Qualitative and quantitative structure activity relationships for the inhibitory effects of cationic head groups, functionalised side chains and anions of ionic liquids on acetylcholinesterase
Jürgen Arning*a, Stefan Stoltea, Andrea Böschena, Frauke Stockb, William-Robert Pitnerc, Urs Welz-Biermannc, Bernd Jastorffa and Johannes Rankea
aUFT - Centre for Environmental Research and Technology, University of Bremen, Leobener Straße, D-28359, Bremen, Germany. E-mail: jarning@uni-bremen.de
bUmweltbundesamt, Postfach 1406, D-06813, Dessau, Germany
cMerck KGaA, Frankfurter Straße 250, D-64293, Darmstadt, Germany
First published on 19th October 2007
Abstract
To contribute to a deeper insight into the hazard potential of ionic liquids to humans and the environment, an acetylcholinesterase (AchE) inhibition screening assay was used to identify toxicophore substructures and interaction potentials mediating enzyme inhibition.
The positively charged nitrogen atom, a widely delocalised aromatic system, and the lipophilicity of the side chains connected to the cationic head groups can be identified as the key structural elements in binding to the enzymes active site. With respect to this, the dimethylaminopyridinium, the quinolinium and the pyridinium head groups exhibit a very strong inhibitory potential to the enzyme with IC50 values around 10 µM. In contrast, the polar and non-aromatic morpholinium head group is found to be only weakly inhibiting to the enzyme activity, with IC50 values > 500 µM.
The introduction of polar hydroxy, ether or nitrile functions into the alkyl side chain is shown to be a potent structural alteration to shift the corresponding ionic liquids to a lower inhibitory potential. Supporting this fact, for a series of imidazolium cations, a QSAR correlation was set up by the linear regression of the log IC50versus the logarithm of the HPLC-derived lipophilicity parameter k0.
Additionally, a broad set of anion species (inorganic, organic and complex borate anions), commonly used as ionic liquid counterions, was tested and the vast majority exhibited no effect on AchE. Only the fluoride and fluoride containing anion species which readily undergo hydrolytic cleavage can be identified to act as AchE inhibitors.
Introduction
Especially since the imminent intensification of the new EU chemicals legislation REACH (which was put into force in June 2006),1 the knowledge of (eco)toxicological hazard potentials of chemical substances is attached to increasing importance.This requires efficient testing strategies, similar to those applied in the field of pharmacological drug design, to generate valid datasets for the registration procedure under REACH, and to get a profound insight into mode of toxic action and possible target sites of industrial chemicals. Regarding this issue, we trace a T-SAR2,3 (thinking in terms of structure activity relationships) guided and tiered strategy to:
• Systematically select test compounds and structural elements according to the “testkit concept”.3,4
• Test the selected substances in a flexible (eco)toxicological test battery at different levels of biological complexity (e.g. enzymes, cells, microorganisms and organisms).5
• Improve the molecular understanding of (eco)toxicological results by relating them to physicochemical properties.6
• Identify toxicophore substructures in chemicals and to use this knowledge in the prospective design of inherently safer chemical products.
Following this concept, we are aiming to assess the hazard potential for a set of 79 ionic liquids at the molecular level using an enzyme inhibition test.
The interest in ionic liquids and in their promising physical and chemical properties is still growing rapidly. Diverse applications of this heterogeneous substance class in different fields have been recently described.7–12
Regarding toxicological issues, they are predominantly discussed as “green” or “sustainable” chemicals, just based on their negligible vapour pressure, resulting in reduced inhalatory exposure and the absence of flammability.
However, the knowledge about their (eco)toxicological impacts on humans and the environment is still very basic and restricted to only a few chemical entities out of the enormous pool of available ionic liquids.4 Our attempt to fill this gap of information is to test the ionic liquids systematically in different test systems out of our flexible test battery.13–16
By applying the above mentioned T-SAR based strategy, we subdivide ionic liquids into the cationic head group, the side chain, and the corresponding anion to handle this structural variability and to identify how these individual structural variables may evoke inhibitory effects on the enzyme acetylcholinesterase (AchE).
The enzyme acetylcholinesterase catalyses the rapid degradation of the neurotransmitter acetylcholine in the synaptic cleft—one of the key mechanisms in neurotransmission in nearly all higher organisms, including humans.17,18 Thus, an inhibition of acetylcholinesterase leads to various adverse effects in neuronal processes, such as heart diseases or myasthenia in humans.19,20 Furthermore, acetylcholinesterase represented the main target in the development of potent insecticides based on phosphoric acid esters (e.g. Parathione®) and carbamates (e.g. Carbendazim®) and therefore the activity pattern of this enzyme in different biological matrices and tissues is used as an established biomarker to monitor the pesticide burden in non-target species.17,21,22
We considered the enzyme acetylcholinesterase to be an (eco)toxicologically relevant molecular target for a broad toxicity screening assay with ionic liquids based on several considerations.
i. The acetylcholinesterase can be found in nearly all higher organisms with a highly conserved and well known active site region, which allows for detailed structure activity analysis.18,23
ii. The enzyme is a crucial target in the development of insecticides and in human drug design and thus the structure activity relationships leading to enzyme inhibition have been intensively studied for a variety of chemical entities.24–26
iii. The amino acid residues with their specific interaction potentials building up the catalytic site and the substrate binding pocket are well known from detailed X-ray studies.27
iv. As key features for the inhibitory potential, a positively charged quaternary nitrogen atom, an electron-deficient aromatic system, as well as a certain lipophilicity could be identified in all potent reversibly acting inhibitors of the enzyme.24
v. The inhibition assay can be performed in a microtiter plate format and thus represents a fast and cost effective screening tool for early toxicity testing of industrial chemicals.
