Novel approach for determination of trace metals bound to suspended solids in surface water samples by inductively coupled plasma sector field mass spectrometry (ICP-SFMS)†
Maximilian
Popp
,
Gunda
Koellensperger
,
Gerhard
Stingeder
and
Stephan
Hann
*
University of Natural Resources and Applied Life Sciences—BOKU Wien, Muthgasse 18, A-1190 Vienna, Austria. E-mail: Stephan.Hann@boku.ac.at; Tel: ++43/1/36006-6086
First published on 28th August 2007
Abstract
An approach for rapid determination of ultra-trace concentrations of As, Cd, Cr, Cu, Ni, Pb and Zn in suspended solids present in surface water was developed and validated for three Austrian rivers (Danube, Leitha, Schwechat). Elemental analysis was performed by ICP-SFMS via slurry-type nebulization of non-centrifuged (total elemental concentration) and centrifuged (dissolved elemental concentration) surface water samples. The elemental concentrations in suspended solids (css) were determined, relating the difference of the total elemental concentration and the dissolved elemental concentration to the concentration of suspended solids (gravimetric determination). The quality of the obtained data was evaluated calculating the expanded uncertainty of measurement according to Eurochem/CITAC. The applied analytical procedure yielded values for css associated with low uncertainties for elements with high particle bound fractions (Zn, Pb) and suspended particle concentrations >5 mg L–1. The novel method provided partition patterns and related partition coefficients (K) which were in agreement with available literature data. The investigated elements could be classified into three groups: (i) elements showing both considerable particle bound fractions (Ni 0%–50%, Cr 22%–33%, Cu 37%–100%) and large fractions of dissolved metals; (ii) elements with a pronounced tendency towards particle binding, such as Pb and Zn, showing particle bound fractions of 56%–100% and 71%–83%; and (iii) elements being predominantly present in the liquid phase (As, Se). The concentrations of Ag and Cd were consistently below the limits of detection for all investigated rivers.
Introduction
Water protection is a major objective of environmental protection policy. So far, within the European Union (EU), environmental quality standards (EQS) and required analytical quality standards have been regulated by member states individually. With the introduction of the water framework directive 2000/60/EC (WFD) the EU aimed at harmonized, uniform and high quality goals for surface water bodies with respect to organic and inorganic micropollutants.1 A list of priority substances which comprises mainly persistent organic pollutants and several inorganic pollutants was issued.2 During the current implementation process of the WFD, monitoring of alternative entities (suspended solids, sediment and biota) has been discussed due to their importance with respect to transport budgets, distribution pathways in the environment and assessment of the toxicological impact of water pollution.In recent years there has been great interest in trace metal(loid)s of ecotoxicological relevance, namely As, Cd, Cr, Cu, Hg, Ni and Pb, and the role that suspended matter plays in their distribution and partition in the aquatic environment.3–9 Several programs for monitoring river-born suspended solids and sediments were conducted in Germany and Austria, especially focusing on the evaluation of established measuring programs and the need for monitoring alternative components.10,11
For the analysis of trace metals in suspended solids it is common practice to collect and isolate sufficient amounts of suspended solids. Following freeze-drying, suspended matter is prepared for elemental analysisvia acidic digestion.12 Elemental concentrations are usually determined by atomic spectrometry using either inductively coupled plasma mass spectrometry (ICP-MS), inductively coupled plasma atomic emission spectrometry (ICP-AES) or atomic absorption spectroscopy (AAS).10 Collection of suspended solids is commonly performed with sedimentation traps, which require long sampling periods and are therefore prone to significant sample changes due to ageing. Alternative approaches consist in the filtration of large volumes of surface water by continuous flow centrifuges or in-situfiltration.10,13–15 A common drawback is that the implemented procedures are tedious, time consuming and, more importantly, prone to contamination.16
Alternatively, some elaborated schemes for investigation of trace metal partition using size fractionation techniques to distinguish between particulate, colloidal and “truly dissolved” metal have been tested.17–19 So far, no scheme has been suggested for the determination of trace metal concentrations in suspended matter through measurement of native surface water.
