Cellulose dissolution with polar ionic liquids under mild conditions: required factors for anions†
Yukinobu
Fukaya
a,
Kensaku
Hayashi
a,
Masahisa
Wada
b and
Hiroyuki
Ohno
*a
aDepartment of Biotechnology, Tokyo University of Agriculture & Technology, 2-24-16 Naka-cho, Koganei, Tokyo, 184-8588, Japan. E-mail: ohnoh@cc.tuat.ac.jp; Fax: +81-42-388-7024; Tel: +81-42-388-7024
bDepartment of Biomaterials Science, Graduate School of Agricultural and Life Sciences, The University of Tokyo, Yayoi 1-1-1, Bunkyo-ku, Tokyo, 113-8657, Japan
First published on 12th November 2007
Abstract
A series of alkylimidazolium salts containing dimethyl phosphate, methyl methylphosphonate, or methyl phosphonate prepared by a facile, one-pot procedure were obtained as room temperature ionic liquids, which have the potential to solubilize cellulose under mild conditions. Especially, N-ethyl-N′-methylimidazolium methylphosphonate enables the preparation of 10 wt% cellulose solution by keeping it at 45 °C for 30 min with stirring and rendered soluble 2–4 wt% cellulose without pretreatments and heating.
Cellulose is the major carbohydrate produced by plant photosynthesis, and is therefore the most abundant organic polymer produced on Earth. Cellulose can be used as a ‘green’ polymer for fabricating new and attractive materials, by chemical modification or mixing with other components.1 Cellulose consists of linear chains of 1,4-linked β-D-glucose units, so that its biodegradation has a great impact on the recovery of natural resources, as well as the production of electrochemical energy by bio-fuel cells. It should be noted here that the total energy needed to produce useful energy from biomass is very important. Accordingly, its treatment with low energy should be important. Cellulose is hardly soluble in conventional solvents because of its many intermolecular hydrogen bonds. Solvent systems2 currently used for cellulose suffer drawbacks such as volatility or generation of poisonous gas. Furthermore, to fully dissolve cellulose, multi-step pretreatments are needed, followed by prolonged stirring.
The low melting point organic salts known as ionic liquids (ILs) are attracting increasing attention as a new class of solvents.3 Because of the diversity of their component organic ions, it is possible to tune their physico-chemical properties, including polarity, viscosity and melting point. This promising diversity suggests that appropriate ILs would be non-volatile polar solvents for cellulose. ILs such as N,N′-dialkylimidazolium chloride salts ([RR′im][Cl]) dissolve cellulose4 and other biomacromolecules.5 However, most [RR′im][Cl] salts are solid or a sticky paste at room temperature,6 and it is necessary to handle them at high temperatures. For more efficient energy production from biomass, heating should be avoided to reduce the energy cost. Recently, we have reported the N,N′-dialkylimidazolium formates ([RR′im][HCOO]) as new solvents for cellulose.7 These [RR′im][HCOO] ILs were obtained as low viscosity liquids at room temperature, but they showed relatively poor thermal stability because of decarboxylation. Moreover, [RR′im][HCOO] IL is prepared by a two step reaction, in which the halide counter anion of the imidazolium cation is first converted to hydroxide, then coupled with the desired HCOO anion. To overcome the above drawbacks, we need a new class of easily-preparable ILs with sufficient ability to dissolve cellulose. Previous studies of the solubilization of cellulose indicate the choice and design of anions are important for preparation of polar ILs to solubilize cellulose. Accordingly, in this study, we prepared a series of ILs with a fixed cation, N-ethyl-N′-methylimidazolium ([C2mim]) with a variety of anions by one-pot procedure. Here, we focused on the effect of the anion structure of ILs on the solubilization of cellulose under mild conditions.