With respect to this, we could recently show that the nitrogen containing imidazolium and pyridinium head groups in ionic liquids act as potent inhibitors of electric eel acetylcholinesterase. In addition, a correlation between an increasing chain length of the side chains connected to the cationic head groups and an enhanced inhibitory potential of the ionic liquids was found.28
The testkits presented here, consisting of a larger variety of head groups, functionalised side chains, anions and substitution patterns, are combined to identify the acetylcholinesterase inhibitory potential of ionic liquids in more detail. Furthermore, the necessity of certain molecular interaction potentials in the tested substances to interact specifically with the enzyme is demonstrated by comparing a potent imidazolium based inhibitor ionic liquid with structural analogues lacking the positively charged nitrogen moiety. And finally, the previously described side chain effect14,28 could be confirmed in the enzymatic test system with a quantitative structure activity correlation.
Characterising such structural features responsible for the toxic mode of action of chemical substances, the so called toxicophores, can help to achieve the goal of a sustainable design of new chemical products by reducing or eliminating toxic or harmful structural elements of an industrial chemical. Additionally, such structural insights provide useful knowledge to concurrently maintain or even to improve the desired technical features of a substance, described by the so called technicopohres, and to reduce the toxicity of the resulting product.
The testkit compounds
To investigate the influence of the cationic head groups on the inhibitory potential of the corresponding ionic liquid, thirteen different commonly used aromatic, heterocyclic and non-cyclic quaternary nitrogen containing structures were selected (Fig. 1). Additionally, the results for a quaternary phosphonium head group is presented for comparison.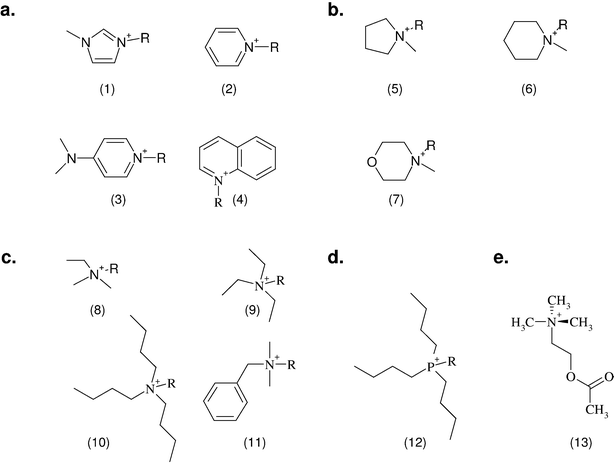 | ||
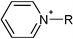
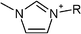
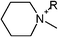
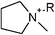
| Fig. 1 All tested cationic head group structures of ionic liquids and the natural substrate of the enzyme are presented, grouped by their specific core elements. The side chain is replaced by the “R” marker. (a) Aromatic quaternary ammonium compounds: (1) imidazolium, (2) pyridinium, (3) dimethylaminopyridinium and (4) quinolinium. (b) Heterocyclic quaternary ammonium compounds: (5) pyrrolidinium, (6) piperidinium and (7) morpholinium. (c) Non-cyclic quaternary ammonium compounds: (8) ethyl-dimethyl-ammonium, (9) triethylammonium, (10) tributylammonium and (11) dimethylbenzylammonium. (d) Quaternary phosphonium compounds: (12) tributylphosphonium. (e) The natural substrate of acetylcholinesterase: (13) acetylcholine. | ||
To test whether the anion species frequently used in ionic liquids exhibit any intrinsic inhibitory effect on the acetylcholinesterase, a selection of the sodium or lithium salts (depending on their availability) of different anions (Fig. 2) was investigated in the enzyme assay and the results are presented.
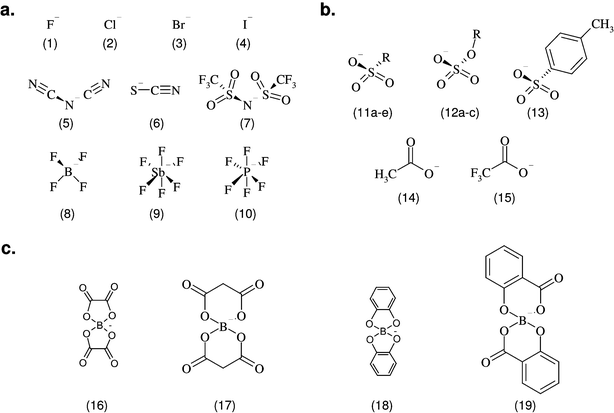 | ||
| Fig. 2 The structures or element symbols of all investigated anion species are shown grouped into inorganic (a), organic (b) and complex borate (c) anions. The “R” marker replaces different side chains. The names and the corresponding log IC50 (µM) values of the tested sodium or lithium salts of the anion species, together with the 95% confidence intervals in parentheses, are listed in Table 1. | ||
Additionally, a testkit comprising five cationic head groups and eleven alkyl and functionalised alkyl side chains containing ether (in varying positions), terminal hydroxy and nitrile functions (Table 3) was set up to demonstrate the impact of the side chain on the enzyme inhibitory potential of the ionic liquids.