Nevertheless, a recent Austrian surface water monitoring program confirmed the necessity of measuring particle bound metal fractions for the assessment of ecotoxicological relevance.20
In the presented study a novel, fast and contamination free sample preparation scheme for measurement of As, Cd, Cr, Cu, Ni, Pb and Zn at ultra-trace levels by ICP-SFMS is presented. The scheme utilizes a combination of centrifugation and slurry-type nebulization for quantification of the particle bound metal fraction of the investigated compounds.
Experimental
Materials and methods
Prior to use, 65% nitric acid of p.a. grade, purchased from Merck, was double-distilled using a duoPUR quartz sub-boiling-unit produced by MLS Lab Systems GmbH (Leutkirch, Germany). Ultra-pure water was used for the preparation of the standards: following reverse-osmosis and ion exchange with a HQ-5 system supplied by REWA GmbH (Gladenbach, Germany) the water was finally processed with a duoPUR quartz sub-boiling-unit. Water and nitric acid were stored in PFA bottles under clean room conditions until use.
![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000 and class 10
000 and class 10![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000, respectively) with temperature control (20 °C) and overpressure (+5 Pa). Dilution of samples and addition of internal standard (In) were carried out on clean benches (class 100) situated in the clean room class 10
000, respectively) with temperature control (20 °C) and overpressure (+5 Pa). Dilution of samples and addition of internal standard (In) were carried out on clean benches (class 100) situated in the clean room class 10![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000.
000.
109Ag, 111Cd, 202Hg, 208Pb at low resolution (LR), 52Cr, 60Ni, 65Cu, 66Zn at medium resolution (MR) and 75As, 77Se at high resolution were the selected isotopes for interference-free ICP-SFMS measurement. During all measurements 115In was used as internal standard at all resolutions. Nominal mass resolutions of the Element 2 ICP-SFMS for low resolution (LR), medium resolution (MR) and high resolution (HR) are 350, 4500 and 10![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000, respectively.
000, respectively.
The collection of surface water samples was performed with a metal-free, custom-fitted device for contamination-free sampling. This device consisted of a polypropylene plate attached to a 1.5 m PP-rod. Round holes were drilled into the plate to contain the necks of 500 mL PE bottles, which were fastened by cut-open PE-bottle screw caps. To assure contamination-free sampling pre-cleaned PE wide necked bottles and caps were used. During sampling and sample transport all bottles and devices were handled wearing PE-gloves. Bottles were transported and stored in sealed plastic bags. The actual sampling sites—except for the urban site in Vienna—were situated off-road to avoid traffic related contaminations.
Sample preparation
The concentration of dissolved metal(loid)s (cdis) was determined after centrifugation of the respective surface water samples. Centrifugation of 10 mL sample aliquots was performed with a Sigma laboratory centrifuge 2–5 (Sigma Laborzentrifugen GmbH, Osterode am Harz, Germany) in acid-cleaned polypropylene tubes (10 mL) at 3900 rpm for 30 min. The centrifugates were transferred into pre-cleaned PP-tubes with an Eppendorf pipette and stabilized by addition of ultra-pure double-distilled nitric acid (pH < 1.5). Aliquots of the original non-centrifuged surface water samples representing the concentration of total metal(loid) were transferred to pre-cleaned PP-tubes and stabilized by volumetric addition of 10 µL ultra-pure nitric acid per mL of sample (final pH < 1.5).As the reference method for the determination of the total concentration of metal(loid)via slurry-type nebulization, surface water sample aliquots were submitted to microwave digestion with H2O2–HNO3 in parallel. Sample aliquots of 2 mL plus 500 µL of ultra-pure nitric acid and 200 µL of 30% H2O2 solution (ultra-pure, Merck) were filled into acid-steam-cleaned PFA-microwave digestion vessels and closed with pre-cleaned PTFE-caps. The digestion was performed by applying a microwave program employing maximum microwave power of 450 W. After cooling, the digested samples were transferred to PP-vials and filled up to 10 mL with ultra-pure water. Microwave digestion blanks were prepared using 2 mL of ultra-pure water.
