconia entities. In the work presented in this article, the COOH groups of the polyDCL shell of the starting composite nanoparticles were exploited to further coordinate the zirconium center (Scheme 2) prior to sol–gel processing. The synthesis procedure is shown in Scheme 1.
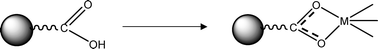 | ||
| Scheme 2 Coordination of a carboxylic acid group onto a metal center M. | ||
PolyDCL-capped silica nanoparticles, dispersed in freshly distilled ethanol (1.1 mg of nanoparticles per mL of ethanol for a total volume of 40 mL), were added to an 80 wt% solution of Zr(OtBu)4 in nBuOH (1.7 g solution of zirconium alkoxide per g of dispersed nanoparticles, i.e. 1.4 g of zirconium alkoxide per g of dispersed nanoparticles) in an inert argon atmosphere. Four equivalents of water and 0.01 equivalent of acetic acid per mol of Zr(OtBu)4 were added to this mixture under stirring.
Reaction of 100 nm average diameter-sized polyDCL-capped silica nanoparticles with the zirconium alkoxide, followed by hydrolysis, led to spherical nanoparticles of increased diameters. As a matter of fact, particles with diameters ranging from 100 to 400 nm, centered at 200 nm, were observed by DLS (dispersion in ethanol, Fig. 3). The observed increase of the particle diameter by a factor of 1.5 to 2 indicates that the reaction of polyDCL-capped silica particles with the zirconium alkoxide led to a new composite material consisting of ZrO2, formed by sol–gel processing and attached to the polyDCL-capped silica particles.
 | ||
| Fig. 3 DLS graph of the size and size distribution of the new silica/polyDCL/zirconia particles (dispersion in ethanol). | ||
The time-dependent colloidal stability was evaluated by DLS after three months. The modified nanoparticles were stored at room temperature in the 40 mL ethanol solution used for the synthesis. Size and size distribution features were unchanged (Fig. 4).
 | ||
| Fig. 4 DLS graph of the size and size distribution of the silica/polyDCL/zirconia nanoparticles after 3 months. | ||
The efficiency of the metal alkoxide coordination by the polyDCL carboxylic acid groups (Scheme 2) can be highlighted by comparison of the FT-IR spectra of samples before and after modification by sol–gel processing. The modified three-component nanoparticles, SiO2/polyDCL/ZrO2, show merged bands corresponding to both silica and zirconia at 1460 and 1330 cm−1. In addition, newly formed Zr–OH bonds arising from sol–gel processing cause a broadening of the 3490 cm−1 band. Except the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O band at 1720 cm−1, all the other bands characteristics of the polyDCL are still observable (O–H groups around 3490 cm−1, 3050 and 2940 cm−1 vibration bands relating to N–H and C–H groups respectively, the peak at 1600 cm−1 indicating the presence of C
O band at 1720 cm−1, all the other bands characteristics of the polyDCL are still observable (O–H groups around 3490 cm−1, 3050 and 2940 cm−1 vibration bands relating to N–H and C–H groups respectively, the peak at 1600 cm−1 indicating the presence of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds). The disappearance of the strong peak at 1720 cm−1 (located by an arrow in Fig. 2, top) indicates the efficient coordination of polyDCL carboxylic acid groups onto the Zr metal center (Fig. 2, bottom).
C double bonds). The disappearance of the strong peak at 1720 cm−1 (located by an arrow in Fig. 2, top) indicates the efficient coordination of polyDCL carboxylic acid groups onto the Zr metal center (Fig. 2, bottom).
The size, and size distribution of the resulting three-compound nanoparticles indicated by DLS measurements was confirmed by TEM (Fig. 5). No condensation between particles was observed, i.e. hydrolytic polycondensation of polycarboxylate-substituted Zr(OtBu)4 occurs only within the polymeric shell of the silica/polyDCL particles. The presence of both SiO2 and ZrO2 components in the new hybrid particles was also proven by EDX analysis (Fig. 5 left, at three positions denoted 1–3). The illustrative EDX spectrum of position 3 is disclosed in Fig. 5 (right). From these observations, it clearly appears that each nanoparticle is composed of both silica and zirconia structures.