Since many kinds of acid esters are commercially available, we prepared a series of IL candidates by the one step quarternization of tertiary amines with acid esters. While there are several alkylimidazolium salts having alkylsulfate,8 alkylsulfonate,9 and alkylphosphate10 anion derivatives prepared by one step quarternization, there has been no evaluation of these ionic liquids as solvents for cellulose. As model experiments, we thus prepared [C2mim] salts with methanesulfonate ([MeSO3]), methylsulfate ([MeOSO3]), ethylsulfate ([EtOSO3]), and dimethylphosphate ([(MeO)2PO2]). Microcrystalline cellulose (MCC; DP = 200–250) was added to them to a final concentration of 2.0 wt%. Only [C2mim][(MeO)2PO2] dissolved MCC at 45 °C. In the 1H and 13C NMR spectra of neat [C2mim][(MeO)2PO2]/MCC solution, all chemical shifts and the integrated peak ratios were in good agreement with those of the neat IL, implying that no decomposition of the IL occurred during the dissolution process. Moreover, cellobiose, a model compound of cellulose, exhibited identical 1H and 13C NMR spectra even after regenerating from IL solution, suggesting that [C2mim][(MeO)2PO2] solubilized cellobiose without derivatization (see Electronic Supplementary Information†). We then analyzed other [C2mim][RR′PO2] salts (Chart 1) in an attempt to improve the solubilizing ability.
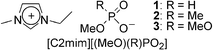 | ||
| Chart 1 Chemical structure of ionic liquids examined. | ||
Both 111 and 2 were readily prepared by the reaction of N-ethylimidazole with the corresponding acid esters. These ILs were all found to be room temperature ILs (RTILs). Differential scanning calorimetry (DSC) found that the melting temperature (Tm) of [C2mim][(MeO)2PO2] (i.e., 3) was 21 °C, whereas the others had only the low glass transition temperature (Tg) of –66 °C (2) and –86 °C (1) (Table 1). Melting and freezing behaviour could not be detected, even when these ILs were slowly cooled or heated at 1 °C min–1 in the DSC measurements; this suggests that they were in stable molten or supercooled states. The asymmetric structure of the anions for 1 and 2 might give rise to poor packing of ions. The thermal stability of [C2mim][(MeO)(R)PO2] was generally better than that for previously reported polar ILs. In particular, the temperature at 10% weight loss (Tdec) of [RR′im][HCOO] was approximately 210 °C, whereas that of [C2mim][(MeO)(R)PO2] was above 260 °C. This might be due to the thermal stability of phosphorus containing ILs.12
Table 1 also shows the Kamlet–Taft parameters13 (α: hydrogen bond acidity, β: hydrogen bond basicity, and π*: dipolarity) for [C2mim][(MeO)(R)PO2]. The ILs prepared here displayed high β values (above 1.0): in particular, the β value of 2 was 1.07, whereas the β value of [RR′im][Cl] ILs and [RR′im][HCOO] ILs was around 0.84 to 0.99, respectively.7 The ILs were revealed to have moderate or low viscosity in spite of their greater hydrogen bonding ability. N-Allyl-N′-methylimidazolium chloride, which is sufficiently polar to solubilize MCC, has lower viscosity than any previously discovered chloride salt known to date. The viscosity at 25 °C of [C2mim][(MeO)(R)PO2] is in the range 100–500 cP, which is lower than that for polar chloride salts. This low viscosity can be attributable to small ion size, low Tg value, and the flat shape of the cation.
We first determined the solubility of MCC in these ILs using stirring for 30 min. As shown in Fig. 1, each IL successfully solubilized MCC under mild conditions. The temperature needed to solubilize the same concentration of MCC depends strongly on the anion structure of the ILs. For example, 2 required heating to 55 °C to solubilize 10 wt% of MCC, whereas the corresponding temperature for 3 was 65 °C. Recent NMR studies on the dissolution mechanism of cellulose in [C4mim][Cl] indicates that the anion of ILs acts as a hydrogen bond acceptor which interacts with the hydroxyl group of cellulose.14 Since 2 has stronger hydrogen bond basicity (β value = 1.07) than 3 (β value = 1.00), the greater ability to dissolve MCC of 2 compared with 3 is easily comprehended. Unexpectedly, we found that 1 dissolved MCC better at lower temperatures than any other of the ILs prepared in this study. The polarity parameters of 1 are similar to those of 3. Also, since 1 had lower viscosity than 3, MCC was more easily dispersed in 1 than in 3. Hence, MCC can be solubilized in 1 more readily than in 2 or 3. To minimize the effect of viscosity on the solubilization, we examined the solubilization of MCC with stirring for 60 min. As is shown in Fig. 1, the sample of [C2mim][(MeO)(R)PO2] required heating at 35–55 °C with stirring for 30 min to solubilize 6 wt% MCC, whereas only 30–40 °C was enough to solubilize the same amount of MCC when they were mixed for 60 min. The temperature of the mixed solution was maintained and there was no additional heat effect generated by mechanical stirring.