| Number | Name | Log IC50 AchE/µM |
|---|---|---|
| 1 | Fluoride | 2.76 (2.69–2.82) |
| 2 | Chloride | >3 |
| 3 | Bromide | >3 |
| 4 | Iodide | >3 |
| 5 | Dicyanamide | >3 |
| 6 | Thiocyanate | >3 |
| 7 | Bis(trifluoromethylsulfonyl)amide | >3 |
| 8 | Tetrafluoroborate | >3 |
| 9 | Hexafluoroantimonate | 2.34 (2.28–2.39) |
| 10 | Hexafluorophosphate | 2.16 (2.12–2.19) |
| 11a | 1-Methanesulfonate | >3 |
| 11b | Trifluoromethanesulfonate | >3 |
| 11c | 1-Butanesulfonate | >3 |
| 11d | 1-Hexanesulfonate | >3 |
| 11e | 1-Octanesulfonate | >3 |
| 12a | 1-Methylsulfate | >3 |
| 12b | 1-Octylsulfate | >3 |
| 12c | 1-Dodecylsulfate | 2.96 (2.91–3.00) |
| 13 | Toluene-4-sulfonate | >3 |
| 14 | Acetate | >3 |
| 15 | Trifluoroacetate | >3 |
| 16 | Bis[oxalato(2-)]-borate | >3 |
| 17 | Bis[malonato(2-)]-borate | >3 |
| 18 | Bis[1,2-benzenediolato(2-)-O1,O2]borate | >3 |
| 19 | Bis[2-hydroxybenzoato(2-)-O1,O2]borate | >3 |
For a series of 1-butylpyridinium ionic liquids the side chain was kept constant and the methyl substitution pattern at the aromatic core structure was altered to elucidate regioselective impacts on the inhibitory potential of the pyridinium containing ionic liquids (see Table 4a).
To demonstrate the need of certain molecular interaction potentials to bind to the active site of electric eel acetylcholinesterase, a testkit of three substances was arranged containing the potent inhibitor 1-methyl-3-octylimidazolium and two uncharged structurally related octyl compounds (see Table 4b).
Acronyms for the ionic liquids
The following system of acronyms is used to facilitate the notation of the ionic liquids. The cation is abbreviated according to the type of the head group; “Py-4NMe2” (dimethylamino)pyridinium, “Py” (pyridinium), “IM” (imidazolium), “Mor” (morpholinium), “Pip” (piperidinium), “Pyr” (pyrrolidinium) and “N” (quaternary ammonium). The substituents at the nitrogen atom(s) of the head group are given as numbers corresponding to their alkyl chain length. For example, the 1-butyl-3-methylimidazolium cation has the shorthand notation IM14. Ether containing side chains are indicated by splitting the chain in alkyl units with the symbol “O” for the oxygen in between (e.g. IM11O2 for 1-(ethoxymethyl)-3-methylimidazolium). Terminal hydroxy or nitrile groups are shortened as OH (e.g. IM14OH is 1-(4-hydroxybutyl)-3-methyl-imidazolium) or CN (e.g. IM11CN is 1-cyanomethyl-3-methylimidazolium, see also Table 3). The acronyms used for the halides are as in the periodic table.The identifiers for the cation and for the anion separated by a white space represent the complete acronym for an ionic liquid.
Statistics and effect data modelling
All enzyme inhibition experiments were carried out at least in triplicates, with three replicates in each. The normalised (0 to 100% enzyme activity) concentration response curves were fitted to the multinominal data with the R language and the environment for statistic computing using the probit model for the relation of enzyme activity to the decadic logarithm of the tested concentrations.29 Confidence intervals (α = 0.05) of the calculated IC50 values and the linear regression parameters of the logarithm of the IC50 values versus the logarithm of the lipophilicity parameter k0 were calculated with the R language as well.Results
The data for the tested anion species are summarised in Table 1. All calculated IC50 values for the ionic liquids and the corresponding confidence intervals are presented in Table 2. In the following subsections the results obtained for the influence of anions, head groups, side chains and regioselective effects on the enzyme inhibitory potential of ionic liquids are systematically presented.A summary of all acetylcholinesterase inhibition data of ionic liquids generated in our test battery has recently been published in Ranke et al.30 All relevant results discussed in the following sections are summarised in Table 2.
Influence of the anion species
For the vast majority of the tested anion species, no influence on the activity of the acetylcholinesterase was detectable up to the highest tested concentration range of 1000 µM. Only for fluoride and the fluoride containing hexafluoroantimonate and hexafluorophosphate, was a significant inhibition found, with IC50 values of 575 µM (F–), 219 µM (SbF6–) and 145 µM (PF6–), respectively (Table 1). The concentration response curves demonstrate the range of the inhibitory potential of the three substances, with the PF6– anion acting as the strongest inhibitor and the fluoride ion at the upper end of the scale (Fig. 3).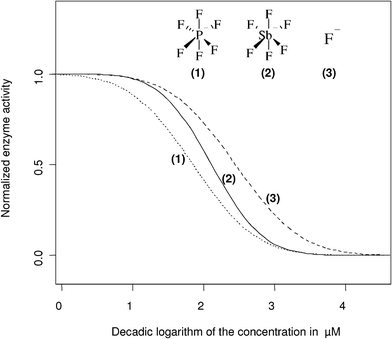 | ||
| Fig. 3 The fitted concentration response curves for the fluoride and two fluoride containing anion species exhibiting an inhibitory effect on the acetylcholinesterase are shown. The exact log IC50 (µM) values and the corresponding 95% confidence intervals are given in Table 1. | ||
Additionally, the 1-dodecylsulfate anion was found to be a weak (IC50 = 912 µM) inhibitor of the enzymes activity. Since all cationic head groups discussed in the following sections were exclusively tested with either halides (chloride, bromide or iodide) or with the non-inhibiting tetrafluoroborate as counterions, it was concluded that all observed inhibitory effects on the enzyme can be exclusively attributed to the cationic moiety.