For quantification multi-element standards were prepared from Merck ICP-single element standard in PFA bottles and vials through dilution in ultra-pure water and addition of 1% nitric acid. The acid content was matched with the content in the respective samples. Prior to ICP-SFMS measurement, In was added to all samples and standards as internal standard at a final concentration of 1 µg L–1. TM 27.2 and SLRS 4 were used for calibration quality control. The certified values and the obtained values agreed within measurement uncertainty for both tested CRMs.
Gravimetric determination of xss was performed according to established methods.21 Sample volumes of 1 L were filtered through glass fibre filters (GF A, Whatman, Brentford, Middlesex, UK) using a polycarbonate filter unit manufactured by Sartorius AG (Göttingen, Germany). The loaded filters were dried at 105 °C in a drying cupboard, applying a fixed drying time of 120 min, and subsequently placed in an evacuated exsiccator for equilibration to room temperature. xss was obtained via differential weighing using an MC-5 micro-balance by Sartorius AG (Göttingen, Germany).
Results and discussion
In this study a novel approach for the determination of elemental concentrations in suspended solids via ICP-SFMS was validated and applied. We designed a simple and straightforward sample preparation protocol for assessment of dissolved and total concentration of metals and metalloids, respectively. The proposed methodology exploits the unique possibility of slurry-type nebulization, thereby permitting the determination of total metal(loid) content without digestion. Table 1 details the implemented approach.The concentration of the dissolved metal(loid) (cdis) was determined from centrifuged and subsequently acidified river water, while the concentration of total metal(loid) (ctot) was measured by introduction of untreated acidified river watervia slurry-type nebulization (V-groove nebulizer). Consequently, the particle bound fraction of metal(loid) was calculated as the difference of these two experimental parameters with respect to the concentration of suspended solid (xss), which was determined gravimetrically.
Method validation
| Surface water sample | ||
|---|---|---|
| Sample aliquot 1 | Sample aliquot 2 | Sample aliquot 3 |
| Centrifugation (3900 rpm for 30 min) | Acidification of surface water aliquot (pH < 1.5) with ultra-pure nitric acid | Determination of concentration of suspended solids according to DIN 38409-H2 |
| Acidification of centrifugate (pH < 1.5) with ultra-pure nitric acid | Addition of internal standard | |
| Addition of internal standard | ICP-SFMS with slurry-type nebulization | |
| ICP-SFMS with slurry-type nebulization | ||
| Concentration of dissolved metal(loid) (cdis) | Concentration of total metal(loid) (ctot) | Concentration of suspended solids (xss) |
| Calculation of metal concentration in suspended solids css (in µg g–1) using ctot, cdis (both in µg L–1) and xss (in g L–1) in a differential approach: css = (ctot – cdis)/xss | ||
| Element | LODA/µg L–1 | LOQA/µg L–1 | LODB/µg L–1 | LOQB/µg L–1 | AA-EQS/µg L–1 |
|---|---|---|---|---|---|
| a For class 1: 40 – < 100 mg L–1 CaCO3. b The values represent AA-EQS deduced using an added risk approach based on maximum permissible addition (MPA) and assuming a negligible natural background concentration in Austria. | |||||
| Ag | 0.002 | 0.007 | 0.0004 | 0.001 | n.a. |
| As | 0.005 | 0.0167 | 0.005 | 0.017 | n.a. |
| Cd | 0.05 | 0.167 | 0.0003 | 0.001 | ≤0.08a |
| Cr | 0.011 | 0.037 | 0.004 | 0.015 | n.a. |
| Cu | 0.023 | 0.075 | 0.004 | 0.013 | n.a. |
| Hg | 0.017 | 0.057 | 0.014 | 0.048 | 0.05 |
| Ni | 0.039 | 0.13 | 0.004 | 0.014 | 1.7b |
| Pb | 0.003 | 0.010 | 0.002 | 0.007 | 2.1b |
| Se | 0.040 | 0.117 | 0.053 | 0.177 | n.a. |
| Zn | 0.1 | 0.133 | 0.021 | 0.069 | n.a. |
Corresponding experiments addressing filtration by acid cleaned Teflon filters for separation of suspended particles showed a significant increase of blank levels (up to 5–8 times for all investigated elements except Ag). Consequently, centrifugation of samples instead of filtration is the sample preparation method of choice for trace and ultra-trace analysis of dissolved metals and metalloids.