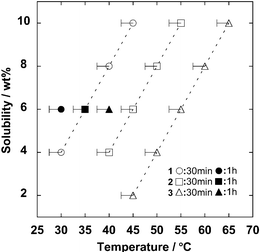 | ||
| Fig. 1 Solubility of cellulose in 1, 2 and 3. | ||
These findings were promising enough for us to examine the solubilization of MCC without heating. We found that 2 wt% MCC dissolved completely in these ILs at room temperature (25 °C) within 3 hr, and 4 wt% within 5 hr. Fig. 2 shows the solubilization of filter paper (1.0 wt%) into IL 1 without heating. From this figure, it is clear that the paper was dissolved in it within 3 hr. Again, no rise in temperature of the solution was found during mixing.
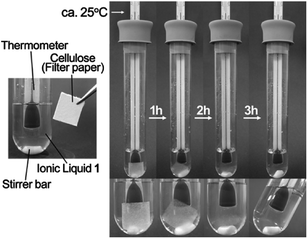 | ||
| Fig. 2 Solubilization of cellulose (filter paper: 1 wt%) in IL 1 at room temperature. | ||
While ILs prepared here are potential candidates for low energy solubilization of cellulose, further investigations on the degradation of cellulose during the solubilization and toxicology of the precursors and the resulting ILs should be addressed. These topics are now being investigated and will be reported elsewhere.
In conclusion, a [C2mim][(MeO)(R)PO2] series was prepared by an easy one-pot procedure. These were polar RTILs with relatively low viscosity and good thermal stability. These polar ILs successfully solubilized cellulose at high concentrations and can dissolve cellulose at room temperature.‡
This study was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan (No. 17205020 and 17073005), and the 21st Century COE program of “Future Nano-Materials” in Tokyo University of Agriculture & Technology.
Notes and references
- Cellulose Derivatives Modification, Characterization, and Nanostructures, ACS Symposium Series 688, eds. T. H. Heinze and W. G. Glasser, American Chemical Society, Washington, DC, 1997 Search PubMed.
- (a) C. L. McCormick, P. A. Callais and B. H. Hutchinson, Jr., Macromolecules, 1985, 18, 2394–2401 CrossRef CAS; (b) H. Chanzy, A. Peguy, S. Chaunis and P. Monzie, J. Polym. Sci., Polym. Phys. Ed, 1980, 18, 1137–1144 CrossRef CAS; (c) J. Cai and L. Zhang, Macromol. Biosci., 2005, 5, 539–548 CrossRef CAS; (d) M. Yanagisawa, I. Shibata and A. Isogai, Cellulose, 2004, 11, 169–176 CrossRef CAS.
- Ionic Liquids in Synthesis, eds. P. Wasserscheid and T. Welton, Wiley-VCH, Weinheim, Germany, 2003 Search PubMed.
- (a) R. P. Swatloski, S. K. Spear, J. D. Holbrey and R. D. Rogers, J. Am. Chem. Soc., 2002, 124, 4974–4975 CrossRef CAS; (b) H. Zhang, W. J. Zhang and J. He, Macromolecules, 2005, 38, 8272–8277 CrossRef CAS; (c) M. B. Turner, S. K. Spear, J. D. Holbrey and R. D. Rogers, Biomacromolecules, 2004, 5, 1379–1384 CrossRef CAS.