Influence of the head group
In general, it could be shown that all investigated cationic head groups containing the butyl side chain affected the activity of electric eel acetylcholinesterase in an inhibitory manner. Furthermore, the two ammonium based cations tetraethylammonium and decylbenzyldimethylammonium were found to inhibit the enzyme’s activity (Table 2). To investigate this influence of the cations on the enzyme inhibitory potential of ionic liquids in more detail, a subset of different core structures (Fig. 4) was combined, all carrying the butyl side chain as a reference standard.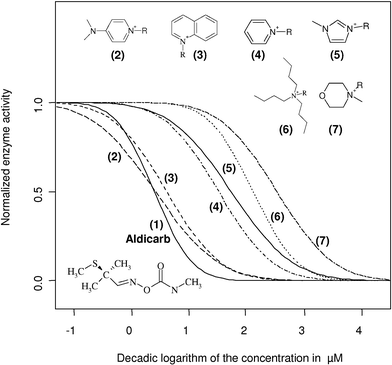 | ||
| Fig. 4 To demonstrate that the cationic head group acts as a key structural element in the interaction with the active site of the acetylcholinesterase, the fitted concentration response curves of some butyl (“R” = C4H9) cations are presented. For comparison, the concentration response curve for the strong carbamatype acetylcholinesterase inhibitor Aldicarb® (2-methyl-2-(methylthio)propionaldehyde-O-methylcarbamoyloxime) was added. As counterions for the cationic head groups, the halides chloride, bromide and iodide were used. The exact log IC50 (µM) data with the corresponding confidence intervals are listed in Table 2. | ||
Comparing all these tested butyl-containing head groups, the range of the measured IC50 values spans nearly three orders of magnitude (Fig. 4), within which the most striking inhibitory effect could be detected for the N-dimethylamminopyridinium and the quinolinium head groups, where IC50 values of 4 µM and 6 µM, respectively, were calculated from the concentration response data. These values are in the same range as the IC50 of the strong and specific carbamate-type acetylcholinesterase inhibitor Aldicarb® (IC50 = 5 µM), which was used as a positive standard in our assay. Thus, the N-dimethylamminopyridinium and the quinolinium moiety exhibited an inhibitory potential one or even two orders of magnitude higher than that for all other tested butyl-containing ionic liquid head groups in this study.
Looking at the other end of the scale, one could find that the polar and non-aromatic morpholinium head group, as well as the sterically bulky tetrabutylammonium cation, exhibited the lowest inhibitory potential to the enzyme, corresponding to IC50 values of 513 µM and 197 µM, respectively.
Grouping the remaining cations into that range of enzyme inhibition potential, one could find the aromatic pyridinium and imidazolium head groups, as well as the heterocyclic but non-aromatic piperidinium and pyrollidinium moieties, to show lower IC50 values lying closely together in the range from 50 µM (“Py”) to 83 µM (“Pyr”). The butylethyldimethylammonium head group, which is structurally closely related to the quaternary ammonium moiety in the natural substrate acetylcholine (see Fig. 1), exhibited a middle inhibitory potential (IC50 = 115 µM) ranging significantly lower than those of the pyridinium (IC50 = 50 µM) and imidazolium (IC50 = 82 µM) cations. Looking at the solely tested phosphonium based head group, one could observe that the tetrabutylphosphonium cation was significantly less active, with an IC50 of 411 µM, than its structural quaternary nitrogen analogue, the tetrabutylammonium head group (Table 2).
Comparing the tetraethylammonium cation (IC50 = 637 µM) with the imidazolium, pyridinium and dimethylaminopyridinium head groups carrying the ethyl side chain (see Table 2), it is noticeable that the relatively small non-cyclic quaternary ammonium cation exhibited a very weak inhibitory potential towards the acetylcholinesterase.
In contrast, the decylbenzyldimethylammonium cation (IC50 = 5 µM) exhibited a slightly stronger effect on the enzyme activity, compared to the aromatic imidazolium cation IM1-10 (IC50 = 12 µM).
Influence of the side chain
When first looking at the alkyl side chains, the previously reported side chain effect could generally be confirmed for the imidazolium cations (IM12–IM1-18) and for the pyridinium moiety (Py2–Py8, see Table 2). Remarkably, the butyl side chain seemed to represent a local minimum in the series of the IC50 values. The side chain effect for the imidazolium cations is analysed in more detail in the following.Furthermore, it could be demonstrated that for the strong inhibiting dimethylaminopyridinium (Py2-4NMe2–Py6-4NMe2) and quinolinium (Quin4–Quin8) head groups, the side chain effect was only marginal compared to the less inhibitory imidazolium (IM12–IM18) and pyridinium (Py2–Py8, see Table 2) cations. This means that the strong effect on the enzyme activity for the dimethylaminopyridinium and the quinolinium cations is mainly dominated by the cationic core structure, whereas for the less active head groups the lipophilicity of the side chain is the dominating factor mediating the inhibitory potential.
Focusing now on the influence of functionalised side chains on the enzyme activity, the results for the ethyl, propyl, butyl and pentyl side chains are presented together with their structurally related (with respect to the chain length) terminal hydroxy, ether and nitrile analogues (Table 3).
In general, one could find a consistent pattern in which the more polar functionalised side chains exhibited a lower inhibitory potential than their lipophilic alkyl references. The hydroxy-functionalised side chains, which provide a donor and an acceptor potential for hydrogen bonding—and thus are the most polar derivatives tested—showed the weakest inhibitory potential compared to the less polar (only hydrogen bonding acceptor potential) ether analogues. The short and polar nitrile side chain showed for all tested head groups, relatively high IC50 values comparable to those for the other polar oxygen containing ether and hydroxy residues.