Summarizing for all elements the method LODs obtained with both experimental set-ups, i.e., self-aspirating PFA after centrifugation or slurry nebulization, were below the current EQS and hence suitable for the concentrations to be monitored in Austrian rivers.
Fig. 1 summarizes the results of this stability study. It reveals that most of the investigated elements show clear evidence of sample ageing as the dissolved fraction significantly decreased with increasing storage time. Based on these findings a maximum time of 24 h between sampling and centrifugation is proposed for accurate determination of cdis.
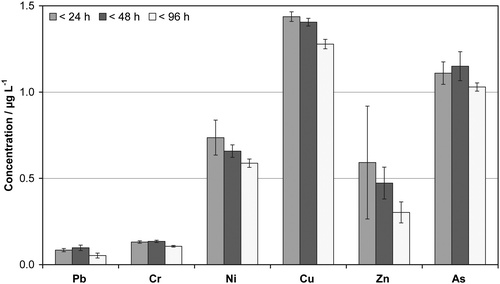 | ||
| Fig. 1 Stability of metal(loid)s in native surface water (Danube) during storage at 5 °C prior to sample preparation. Data were obtained with ICP-SFMS using ultra-pure set-up B after separation of suspended solids viacentrifugation and subsequent acidification (1% HNO3). | ||
Moreover, storage of processed samples (i.e., centrifuged and acidified samples representing the dissolved metal(loid) fraction) was investigated with respect to wall adsorption. The samples were stored at 5 °C for 2 weeks in PP tubes showing no significant loss except for Hg. Mercury loss would be expected from plastics, which is why samples for mercury determination are stored in glass and generally preserved on-site, for instance by addition of chromate to the sample which would hamper the analysis of Cr. Hg was therefore excluded from our study and will be investigated separately in future monitorings (see conclusions).
As a first step, sedimentation of suspended solids in the sample vials was considered as a potential source of error in the analysis of the total metal(loid) concentration by slurry nebulization. An experiment was designed comparing river water sample aliquots, which were either stirred during ICP-SFMS analysis using set-up A or measured without stirring. Lead was selected as the reference element in this experiment, because it was found to be predominantly adsorbed to suspended matter. When the samples were continuously mixed during the ICP-SFMS measurements using slurry-type nebulization a concentration of 0.567 ± 0.025 µg L–1 (n = 6) was obtained. Without stirring the measured concentration was 0.544 ± 0.123 µg L–1 (n = 6). Accordingly, the influence of sedimentation was found to be negligible for the investigated river water samples.
For validation of the accuracy of the slurry nebulization procedure, acidified river water samples were measured directly by slurry-type nebulization as well as after microwave assisted digestion. A scheme of the sample preparation for the respective experiments is given in Table 3. Measurement of slurry-type nebulized and acid digested samples did not reveal significant differences in metall(loid) concentrations. The results of the method comparison are given in Table 4. Only in the case of Ni were the values obtained by microwave digestion significantly lower than those obtained by slurry nebulization, which is not understood yet and needs further investigation. The high standard uncertainty of Zn can be explained by a possible contamination during microwave digestion. For Ag and Cd the concentrations in the river water samples ranged below the respective detection limits for both investigated types of sample preparation. However, our findings confirm the suitability of slurry-type nebulization for the determination of the total metal content in river water containing suspended solids.
