Making diazomethane accessible for R&D and industry: generation and direct conversion in a continuous micro-reactor set-up†
Michael
Struempel
a,
Bernd
Ondruschka
a,
Ralf
Daute
b and
Annegret
Stark
*a
aFriedrich-Schiller University of Jena, Institute for Technical Chemistry and Environmental Chemistry, Lessingstr. 12, 07743 Jena, Germany. E-mail: annegret.stark@uni-jena.de; Fax: +49 3641 948402; Tel: +49 3641 948413
bCHEMTEC LEUNA, Gesellschaft für Chemie und Technologie mbH, Am Haupttor, 06237 Leuna, Germany
First published on 9th November 2007
Abstract
Micro-reaction technology offers a safe way to deliver diazomethane on demand both on the R&D and the industrially relevant scale, including in situ conversion to the desired product to avoid handling and storage of explosive diazomethane solutions, thus making this potent compound accessible for the pharmaceutical and fine chemical sector.
Diazomethane, which is generally liberated from the more stable1,2 and commercially available precursor N-methyl-N-nitroso-p-toluenesulfonamide (Diazald®) by treatment with a strong base, is an extremely reactive substance. Although it features interesting and versatile chemistries under mild conditions,3–10 and can perform as methylating agent, 1,3-dipolar compound or carbene source (Scheme 1), its industrial relevance, for example in the derivatisation of labile biogenic substances and complex pharmaceutical compounds,5,11 is nil. In the laboratory, its use is mostly avoided, as the hazardous properties of diazomethane, such as explosiveness, toxicity, and carcinogenicity,12–14 make special techniques and safety precautions necessary during its preparation and handling, such as explosion protection and fire-polished glassware.1
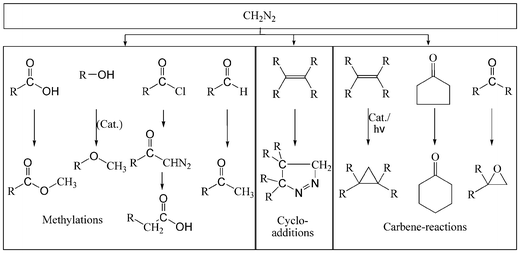 | ||
| Scheme 1 Overview of the reaction types of diazomethane. | ||
In order to spur diazomethane chemistry for both laboratory and industrial applications, especially for fine chemicals, specialities and pharmaceutical products, the use of micro-reaction technology15–18 was envisaged as a consummate tool to overcome any safety issues: micro-reactor technology features extremely small internal volumes, resulting in uncritically low spatiotemporal concentrations of diazomethane. The concise arrangement of the reactor and peripheral equipment allows for the installation of additional safety equipment, such as a surrounding acid bath, and temperature and pressure sensors. Thus, the customarily hazardous batch distillation for the generation of diazomethane from the precursor Diazald®, and successive conversion with a reactant to the desired product, was replaced by a safe two-stage continuous micro-reaction process in which the diazomethane is produced on demand only.19
In addition to the advantages relating to process safety, another benefit arises in the context of effective process development: due to the optimisation of the continuous production at an early R&D stage, the scale-up to relevant (and flexible) product quantities is achieved by simple numbering-up to multiple manufacturing lines running in parallel.
As a model reaction to demonstrate the practicability of this concept, the generation of diazomethane from Diazald®, and its rapid, direct conversion with benzoic acid5 to benzoic acid methyl ester (Scheme 2), was chosen. For a micro-reaction process, it is of crucial importance to ensure particle-free conditions to avoid clogging due to precipitation from feeds with high saline load. Therefore, before studying the influence of residence times, flow rate and temperature, a thorough solvent study was carried out. Finally, a long-term test of the optimised system was carried out to demonstrate the stability of the process for industrial applications.