For the imidazolium head group connected to a four atom containing side chain, it could be shown that the introduction of a hydroxy function into the alkyl side chain was able to shift the IC50 one order of magnitude to the side of lower enzyme inhibition. The ether-containing side chains are lying in between the highly polar hydroxy side chain and the butyl reference and no significant regioselective effect with respect to the position of the ether bridge was observable (Fig. 5).
| R | IC50 AchE/µM | |||||
|---|---|---|---|---|---|---|
| Structure | Abbreviations |
|
|
|
|
|
| “Py” | “IM1” | “Mor1” | “Pip1” | “Pyr1” | ||
| –C2H5 | 2 | 125 | 115 | |||
| –C3H7 | 3 | 164 | 185 | |||
| –CH2CH2–OH | 2OH | 913 | 919 | 221 | 430 | |
| –CH2CN | 1CN | 776 | >1000 | 267 | 767 | |
| –C4H9 | 4 | 82 | 513 | 68 | 83 | |
| –CH2CH2CH2–OH | 3OH | 990 | >1000 | 342 | 731 | |
| –CH2–O–CH2CH3 | 1O2 | 407 | 920 | 139 | 72 | |
| –CH2CH2–O–CH3 | 2O1 | 379 | 999 | 114 | 239 | |
| –C5H11 | 5 | 92 | ||||
| –CH2CH2–O–CH2CH3 | 2O2 | 187 | >1000 | 401 | 400 | |
| –CH2CH2CH2–O–CH3 | 3O1 | 405 | >1000 | 158 | 545 | |
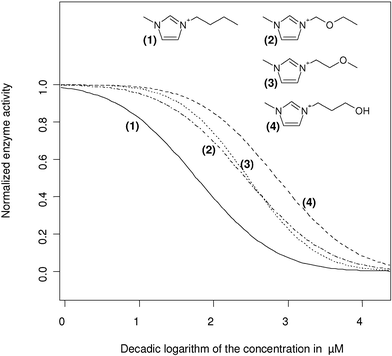 | ||
| Fig. 5 Fitted concentration response curves for 1-butyl-3-methylimidazolium and three 3-methylimidazolium headgroups containing functionalised butyl side chain analogues (ether (2),(3) and hydroxy (4) functions). The chloride serves as a counterion for all shown cations. For the exact IC50 values in µM, see Table 3. | ||
The above presented results for the alkyl and functionalised side chains reinforce the assumption that the lipophilicity of the side chain for one cationic head group is a key parameter in predicting the acetylcholinesterase inhibitory potential of the corresponding ionic liquid when looking at a relatively weak inhibiting head group (e.g. imidazolium, pyrrolidinium or morpholinium). With respect to this, for a series of imidazolium head groups connected to different alkyl and functionalised side chains, a quantitative structure activity relationship was derived by the linear regression of the log IC50 values versus the logarithm of the HPLC-derived lipophilicity parameter k0 of the ionic liquids cations (Fig. 6). A good correlation (r2 = 0.79) with three outliers could be found for a decrease in the IC50 values, with increasing lipophilicity of the side chain.
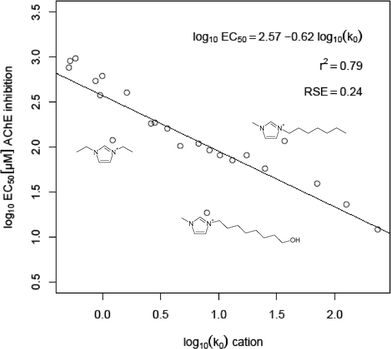 | ||
| Fig. 6 Quantitative structure activity relationship between the decadic logarithm of the lipophilicity parameter k0 and the log IC50 values (µM) of a series of imidazolium headgroups (see Table 2). The three prominent outliers are indicated by their structural formula. The calculated regression function is specified together with the corresponding quadratic correlation coefficient (r2) and the residual standard error (RSE). | ||
Regioselective and general structural considerations
To demonstrate the strong influence a simple structural alteration in a chemical entity can exert on the interaction with a biomolecule, the IC50 values for a series of 1-butylmethylpyridinium cations are presented (Table 4a). The introduction of a methyl substituent at the aromatic core structure significantly increased the inhibitory potential of the 1-butylpyridinium cation. Additionally, it could be observed that the IC50 values decreased when going from the linear 1-butyl-4-methylpyridinium to the angled 1-butyl-2-methylpyridinium configuration, showing more structural analogy to the natural substrate acetylcholine. Looking at the toxicity range of the substituted pyridinium type ionic liquids, one could state that they all exhibited a rather high acetylcholinesterase inhibitory potential with a subtle regioselective influence.However, comparing the observed IC50 values for the positively charged aromatic 1-methyl-3-octylimidazolium, the uncharged (under the assay conditions) 1-octylimidazol and the aromatic, electron-deficient and highly reactive 4,5-dichloro-2-octylisothiazol-3-one, the necessity of certain molecular interaction potentials for the binding to the active site of acetylcholinesterase becomes obvious (Table 4b). Neither the structurally closely related 1-octylimidazol, nor the also octyl-substituted isothiazol-3-one structure exhibited any inhibitory effect on AchE up to the highest concentration tested. Thus, for a strong specific interaction of a compound with the active site of acetylcholinesterase, the positively charged nitrogen atom could be identified to act as the key molecular interaction potential. The lipophilicity of the side chain and the presence or absence of an aromatic ring system modulated the strength of the resulting inhibitory potential.