- (a) D. M. Phillips, L. F. Drummy, D. G. Conrady, D. M. Fox, R. R. Naik, M. O. Stone, P. C. Trulove, H. C. De Long and R. A. Mantz, J. Am. Chem. Soc., 2004, 126, 14350–14351 CrossRef CAS; (b) H. Xie, S. Li and S. Zhang, Green Chem., 2005, 7, 606–608 RSC; (c) K. Fujita, Y. Fukaya, N. Nishimura and H. Ohno, in Electrochemical Aspects of Ionic Liquids, ed. H. Ohno, Wiley-Interscience, New York, 2005, pp 157–167; Search PubMedS. A. Forsyth, D. R. MacFarlane, R. J. Thomson and M. V. Itzstein, Chem. Commun., 2002, 714–715 Search PubMed; D. W. Armstrong, L. He and Y.-S. Liu, Anal. Chem., 1999, 71, 3873–3876 Search PubMed.
- T. Mizumo, E. Marwanta, N. Matsumi and H. Ohno, Chem. Lett., 2004, 33, 1360–1361 CrossRef CAS.
- Y. Fukaya, A. Sugimoto and H. Ohno, Biomacromolecules, 2006, 7, 3295–3298 CrossRef CAS.
- A. J. Carmichael, M. Deetlefs, M. J. Earle, U. Frohlich and K. Seddon, in Ionic Liquids as Green Solvents, Progress and Prospects, ACS Symposium Series 856, eds. R. D. Rogers and K. R. Seddon, American Chemical Society, Washington, 2003, pp 14–31 Search PubMed.
- S. Himmler, S. Hörmann, R. V. Hal, P. S. Schulz and P. Wasserscheid, Green Chem., 2006, 10, 887–894 Search PubMed.
- E. Kuhlmann, S. Himmler, H. Giebelhaus and P. Wasserscheid, Green Chem., 2007, 3, 233–242 Search PubMed.
- C. Anding, S. Trinh and J.-M. Gaulliard, French Patent, vol. 80, p. 15323 Search PubMed.
- R. E. Del Sesto, C. Corley, A. Robertson and J. S. Wilkes, J. Organomet. Chem., 2005, 690, 2536–2542 CrossRef.
- (a) L. Crowhurst, P. R. Mawdsley, J. M. Perez-Arlandis, P. A. Salter and T. Welton, Phys. Chem. Chem. Phys., 2003, 5, 2790–2794 RSC; (b) S. N. Baker, G. A. Baker and F. V. Bright, Green Chem., 2002, 4, 165–169 RSC.
- (a) R. C. Remsing, R. P. Swatloski, R. D. Rogers and G. Moyna, Chem. Commun., 2006, 1271–1273 RSC; (b) J. S. Moulthrop, R. P. Swatloski, G. Moyna and R. D. Rogers, Chem. Commun., 2005, 1557–1559 RSC.
Footnotes | |||||||||||||||||
| † Electronic supplementary information (ESI) available: experimental details as well as 1H and 13C-NMR charts of ILs. See DOI: 10.1039/b713289a | |||||||||||||||||
‡ (1) Synthesis and characterization of ionic liquids. As the general preparation procedure of [C2mim][(MeO)(R)PO2], synthesis of IL 1 is mentioned as follows. To a THF solution of N-ethylimidazole, dimethyl phosphite in THF was added dropwise under argon gas atmosphere at room temperature. The reaction mixture was stirred with reflux at 90 °C for 2 days. After removal of THF under reduced pressure, the resulting liquid was washed with an excess amount of diethyl ether repeatedly. The residual liquid was dissolved in dichloromethane, and the resulting solution was passed through a column filled with neutral activated alumina. After removal of dichloromethane, residual liquid was dried in vacuo at 80 °C for 24 h to give 1 as a colourless liquid in 95% yield. 1H-NMR (400 MHz; CDCl3; Me4Si) δ = 1.58 (3H, t, J = 7.3 Hz, NCH3), 3.55 (3H, d, J = 11.9 Hz, POCH3), 4.06 (3H, s, NCH3), 4.36 (2H, q, J = 7.3 Hz, NCH2CH3), 6.92 (1H, d, J = 588.5 Hz, PH), 7.58 (2H, d, J = 11.3 Hz, NCHCHN), 10.66 (1H, s, NCHN). 