| Surface water | ||
|---|---|---|
| Aliquot A | Aliquot B | Aliquot C |
| Processing of aliquots (10 mL): | Microwave digestion in PFA-inlets: | |
| –Acidification with ultra-pure HNO3 (1% or pH < 1.5) | –2 mL sample | |
| –Storage at room temperature (20 °C) until measurement with ICP-SFMS | –0.5 mL HNO3 | |
| –0.25 mL H2O2 | ||
| Stirred during measurement | Measured without stirring | Filled up to 10 mL and measured |
| Unfiltered sample (stirred) | Unfiltered sample (no stirring) | Microwave digestion |
| Element | Non-centrifuged sample with set-up A/µg L–1 | Microwave digestion with set-up B/µg L–1 |
|---|---|---|
| Ag | <LODA | <LODB |
| As | 1.23 ± 0.13 | 1.10 ± 0.10 |
| Cd | <LODA | <LODB |
| Cr | 0.712 ± 0.059 | 0.807 ± 0.141 |
| Cu | 2.19 ± 0.23 | 2.05 ± 0.02 |
| Hg | 0.022 ± 0.001 | 0.025 ± 0.035 |
| Ni | 1.19 ± 0.16 | 0.614 ± 0.153 |
| Pb | 0.612 ± 0.063 | 0.569 ± 0.067 |
| Se | 0.145 ± 0.001 | 0.100 ± 0.102 |
| Zn | 2.76 ± 0.34 | 3.35 ± 1.35 |
Determination of metal(loid)s in suspended solids of the rivers Danube, Schwechat and Leitha
The distribution of the elements Ag, As, Cd, Cr, Cu, Ni, Pb, Se and Zn between suspended solids and the liquid phase was determined in the rivers Danube, Schwechat and Leitha. The measurements were performed according to the validated analysis scheme as shown in Table 1. Total elemental concentrations (ctot), concentrations of the dissolved elemental fraction (cdis) and the concentration of suspended solids (xss) were determined.For determination of cdis a non-acidified sample aliquot was centrifuged and the centrifugate was acidified prior to ICP-SFMS measurement. ctot was determined via ICP-SFMS using slurry-type nebulization of an unfiltered, acidified sample aliquot. The values of ctot and cdis obtained for the rivers Schwechat, Danube and Leitha are summarized in Fig. 2–4, respectively.
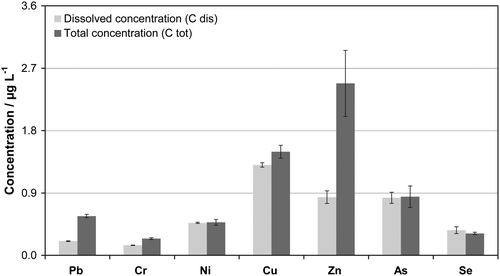 | ||
| Fig. 2 Investigation of total concentrations (ctot) and concentration of dissolved metal(loid)s (cdis) in the river Schwechat (concentration of suspended solids (xss) = 4.3 mg L–1) according to the proposed scheme using slurry-type nebulization. | ||
 | ||
| Fig. 3 Investigation of total concentrations (ctot) and concentration of dissolved metal(loid)s (cdis) in a side arm of the river Danube in the urban area of Vienna (xss = 14.9 mg L–1) according to the proposed scheme using slurry-type nebulization. | ||
 | ||
| Fig. 4 Investigation of total concentrations (ctot) and concentrations of dissolved metal(loid)s (cdis) in the river Leitha (xss = 9.9 mg L–1) according to the proposed scheme using slurry-type nebulization. | ||
The results were first evaluated with respect to elemental partitioning between the solid and the liquid phase. The investigated elements could be classified into three groups: Group 1 consisted of elements mainly bound to suspended solids (Pb, Zn), the second group (Cr, Cu, Ni) exhibited considerable dissolved and particle bound fractions, while the third group (As, Se) was predominantly present in the liquid phase. Ag and Cd were consistently below the LODs for all three rivers.
The detailed results of elemental fractions bound to suspended solids expressed as percentage of total elemental concentration are given in Table 5.