| (a) To demonstrate regioselective impacts on the enzyme inhibitory potential, three methyl substituted 1-butylpyridinium cations with their corresponding IC50 values in µM are presented. The unsubstituted 1-butylpyridinium serves as reference. The 95% confidence intervals are given in parenthesis and for all cations the chloride serves as the counterion | ||
|---|---|---|
| Structure | Name | IC50 AchE/µM |
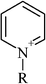 | 1-Butylpyridinium | 50.0 (48.3–51.5) |
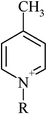 | 1-Butyl-4-methylpyridinium | 27.4 (26.3–28.6) |
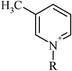 | 1-Butyl-3-methylpyridinium | 14.1 (13.5–14.8) |
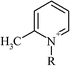 | 1-Butyl-2-methylpyridinium | 5.1 (4.55–5.59) |
| (b) To show the necessity of certain molecular interaction potentials of a substance to interfere with the active site of the AchE, the potent inhibitor 1-methyl-3-octylimidazolium chloride is compared with its mainly uncharged (pKs ∼ 6 and pH 8.0 of the test buffer) analogue 1-octylimidazol and with an aromatic, heterocyclic and highly reactive 2-octyl-isothiazol-3-one biocide. The IC50 value in µM of the imidazolium compound is given with the 95% confidence interval in parenthesis. For the two remaining substances, the highest tested concentration in the enzyme inhibition assay is presented | ||
|---|---|---|
| Structure | Name | IC50 AchE/µM |
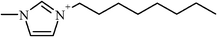 | 1-Methyl-3-octylimidazolium | 39.4 (36.7–42.3) |
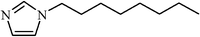 | 1-Octylimidazol | >2000 |
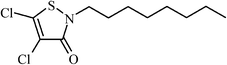 | 4,5-Dichloro-2-octylisothiazol-3-one | >1000 |
Discussion
It is the aim of this study to get a deeper insight into the (eco)toxicological impacts of structural variations in ionic liquid substructural elements, built up by the positively charged head group, substituted with one or more different side chains, and the corresponding anionic species, in an (eco)toxicologically relevant molecular test system.To discuss the above presented results, the catalytic cycle of the enzyme and the essential amino acid residues involved in substrate binding are presented. The active site of acetylcholinesterase is located at the bottom of a narrow gorge. The gorge is lined with lipophilic aromatic amino acid residues and the entrance is built up with negatively charged residues. The active centre can be divided into the catalytic esteratic site where the acetyl group of the substrate is bound, and the anionic site where the quaternary ammonium moiety of the acetylcholine is stabilised via a cation–π interaction with the essential tryptophane residue Trp 84.31 Additionally, a peripheral anionic site (PAS) could be identified at the entrance of the narrow gorge, where the substrate acetylcholine is bound to Trp 279, again via cation–π interactions, and is thereby orientated towards the active centre.32 The catalytic cycle of the enzyme can be described in three steps. At first, the substrate is attracted by the negative potential surrounding the entrance of the gorge and binds to Trp 279. The so orientated substrate molecule is subsequently transferred through the lipophilic gorge and bound to the active centre with the positively charged nitrogen moiety interacting with the Trp 84 and the acetyl group lying at the esteratic site. The ester bond is hydrolysed and the resulting choline moiety leaves the catalytic site via the gorge. In the last step, a water molecule regenerates the acetylated enzyme and the acetate anion is expelled via the channel formed by the lipophilic gorge.33
Thus, competitive inhibitors of acetylcholinesterase can act via two distinct mechanisms. They can either bind directly to the active site and thereby inhibit the cleavage of the natural substrate or inhibitors can bind to the PAS and block substrate traffic into and out of the catalytic centre by steric interference or allosteric alteration of the enzymes active centre.32
With respect to this, the identified molecular interaction potentials found for the inhibiting ionic liquids can be interpreted. The cationic head groups are attracted by the negative surface potential of the enzyme and bind via the positively charged nitrogen atom, in a competitive manner, to the essential tryptophane residues at the catalytic site or the PAS. Especially, the quinolinium and dimethylaminopyridinium head groups can bind strongly via π–π interactions to the Trp 279 at the PAS, due to their large aromatic systems. The natural substrate acetylcholine or the acetylthiocholine used in our assay are only weakly bound to the PAS, which explains the strong inhibiting effect of these two aromatic ionic liquid head groups. The aromatic stacking interactions for the quinolinium and the dimethylaminopyridinium head groups are that strong that the side chain effect is negligible.
For the remaining aromatic head groups—the pyridinium and imidazolium cations—the side chain enforces the weaker π–π interactions of the smaller aromatic systems by lipophilic interactions with the amino acid residues lining the narrow gorge. Thus, the correlation of increasing side chain lipophilicity and decreasing IC50 values for the different head groups can be explained and even quantified in a QSAR correlation in the case of the imidazolium cations. The most prominent outlier in the linear regression, the IM18OH, may be explained by an additional interaction at an allosteric subsite, far away from the active site.
The morpholinium head group is lacking the aromatic π–π interaction potential and provides the lowest lipophilic interaction potential compared to all other tested cations and therefore exhibits the observed low inhibitory potential. The introduced free electron pairs at the oxygen atom of the morpholinium head group are only weak donors or acceptors for π–π interactions. It is more likely that they are involved in strong hydrogen bonding interactions to water molecules, making the morpholinium head group even more hydrophilic.
The quaternary ammonium head groups, as well as the remaining heterocyclic cations, are also able to bind only via cation–π and lipohilic interactions to the tryptophane residues and the aromatic gorge. The significance of a π–π interaction for a tight binding to the enzyme can also be demonstrated when comparing the non-aromatic quaternary ammonium compound decylbenzyldimethylammonium with the 1-decyl-3-methylimidazolium cation. The benzyl residue connected to the ammonium moiety interacts slightly stronger with the tryptophane ring system than the smaller aromatic imidazolium system.