13C-NMR (100 MHz; CDCl3; Me4Si) δ = 15.22 (NCH2CH3), 35.87 (NCH3), 44.57 (NCH2CH3), 50.05 (POCH3), 121.35, 123.17 (NCHCHN), 138.40 (NCHN). ESI-TOF-MS: calcd for C7H15N2O3P [M]+: m/z = 111.17; found: 111.37. Elemental analysis: calcd for C7H15N2O3P: C, 40.78; H, 7.33; N, 13.59; found: C, 40.72; H, 7.47; N, 13.72. Ion chromatography: cation 99.98 (area%), anion 99.65 (area%). IL 2 (in 96% yield): 1H-NMR (400 MHz; CDCl3; Me4Si) δ = 1.28 (3H, d, J = 16.0 Hz, PCH3), 1.57 (3H, t, J = 7.3 Hz, NCH2CH3), 3.58 (3H, d, J = 10.1 Hz, POCH3), 4.08 (3H, s, NCH3), 4.38 (2H, q, J = 7.3 Hz, NCH2CH3), 7.47 (2H, d, J = 14.2 Hz, NCHCHN), 10.92 (1H, s, NCHN). 13C-NMR (100 MHz; CDCl3; Me4Si) δ = 11.24, 12.56 (PCH3), 15.31 (NCH2CH3), 35.92 (NCH3), 44.60 (NCH2CH3), 50.05 (POCH3), 121.00, 121.96 (NCHCHN), 139.28 (NCHN). ESI-TOF-MS: calcd for C8H17 N2O3P [M]+: m/z = 111.17; found: 111.37, [M]–: m/z = 109.04; found: 108.91. Elemental analysis: calcd for C8H17N2O3P: C, 43.63; H, 7.78; N, 12.72. Found: C, 43.56; H, 7.91; N, 12.95. Ion chromatography: cation 99.99 (area%), anion 99.98 (area%). IL 3 (in 94% yield): 1H-NMR (400 MHz; CDCl3; Me4Si) δ = 1.57 (3H, t, J = 12.0 Hz, NCH2CH3), 3.59 (6H, d, J = 8 Hz, POCH3), 4.06 (3H, s, NCH3), 4.36 (2H, q, J = 24 Hz, NCH2CH3), 7.52 (2H, d, J = 24 Hz, NCHCHN), 10.57 (1H, s, NCHN). 13C-NMR (100 MHz; CDCl3; Me4Si) δ = 15.28 (NCH2CH3), 35.93 (NCH3), 44.61 (NCH2CH3), 52.10 (POCH3), 121.40, 123.36 (NCHCHN), 138.20 (NCHN). ESI-TOF-MS: calcd for C8H17N2O4P [M]+: m/z = 111.17; found: 111.37, [M]–: m/z = 124.89; found: 125.04. Elemental analysis: calcd for C8H17N2O4P: C, 40.68; H, 7.25; N, 11.86. Found: C, 40.65; H, 7.86; N, 11.69. Ion chromatography: cation 99.97 (area%), anion 100 (area%). The amount of water for all ILs prepared in this study was confirmed at less than 1000 ppm.(2) Measurement of the Kamlet–Taft parameters of ionic liquids. Measurement of the Kamlet–Taft parameters of a series of ionic liquids was carried out as follows. The solvatochromic dyes, (2,6-dichloro-4-(2,4,6-triphenyl-1-pyridinio)phenolate (Reichardt's dye 33) (from Fluka), 4-nitroaniline (from Tokyo Chemical Industries Co., Ltd), and N,N-diethyl-4-nitroaniline (from Kanto Chem.), were used as received. To 0.2 ml of ionic liquid, the dye was added as a concentrated dry methanol solution. The methanol was then carefully removed by vacuum drying at 40 °C for 6 h. To avoid dye aggregation, the dye concentration in a series of ionic liquids was such that it had an absorbance between 0.15 and 0.30. These ionic liquid solutions were placed into quartz cells with 1 mm light-path length. Temperature of the quartz sample cell was maintained at 25 °C by water circulation. From the wavelength at the maximum absorption (λmax) determined, the α, β and π* values were calculated by use of the following equations:
|
| This journal is © The Royal Society of Chemistry 2008 |

![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 592/
592/