| As | Cr | Cu | Ni | Pb | Zn | |
|---|---|---|---|---|---|---|
| River Danube | ||||||
| F ss (%) | 31 | 78 | 40 | 49 | >97 | 83 |
| c ss/µg g–1 | 25 | 37 | 59 | 41 | 41 | 154 |
| U (k = 2) | 79% | 22% | 52% | 53% | 20% | 34% |
| c sed/µg g–1 | n.a. | 50 | 42 | 34 | 53 | 104 |
| River Schwechat | ||||||
| F ss (%) | <10 | 40 | 13 | n.a. | 64 | 66 |
| c ss/µg g–1 | n.a. | 22 | 44 | n.a. | 85 | 384 |
| U (k = 2) | n.a. | 31% | 105% | n.a. | 15% | 59% |
| c sed/µg g–1 | <5.0 | 53 | 64 | 28 | 53 | 200 |
| River Leitha | ||||||
| F ss (%) | <10 | 34 | 48 | 14 | 56 | 71 |
| c ss/µg g–1 | n.a. | 17 | 62 | 21 | 55 | 224 |
| U (k = 2) | n.a. | 23% | 37% | 68% | 25% | 25% |
| c sed/µg g–1 | 13.2 | 66.2 | 52.8 | 33.5 | 49.2 | 187 |
Our distribution patterns are in accordance with those found in another study on suspended matter in the river Elbe, carried out by Prange and co-workers.11 In this work dissolved fractions were determined from filtered surface water, while fractions bound to suspended matter were determined in microwave digested aliquots of collected suspended matter. Elemental analysis was carried out using ICP-AES and ICP-quadrupole-MS, respectively.
Ag, Cd, Hg, Pb and Zn were found to be associated with suspended solids, while for other elements (Cu, Cr, Ni) large dissolved fractions were reported. These findings correlate with the observations made in our study: For Pb and Zn large particle-bound fractions of 56%–100% and 71%–83%, respectively, were found in all three rivers. For Ni (0%–50%), Cr (22%–33%) and Cu (37%–100%) a wide range of values for bound metal fractions was obtained for the rivers included in this survey. Prange et al. found As predominantly in the dissolved fractions (>70%), which is supported by dissolved fractions >69% during this study in all investigated rivers. Selenium, which in our study was almost exclusively found in dissolved form in all three rivers, was found in very low concentrations in the Elbe in suspended solids and could not be detected in the liquid phase.
The concentration of metal(loid)s in the suspended solids css was calculated according to eqn (1) in Table 1. In Table 5 the values are given with the total combined uncertainties (U) calculated according Eurochem/CITAC with a coverage factor of 2 by error propagation of all experimental parameters.22
As the differences between the dissolved As concentration and the total As concentration were not significant in the case of the rivers Schwechat and Leitha, no concentration of As in the suspended solids could be given. For the Danube river a concentration of 25 µg g–1 was found. However the expanded uncertainty was 79% with the low concentration of bound arsenic as a major contribution to the uncertainty. Owing to the low concentration of suspended solids in the river Schwechat (4.3 mg L–1) the concentration of Cu in suspended solids showed high uncertainty (105%). This finding is further supported by the fact that for Ni also, a metal with significant binding to particulate matter in the other studied rivers, no significant differences between dissolved and total concentrations were found in the samples of the river Schwechat. For Se no significant bound fractions were present in any of the studied rivers. Summarizing, in terms of analytical figures of merit, the applied analytical procedure yields css values associated with low uncertainties for elements with high particle bound fractions and suspended particle concentrations >5 mg L–1.
During a study (Joint Danube Survey) suspended solids were collected from the Danube at several sites along the stream. Average values for metalloids in suspended solids for As (18.2 µg g–1), Cr (62.3 µg g–1), Cu (52.7 µg g–1), Ni (47.3 µg g–1), Pb (34.6 µg g–1) and Zn (166 µg g–1) were reported.23 Considering the uncertainties attached, these values agree very well with our findings.
As data on the concentration in suspended matter are only available for the Danube, the results of the other two rivers are discussed with respect to available sediment data.24 At all sampling sites river sediment in the size fraction below 40 µm (csed) was monitored during the past 10–15 years on several occasions by the Austrian Federal Ministry of Agriculture, Forestry, Environment and Water Management (BMLFUW). The data from the last campaign at the respective sites were compared with the data obtained within this study. For rivers Schwechat and Leitha river sediment was investigated in 2005, while Data for Danube sediment at the investigated site were comparatively old (1996). As can be readily observed in Table 5 the concentration levels in the suspended solids were in the same range as the concentration levels in the sediment.