The tested phosphonium head group is more bulky and subsequently the positive charge density is decreased compared to its nitrogen containing analogue. Thus, the interactions with the tryptophane residues in the enzyme are smaller, resulting in a higher IC50 for the tetrabutylphosphonium cation.
The local maximum in the inhibitory potential for the butyl side chain observed within the series of imidazolium and pyridinium ionic liquids may be explained by a strong interaction at the catalytic site in addition to the binding at the PAS. The butyl side chain is short enough to fit in the active centre, whereas the longer side chains only allow for binding at the PAS.
Additionally, the observed regioselective effects can be interpreted by the fact that the 1-butylpyridinium compounds can bind directly at the active site. The angled configuration is able to interact stronger with the Trp 84 than the more stretched isomers, due to its structural homology to the choline moiety. This angled configuration allows for an optimised orientation of the 2-methylpyridinium moiety towards the anionic subside in the active centre of the AchE.
Considering the negative surface potential at the entrance to the catalytic centre, one would expect all anion species to exert no effect on the enzyme activity. Our results confirm this presumption, with the only exception of the fluoride anion and the fluoride containing SbF6– and PF6– species. Both species are known to readily undergo hydrolysis in aqueous media34–36 and thus the fluoride seems to be the active compound and acetylcholinesterase inhibition by F– has already been described in the literature.37,38 The relatively low IC50 values for the SbF6– and PF6– anions compared to the fluoride are due to the fact that per mole SbF6– or PF6–, six moles of fluoride may theoretically be released.
The very weak inhibitory potential observed for the 1-dodecylsulfate anion is presumably due to non-specific detergent like inactivation of the acetylcholinesterase.
Conclusion
Using different testkits of anion species, cationic head groups and functionalised side chains connected to these head groups, we were able to identify three molecular key interaction potentials for the inhibitory effect of a broad variety of ionic liquid species on the enzyme acetylcholinesterase. Considering these interaction potentials and the molecular interaction potentials provided by the catalytic centre of the enzyme, the observed structure activity relationships of the tested substances can qualitatively and quantitatively be described. Thus, the applied enzyme inhibition screening assay with the electric eel acetylcholinesterase seems to be a valid and useful tool in a flexible (eco)toxicological test battery to analyse the impact of structural elements on the toxic mode of action of chemical substances. This implies that the AchE inhibition assay can be used to identify toxicophore substructures in a chemical entity, and thereby is able to support the design of new inherently safer and hence sustainable chemical products.In detail, for the ionic liquids the positively charged nitrogen atom, a broad delocalised aromatic ring system and a certain lipophilicity could be shown to be the mediators for the acetylcholinesterase inhibition potential. With respect to this, the dimethylaminopyridinium and the quinolinium head groups were identified to be very strong inhibitors of the enzyme. This trend has also been recently described for the cytotoxicity of these two head groups6,14 and thus the dimethylaminopyridinium and the quinolinium cations should be avoided when aiming at the design of non-toxic ionic liquids. The results obtained for the pyridinium and methylated pyridinium head groups confirm our previous results,28 marking these cations also as strong AchE inhibitors. In contrast, the morpholinium head group was found to be only weakly inhibiting or even inactive, depending on the connected side chain. Again, this is well in accordance with recently published cytotoxicity data generated in our flexible test battery.14
Furthermore, the well known side chain effect28 is confirmed for the imidazolium and pyridinium cations and could even be described by a QSAR correlation for a series of imidazolium ionic liquids. Additionally, the lipophilicity of the side chain was identified to be a potent structural element to alter the enzyme inhibitory potential of a broad spectrum of ionic liquid head groups. For example, the IC50 of IM14 is decreased one order of magnitude by the introduction of a hydroxy function into the side chain, resulting in the IM13OH cation.
Since the vast majority of the tested anion species exhibited no inhibiting effect to the enzyme, this structural element can be used to tune and improve the technicophore properties of the ionic liquids. Only fluoride or fluoride containing anions, which readily undergo hydrolytic cleavage, should be avoided.
Putting together our results, we have found a set of structural elements which allows for the rough and fine tuning of the molecular toxicity towards the electric eel acetylcholinesterase. The inherent head group effect can be modulated to lower inhibitory potentials by choosing polar, non-aromatic head groups or incorporating polar hydroxy, ether or nitrile functions into the side chains connected to the cationic core structure. The anion species represents the most promising structural element to tune the technical properties of the ionic liquids because a big pool of different anions (inorganic, organic and complex borate species) was shown to be inactive in the AchE inhibition assay.
However, it should be mentioned that the design of inherently safer chemical products with optimised technological and economical features is not an easy task and often leads to goal conflicts. These conflicts can only be overcome in close cooperation between industry and academic research groups.
Experimental
Chemicals
All tested ionic liquids, the 1-octylimidazol and the sodium or lithium salts of the investigated anion species were received by the Merck KGaA (Darmstadt, Germany), with the exception of 1-octyl-quinolinium bromide, which was prepared at the ITUC in Jena, Germany.The 4,5-dichloro-2-octylisothiazol-3-one was donated by Rohm and Haas (Philadelphia, USA). Stock solutions of all test substances were prepared in methanol or dimethylsulfoxide, depending on their solubility.
2-methyl-2-(methylthio)propionaldehyde-O-methylcarbamoyloxime (Aldicarb®), acetic acid, acetonitrile, methanol and dimethylsulfoxide, as well as bovine serum albumin, and sodium hydrogen phosphate, were purchased from the Sigma–Aldrich Cooperation (Steinheim, Germany).
Sodium hydrogen carbonate was purchased from GIBCO BRL Life technologies (Eggenstein, Germany) and acetylthiocholine iodide was provided by Fluka (Buchs, Switzerland).