 | ||
| Fig. 5 (left) TEM photomicrograph of the silica/polyDCL/zirconia nanoparticles; (right) EDX spectrum of position 3 in the same TEM photomicrograph. | ||
Moreover, an EDX line scan profile of both Si and Zr elemental distributions across one of the particles (Fig. 6) showed that Si and Zr are present at a relatively constant level. Accordingly, the formation of an outer zirconia shell around the polyDCL-covered silica particles (Scheme 1, top) may be doubtful. Zirconia particulates embedded in the polymeric shell, i.e. resulting in a mixed organic–inorganic outer shell, have to be also considered (Scheme 1, bottom). Nevertheless, the EDX measurement is made on the indicated line but analyzes the entire thickness of the deposited product, thus the constant level of silicon and zirconium elements detected could be a consequence of particles overlapping. The obtained zirconia structures are amorphous, as usually observed for sol–gel materials, and thus show no diffraction pattern.
 | ||
| Fig. 6 (left) TEM photomicrograph of the silica/polyDCL/zirconia nanoparticles; (right) elemental EDX line scan analysis profile of line 1. | ||
When an ethanol dispersion of the obtained silica/polyDCL/zirconia nanoparticles was coated on a glass plate and dried at room temperature under air, a multi-layered film of agglomerated nanoparticles was obtained. SEM analysis (Fig. 7) shows a narrow average size and size distribution of the nanoparticles, as also observed by DLS. Nanoparticle diameters from the TEM range from 130 to 300 nm, centered at 200 nm.
 | ||
| Fig. 7 SEM of three-component silica/polyDCL/zirconia nanoparticles. | ||
The silica/polyDCL/zirconia nanoparticles were treated with a 32 wt% aqueous solution of hydrofluoric acid in order to dissolve the silica core. After 3 h treatment at room temperature, the silica core was completely dissolved and small zirconia particles were observed (Fig. 8).
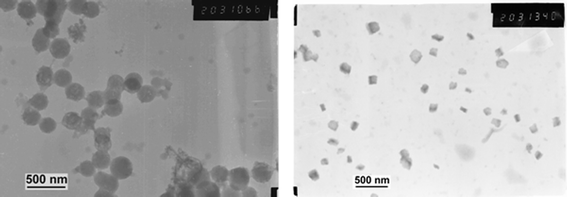 | ||
| Fig. 8 TEM photomicrograph before (left) and after (right) 3 h of HF treatment of silica/polyDCL/zirconia nanoparticles. | ||
They appeared almost cubic although the zirconia phase was amorphous (XRD). The size distribution of the observed 150 nm-sized ZrO2nanoparticles is rather broad (120–250 nm). Their appearance did not allow any definite conclusion to be drawn about whether they constituted part of a broken zirconia shell or isolated nanoparticles originally embedded in the polymeric polyDCL shell.
In conclusion, hybrid organic–inorganic SiO2/polyDCL/ZrO2nanoparticles possessing a complex dual inorganic composition were prepared. They were composed of a silica core and an organic polyDCL layer with pendant carboxylic acid groups. The latter enabled further reaction with a metal alkoxide precursor that resulted in zirconia nanoparticulates after sol–gel processing. The feasibility of this procedure was demonstrated for one zirconium alkoxide precursor, Zr(OtBu)4, but might be readily applicable for equivalent nanosized systems based on different inorganic cores and metal alkoxides.
There are two structural possibilities of how zirconia may attach to polyDCL-coated silica nanoparticles. The first is a zirconia shell covering the silica/polyDCL-capped particles (Scheme 1, top). The second possibility will lead to the formation of a mixed organic (polyDCL)–inorganic (ZrO2) shell (Scheme 1, bottom) deposited around the silica core. The first possibility would require that a significant part of the COOH groups of the polyDCL polymer would be located on the outer surface of the starting hybrid silica/polyDCL nanoparticles. Given the employed ratios used between silica nanoparticles and the zirconium alkoxide precursor, the zirconia shell built around the silica/polyDCL-capped nanoparticles should be very thin. However, the increase of the particle diameter after sol–gel processing is more indicative of the alternative hybrid structure, which is also supported by elemental EDX analysis and dissolution studies. The formation of such a hybrid polyDCL/zirconia hybrid shell around the silica core is also more likely from a chemical point of view, since the COOH groups of the polyDCL shell are not only present onto the particle surface, but also within the organic shell.