Finally the obtained data was used to calculate distribution factors (K) for the metals based on the obtained average values of the concentration of dissolved metalloid cdis and the obtained concentrations in suspended solids css using eqn (1).
| K = css/cdis | (1) |
A comparison of the data (Table 6) reveals that the results obtained in this study agree with values given in the literature, indicating that the developed procedure is well suited for determination of accurate K-values.
| Pb | Zn | Cr | Cu | Ni | As | |
|---|---|---|---|---|---|---|
| Danube | n.a. | 319![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000 000 |
233![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000 000 |
44![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 500 500 |
69![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 900 900 |
29![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 900 900 |
| Schwechat | 412![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000 000 |
457![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000 000 |
153![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000 000 |
33![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 900 900 |
n.a. | n.a. |
| Leitha | 128![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000 000 |
252![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000 000 |
51![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 700 700 |
91![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 600 600 |
16![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 900 900 |
n.a. |
| Literature values25–27 | 146![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000 000 |
110![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000 000 |
191![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000 000 |
30![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 246 246 |
8300 | 10![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000 000 |
Conclusion
The main advantages of the proposed method compared with routine methods used in the field are as follows: (1) Sampling consists of contamination-free collection of native surface water. (2) The samples are only subjected to simple operational steps (pipetting, centrifugation). (3) The use of filter media which are often mentioned as possible source of contamination is avoided in the preparation of samples for trace elemental analysis.The method exhibited limitations for elements with low total concentration and highly dissolved fractions (e.g., As, Se, Ni) with respect to quantification in suspended solids because measurement of significant differences is hampered in these cases. Evidently, the accuracy of quantification is compromised in the case of samples with low concentration of suspended solids (<5 mg L–1) as well.
Determination of elemental distribution between solid and liquid phases is essential for establishing environmental monitoring standards. In our investigations the studied set of metal(loids) exhibited distinct partition patterns. Indeed, Pb and Zn should, in future, not be monitored as dissolved metals only, due to their high particle bound fraction. Arsenic and selenium, on the other hand, were almost exclusively found in the liquid phase and hence can be quantified in the liquid fraction of surface water. Hg could not be successfully included into this procedure due to its high tendency to adsorb on all available surfaces (wall adsorption). As a matter of fact, Hg and its organometallic compounds are strongly recommended for biota-monitoring in several detailed studies.20
Acknowledgements
Financial support from the Austrian Federal Ministry of Agriculture, Forestry, Environment and Water Management (BMLFUW, Project Title: “Determination of trace metals in the water phase and suspended solids of surface water by ICP-SFMS”) is gratefully acknowledged. Dr Martin Wimmer from the division of surface water, BMLFUW, is gratefully acknowledged for many fruitful discussions.References
- Official Journal of the European Communities L 327/1 (22.12.2000): “Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 establishing a framework for Community action in the field of water policy”.
- Proposal for a Directive of the European Parliament and of the Council on environmental quality standards in the field of water policy and amending directive 2000/06/EC (presented by the Commission—Annex 1: Environmental Quality Standards for Priority Substances and Certain Other Pollutants (Part A)).
- R. Poikane, J. Carstensen, I. Dahllof and J. Aigars, Chemosphere, 2005, 60, 216–225 CrossRef CAS.
- R. L. Bibby and J. G. Webster-Brown, Sci. Total Environ., 2005, 343, 177–197 CrossRef CAS.
- H. Pekey, D. Karakas and M. Bakoglu, Mar. Pollut. Bull., 2004, 49, 809–818 CrossRef CAS.
- J. H. Ren and A. I. Packman, Environ. Sci. Technol., 2004, 38, 2901–2911 CrossRef CAS.
- D. Point, G. Bareille, D. Amouroux, H. Etcheber and O. F. X. Donard, J. Environ. Monit., 2007, 9, 157–167 RSC.
- E. H. Rybicka, E. Adamiec and U. Aleksander-Kwaterczak, Limnologica, 2005, 35, 185–198 Search PubMed.
- A. Turner and G. E. Millward, Estuarine, Coastal Shelf Sci., 2002, 55, 857–883 CrossRef CAS.
- Länderarbeitsgemeinschaft Wasser (LAWA), Fließgewässer der Bundes-republik Deutschland—Schwebstoffuntersuchungen—Bestandsaufnahme Stand 1996—Empfehlungen, Kulturbuch-Verlag, Schwerin, Germany, 1999.