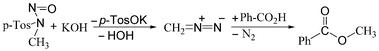 | ||
| Scheme 2 Generation of diazomethane from the precursor Diazald® with KOH, and its reaction with benzoic acid to benzoic acid methyl ester. | ||
Table 1 shows selected results of the preliminary solvent study, which was carried out in batch experiments, see Electronic Supplementary Information (ESI)†, to determine potential solvents from which precipitation of salts does not occur, while giving maximum yields of benzoic acid methyl ester. The relative concentrations of the reactants in the respective solvent was chosen based on the reagent with the lowest solubility, which was used as a concentrated solution. The other feed solutions were prepared in relation to allow for the quantitative conversion of Diazald® to diazomethane, while also providing sufficient benzoic acid for both the neutralisation of any unconverted KOH and the complete conversion of diazomethane to benzoic acid methyl ester. This ratio was found to be Diazald® : KOH : benzoic acid = 1.0 : 1.5 : 4.0.
| Entry | Diazald® | KOH | Benzoic acid | No. liquid phases | Precipitate | Yield (%)a |
|---|---|---|---|---|---|---|
| a Yield of benzoic acid methyl ester. b Carbitol = di(ethylene glycol) ethyl ether. c After addition of benzoic acid. d Before addition of benzoic acid. | ||||||
| 1 | Diethyl ether | Water : carbitol (2 : 1)b | Diethyl ether | 2 | No | 21 |
| 2 | Diethyl ether : carbitol (1 : 1) | Water | Diethyl ether | 2 | No | 30 |
| 3 | Diethyl ether | Water : carbitol (2 : 1) | Carbitol | 1 | No | 20 |
| 4 | Carbitol | Water : carbitol (2 : 1) | Diethyl ether | 2 | No | 23 |
| 5 | Diethyldiglycol | 2-Propanol | 2-Propanol : water (1 : 1) | 1 | No | 75 |
| 6 | Carbitol | 2-Propanol | Diethyl ether | 1 | Yesc | 72 |
| 7 | Tetrahydrofuran | 2-Propanol | Tetrahydrofuran | 1 | Yesc,d | 58 |
| 8 | Toluene | 2-Propanol | Toluene | 1 | Yesc,d | 42 |
| 9 | Carbitol | 2-Propanol | Carbitol | 1 | No | 58 |
Depending on the choice of solvent, a mono- or biphasic liquid–liquid system was obtained. In most instances (except entry 9), the precipitation of solids (either during the liberation of diazomethane, or during its reaction with benzoic acid) was prevented only if at least one feed solvent contained water. The major effect with respect to yield was observed for the solvent selection of the base KOH: if it contained water, yields below 30% were obtained (entries 1–4), whereas for non-aqueous base feeds, e.g., with 2-propanol, yields were increased up to 75% (entries 5–9). On the other hand, the solvents of the organic feeds (Diazald®, benzoic acid) did not crucially influence the product yield. However, they determine the solubility of starting material and products, and their choice is therefore important for both minimising the formation of particles and achieving economically high concentrations of reactants. Thus, diethyldiglycol and carbitol show optimal solubility and minimal formation of particles in combination with high yields (entries 5 and 9), which are comparable to those described previously in the batch synthesis.1
In a first attempt to transpose the reaction into continuous mode, the solvent combination of entry 5 was adopted, as it promises the highest yields. Surprisingly, unlike in batch mode, precipitation did occur in the micro-reactor as a result of locally exceeding the maximum solubility. Therefore, the second favourite alternative, i.e., carbitol–2-propanol (entry 9) was investigated in the micro-reaction set-up (Scheme 3), giving a yield of 60%, which corresponds well with the result in batch modus†. Additionally, it was found that similar to the batch experiments, the process results in low yields when the base is dissolved in water or an aqueous solvent mixture, so that only 5% of benzoic acid methyl ester was obtained when the organic (Diazald®, benzoic acid) and KOH feeds were provided in dichloromethane and water, respectively.