Acknowledgements
This work was supported by the European Commission in the frame of the 6th Framework Integrated Project NAPOLYDE (Contract No. NMP2-CT-2005-515846). The authors thank Emmanuel Scolan, CSEM Switzerland, for SEM investigations.References
- M. Green, Small, 2005, 1, 684 CrossRef CAS.
- D. Lee, D. Omolade, R. E. Cohen and M. F. Rubner, Chem. Mater., 2007, 19, 1427 CrossRef CAS.
- A. Perro, S. Reculusa, S. Ravaine, E. Bourgeat-Lami and E. Duguet, J. Mater. Chem., 2005, 15, 3745 RSC.
- S. Reculusa, C. Poncet-Legrand, S. Ravaine, C. Mingotaud, E. Duguet and E. Bourgeat-Lami, Chem. Mater., 2002, 14, 2354 CrossRef CAS.
- S. Sadasivan and G. B. Sukhorukov, J. Colloid Interface Sci., 2006, 304, 437 CrossRef CAS.
- M. Sastry, A. Swami, S. Mandal and P. R. Selvakannan, J. Mater. Chem., 2005, 15, 3161 RSC.
- A. van Blaaderen, Surfactant Sci. Ser., 2006, 131, 233 Search PubMed.
- Y.-Y. Yang, Y. Wang, R. Powell and P. Chan, Clin. Exp. Pharmacol. Physiol., 2006, 33, 557 CrossRef CAS.
- Functional Coatings by Polymer Microencapsulation, ed. S. K. Ghosh, Wiley-VCH Verlag GmbH & Co., Weinheim, 2006 Search PubMed.
- D. Shukla and A. Mehra, Langmuir, 2006, 22, 9500 CrossRef CAS.
- Z. Lei and S. Bi, Mater. Lett., 2007, 61, 3531 CrossRef CAS.
- D. Holzinger and G. Kickelbick, Chem. Mater., 2003, 15, 4944 CrossRef CAS.
- D. Holzinger, L. M. Liz-Marzan and G. Kickelbick, J. Nanosci. Nanotechnol., 2006, 6, 445 CAS.
- F. N. Crespilho, M. E. Ghica, V. Zucolotto, F. C. Nart, O. N. Oliveira, Jr. and C. M. A. Brett, Electroanalysis, 2007, 19, 805 CrossRef CAS.
- F. N. Crespilho, F. C. Nart, O. N. Oliveira and C. M. A. Brett, Electrochim. Acta, 2007, 52, 4649 CrossRef CAS.
- Y. Haba, C. Kojima, A. Harada, T. Ura, H. Horinaka and K. Kono, Langmuir, 2007, 23, 5243 CrossRef CAS.
- Y. Zhu, H. Zhu, X. Yang, L. Xu and C. Li, Electroanalysis, 2007, 19, 698 CrossRef CAS.
- A. Di Gianni, S. Trabelsi, G. Rizza, M. Sangermano, H. Althues, S. Kaskel and B. Voit, Macromol. Chem. Phys., 2007, 208, 76 CrossRef.
- J. Zhou, M. Chen, X. Qiao and L. Wu, Langmuir, 2006, 22, 10175 CrossRef CAS.
- X. Xia, Y. Liu, V. Backman and G. A. Ameer, Nanotechnology, 2006, 17, 5435 CrossRef CAS.
- H. Fan, Z. Chen, C. J. Brinker, J. Clawson and T. Alam, J. Am. Chem. Soc., 2005, 127, 13746 CrossRef CAS.
- E. Kuperschmidt, Preparation and Characterization of Nanocomposites Based on SiO2 and SiC Nanoparticles and Functional Polypyrrole/polycarbazole Polymers, MSc Thesis, Bar-Ilan University, Ramat-Gan, Israel, 2007 Search PubMed.
- U. Schubert, J. Sol–Gel Sci. Technol., 2003, 26, 47 CrossRef CAS.
- U. Schubert, Chem. Mater., 2001, 13, 3487 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2008 |