- A. Prange, A. Aulinger, H. Böddeker, E. Bössow, B. Erbslöh, R. JablonskiE. Jantzen, P. Krause, P. Leonhardt, P. Niedergesäß, R. Pepelnik, A. Schäfer, M. Schirmacher and W. v. Tümpling, Jr, Erfassung und Beurteilung der Belastung der Elbe mit Schadstoffen—Teilprojekt 2: Schwermetalle—Schwermetallspezies, Final Report BMBF—Forschungsvorhaben 02-WT 9355/4, GKKS—Forschungszentrum Geesthacht GmbH, Geesthacht, Germany, 1997, Band 1/3 Search PubMed.
- M. Baborowski, E. Clausd, K. Friese, F. v. D. Kammer, P. Kasimir, J. Pelzer and P. Heininger, Acta Hydrochim. Hydrobiol., 2005, 33, 404–417 CrossRef CAS.
- F. Odman, T. Ruth and Ch. Ponter, Appl. Geochem., 1999, 14, 301–317 CrossRef CAS.
- F. Odman, T. Ruth, I. Rodushkin and C. Ponter, Appl. Geochem., 2006, 21, 2112–2134 CrossRef.
- M. Grotti, F. Soggia, S. Dalla Riva, E. Magi and R. Frache, Anal. Chim. Acta, 2003, 498, 165–173 CrossRef CAS.
- P. Rosse, D. Vignati and J. Dominik, Hydrol. Processes, 2006, 20, 2745–2754 CrossRef CAS.
- G. Blo, C. Contado, D. Grandi, F. Fagioli and F. Dondi, Anal. Chim. Acta, 2002, 470, 253–262 CrossRef CAS.
- C. Contado, G. Blo, C. Conato, F. Dondi and R. Beckett, J. Environ. Monit., 2003, 5, 845–851 RSC.
- L. Sigg, H. B. Xue, D. Kistler and R. Schonenberger, Aquat. Geochem., 2000, 6, 413–434 CrossRef CAS.
- H. Ruedel, A. Fliedner and M. Herrchen, Machbarkeitsstudie: Strategie für ein stoffangepasstes Wasser-Monitoring—Erfassung potentiell sorbierender oder akkumulierender Stoffe in anderen Kompartimenten (Biota, Sediment, Schwebstoffe), Fraunhofer Institut für Molekularbiologie und Angewandte Oekologie (Fraunhofer IME) Bereich Angewandte Oekologie, Schmallenberg, Germany, 2007 Search PubMed.
- DIN 38409-2, German standard methods for the examination of water, waste water and sludge; parameters characterizing effects and substances (group H); determination of filterable matter and the residue on ignition (H 2), Beuth Verlag, Berlin, Germany, 1987.
- EURACHEM/CITAC Guide CG 4, Quantifying Uncertainty in Analytical Measurement, EURACHEM/CITAC, London, 2nd edn, 2000, ISBN 0 948926 15 5 Search PubMed.
- P. Woitke, J. Wellmitz, D. Helm, P. Kube, P. Lepom and P. Litheraty, Chemosphere, 2003, 51, 633–642 CrossRef CAS.
- M. Kralik, personal communication.
- Common Implementation Strategy, environmental quality standards—substance data sheet “Lead and its compounds”, European Commission, 31st July, 2005.
- Common Implementation Strategy, environmental quality standards—substance data sheet “Nickel and its compounds”, European Commission, 31st July, 2005.
- H. Ruedel, A. Fliedner and M. Herrchen, Machbarkeitsstudie: Strategie für ein stoffangepasstes Wasser-Monitoring—Erfassung potentiell sorbierender oder akkumulierender Stoffe in anderen Kompartimenten (Biota, Sediment, Schwebstoffe), Appendix, Anhang 16: Datenblätter,, Fraunhofer Institut für Molekularbiologie und Angewandte Oekologie (Fraunhofer IME) Bereich Angewandte Oekologie, Schmallenberg, Germany, 2007 Search PubMed.
Footnote |
| † Presented at the 2007 European Winter Conference on Plasma Spectrochemistry, Taormina, Italy, February 18–23, 2007. |
| This journal is © The Royal Society of Chemistry 2008 |