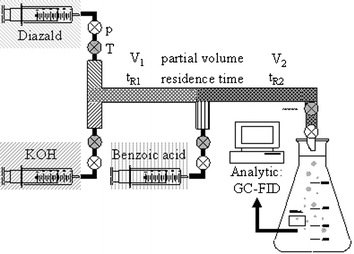 | ||
| Scheme 3 Flow scheme of the experimental set-up for the continuous experiments. | ||
In order to further optimise the process conditions, the influence of the parameters residence time, flow rate and reaction temperature were investigated. Using the optimised solvent system, i.e., 2-propanol for the base and carbitol for the organic feeds, the effect of the first residence time for the generation of diazomethane from the precursor by treatment with the base (tR1) was investigated, and compared with a capillary reactor without mixing elements. Different residence times were achieved by adjustment of the feed flows. The results show a clear dependency of the yield of benzoic acid methyl ester (relative to Diazald®) on tR1 for the use of a capillary as reaction device (Fig. 1). The maximum yield can only be reached at residence times >50 s, whereas the micro-reactor does not show any dependence of the maximum yield with tR1 > 5 s. These results indicate that the mixing efficiency in the micro-reactor is greater than in the capillary, probably due to the mixing elements of the former.
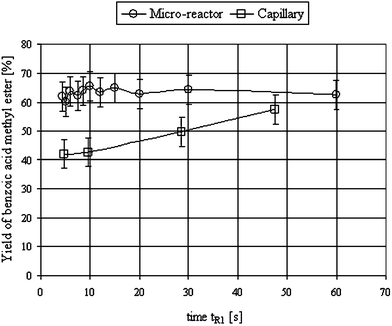 | ||
| Fig. 1 Dependence of the yield of benzoic acid methyl ester (relative to Diazald®) on the residence time tR1; formation of diazomethane, in the micro-reactor and capillary. For experimental conditions, see ESI†. | ||
In contrast to tR1, variation of the second residence time (10 s < tR2 < 70 s) for the reaction of the generated diazomethane with benzoic acid did not exhibit any effect on the yield, and no differences between micro-reactor and capillary became apparent. Likewise, neither the micro-reactor nor the capillary set-up showed an effect on the total flow rate‡ as long as the residence time tR1 was sufficient (micro-reactor > 5 s, capillary > 50 s; Fig. 2).
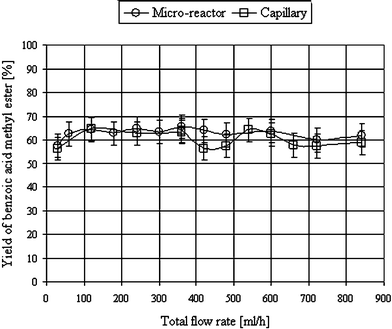 | ||
| Fig. 2 Dependence of the yield of benzoic acid methyl ester on the total flow rate in the micro-reactor in comparison to the capillary. For experimental conditions, see ESI†. | ||
With the inherent safety of the micro-reactor set-up, it was for the first time possible to investigate the dependence of the temperature on diazomethane formation. Under optimised conditions†, a constant yield of >65% of benzoic acid methyl ester is achieved between temperatures of 0 and 50 °C. At temperatures of about 50–85 °C, the yield appears to decrease slightly. This may be due to a starting decomposition.6,12,14 Although the yields of this particular reaction cannot be optimised by adjustment of the temperature, the set-up demonstrates an inherent process safety, even at high temperatures up to 85 °C. Therefore, its application is extendable to both reactions of diazomethane with other, less reactive, substrates (Scheme 1) and to other highly hazardous reactants with potential synthetic relevance.
The final aim to show that the method and technique are suitable for industrial application was achieved by a continuous stability test, which indicated a stable micro-reaction process under optimised conditions: a constant yield of 75% of benzoic acid methyl ester is generated over a period of at least 1 hour, and longer production periods appear feasible without the risk of clogging†. Therefore, it should be possible to produce and directly convert about 2.5 mol d–1 of diazomethane with a single micro-reactor, at a total flow rate of 840 ml h–1. These calculations show that through its continuity and the inherent safety, the process fulfils the requirements for industrial applications. Micro-reaction technology also allows for fast and easy scaling up to flexibly adapt product quantities to market demands (the numbering-up principle).
In conclusion, this study has shown the potential of micro-reactor technology to make previously hazardous chemistries available for the pharmaceutical, fine chemical and speciality sectors, while providing yields which compare well with the classic batch synthesis. As shown for the example of diazomethane, its high reactivity and tolerance to functional groups can be used under mild conditions. Nevertheless, where required, the set-up presented herein may also be used at elevated temperatures. As opposed to when scaling up batch equipments, the micro-reactor can simply be numbered up to produce the required quantities, and is thus highly flexible to the demands of the market.
Chemtec Leuna provided financial support for this project. M.S. would like to acknowledge a fellowship from Deutsche Bundesstiftung Umwelt (DBU), and Dr. Ferstl (Fraunhofer Institut Chemische Technologie Pfinztal) for helpful information given in private correspondence (2006).
Notes and references
- Th. J. de Boer and H. J. Backer, Org. Synth., 1963, Coll. Vol. 4, 250.
- F. Arndt, Org. Synth., 1943, Coll. Vol. 2, 165.
- H. v. Pechmann, Chem. Ber., 1894, 27, 1888 CrossRef.
- H. B. Hopps, Aldrichim. Acta, 1970, 3, 9 CAS.
- T. H. Black, Aldrichim. Acta, 1983, 16, 3.
- J. A. Moore and D. E. Reed, Org. Synth., 1961, 41, 16 CAS.
- H. Meerwein and G. Hintz, Liebigs Ann. Chem., 1930, 484, 1 CrossRef.
- F. Schlotterbeck, Chem. Ber., 1907, 40, 479 CrossRef CAS.
- H. E. Simmons and R. D. Smith, J. Am. Chem. Soc., 1958, 80, 5323 CrossRef CAS.
- W. E. Bachmann and W. S. Struve, Org. Reactions, 1942, 1, 50.
- T. Archibald, Diazomethane, http://www.thomasarchibald.com/adobe/diazomethane.pdf, 28.03.2007.
- Occupational Safety and Health Administration, http://www.osha.gov/SLTC/healthguidelines/diazomethane/recognition.html, 17.02.00; http://www.gifte.de/chemikalien/diazomethan.htm, 17.08.05; International Occupational Safety and Health Information Centre, ICSC 1256, http://www.ilo.org/public/english/protection/safework/cis/products/icsc/dtasht/_icsc12icsc1256.htm, 03.95.
- C. D. Gutsche, Org. Reactions, 1954, 8, 391.
- E. B. LeWinn, Am. J. Med. Sci., 1949, 218, 556 Search PubMed.
- E. Klemm, M. Rudek, G. Markowz and R. Schütte, ‘Mikroverfahrenstechnik’, in Chemische Technik—Prozesse und Produkte, eds. Winnacker, Küchler, Wiley-VCH, Weinheim, 2004 Search PubMed.
- K. Jähnisch, V. Hessel, H. Löwe and M. Baerns, Angew. Chem., Int. Ed., 2004, 43, 406 CrossRef.
- W. Ehrfeld, V. Hessel and H. Löwe, Microreactors—New Technology for Modern Chemistry, Wiley-VCH, Weinheim, 2005 Search PubMed.
- Micro Process Engineering—Fundamentals, Devices, Fabrication, and Applications, ed. N. Kockmann, Wiley-VCH, Weinheim, 2006 Search PubMed.
- W. F. Ferstl, M. S. Schwarzer and S. L. Löbbecke, Chem.-Ing.-Tech., 2004, 76, 1326 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: experimental and analytical details, and graphs detailing both the dependence of yield on reaction temperature and the stability of the set-up during continuous operation. See DOI: 10.1039/b710554a. |
| ‡ The total flow rate is defined as the sum of the three inlet flow rates. The relative ratio of feeds, and the reactant ratios, were kept constant. |
| This journal is © The Royal Society of Chemistry 2008 |