Acetylcholinesterase (AchE, EC 3.1.1.7) from the electric organ of the electric eel (Electrophorus electricus) type VI-S was purchased from Sigma–Aldrich (Steinheim, Germany). The activity was determined to be 463 U mg protein–1.
Acetylcholinesterase inhibition assay
The inhibition of the acetylcholinesterase was measured using a colorimetric assay based on the reduction of the dye 5,5′-dithio-bis-(2-nitrobenzoic acid) (DTNB) by the enzymatically formed thiocholine moiety from the AchE substrate acetylthiocholine iodide. The assay is described in detail in Stock et al.28 Briefly, a dilution series of the test substances in phosphate buffer (0.02 M, pH 8.0) containing max. 1% methanol was prepared directly in the wells of a 96-well microtiter plate. DTNB (2 mM, 0.185 mg mL–1 NaHCO3 in phosphate buffer pH 8.0) and the enzyme (0.2 U mL–1, 0.25 mg mL–1 bovine serum albumin in phosphate buffer pH 8.0) were added to each well. The reaction was started by the addition of acetylthiocholine iodide (2 mM in phosphate buffer). The final test concentrations were 0.5 mM of DTNB and acetylthiocholine iodide, and 0.05 U mL–1 acetylcholinesterase, respectively.Enzyme kinetics were measured at 405 nm in 30 s intervals in a microplate-reader (MRX Dynatech) for a time period of 5 min. The enzyme activity was expressed as OD min–1 from a linear regression. To avoid false positive results in preliminary tests, it was shown that none of the test substances interacts with the formed thiocholine during the assay (data not shown).
Determination of the lipophilicity parameter k0
The lipophilicity parameter k0 of the ionic liquids was derived using a gradient run HPLC method. The method, the theoretical background and the calculation of the log ko values were recently described in Ranke et al.6 Briefly, the HPLC system used for deriving the lipophilicity parameters was a Hewlett Packard system Series 1100, with gradient pump, online degasser, autosampler and a Bruker esquire ESI-MS ion trap detector. The column used was a MetaChem Polaris Ether bridged RP-18 column with 150 mm length, 3 mm inner diameter and 3 µm particle size. A guard column with octadecylsilica material was also used (both Varian, Inc.). The eluent was composed of 0.25% acetic acid (p.a.) in Milipore (TM) water (pH = 3.2), mixed with gradient grade acetonitrile. The column dead time to was calculated from retention time difference of thiourea with and without column. The equipment dwell volume tD was quantified by switching from water to 0.1 mM NaNO3 in 10 min. Cation retention times from a single gradient run with a gradient time tG of 10 min were obtained for all substances listed in Table 2.Acknowledgements
The authors gratefully thank Marianne Matzke, Tanja Juffernholz and Karen Thiele for helpful discussions. Furthermore, thanks are given to the Merck KgaA for their cooperation in a strategic partnership. Annegret Stark is acknowledged for providing the 1-octyl-quinolinium bromide as well as the Rohm and Haas company for donating the 4,5-dichloro-2-octylisothiazol-3-one.The first author gratefully thanks the Deutsche Bundesstiftung Umwelt for funding the work with a PhD scholarship.
References
- http://ec.europa.eu/environment/chemicals/reach/reach_intro.htm, 2007
.
- B. Jastorff, R. Störmann and U. Wölke, Struktur-Wirkungs-Denken in der Chemie, Universitätsverlag Aschenbeck & Isensee, Bremen, Oldenburg, 2004, pp. 15–150 Search PubMed
.
- B. Jastorff, K. Mölter, P. Behrend, U. Bottin-Weber, J. Filser, A. Heimers, B. Ondruschka, J. Ranke, M. Schaefer, H. Schröder, A. Stark, P. Stepnowski, F. Stock, R. Störmann, S. Stolte, U. Welz-Biermann, S. Ziegert and J. Thöming, Green Chem., 2005, 7, 362–372 RSC
.
- B. Jastorff, R. Störmann, J. Ranke, K. Molter, F. Stock, B. Oberheitmann, W. Hoffmann, J. Hoffmann, M. Nuchter, B. Ondruschka and J. Filser, Green Chem., 2003, 5(2), 136–142 RSC
.
- M. Matzke, S. Stolte, K. Thiele, T. Juffernholz, J. Ranke, U. Welz-Biermann and B. Jastorff, Green Chem., 2007 10.1039/b705795d
.
- J. Ranke, F. Stock, A. Müller, S. Stolte, R. Störman, U. Bottin-Weber and B. Jastorff, Ecotoxicol. Environ. Saf., 2006, 107, 2183–2206
.
- J. L. Anderson, D. W. Armstrong and G. T. Wei, Anal. Chem., 2006, 78(9), 2892–2902 CrossRef
.
- F. Endres, Eur.J. Chem. Phys. Phys. Chem., 2002, 3(2), 144–154 Search PubMed
.
- W. M. Liu, C. F. Ye, Q. Y. Gong, H. Z. Wang and P. Wang, Tribol. Lett., 2002, 13(2), 81–85 CrossRef CAS
.
- T. Welton, Coord. Chem. Rev., 2004, 248(21–24), 2459–2477 CrossRef CAS
.
- H. Zhang, J. Wu, J. Zhang and J. S. He, Macromolecules, 2005, 38(20), 8272–8277 CrossRef CAS
.
- H. Zhao, J. Mol. Cat. B: Enzym., 2005, 37, 16–25 Search PubMed
.
- J. Ranke, M. Cox, A. Müller, C. Schmidt and D. Beyersmann, Toxicol. Environ. Chem., 2006, 88(2), 273–285 CrossRef CAS
.
- S. Stolte, J. Arning, U. Bottin-Weber, A. Müller, W. R. Pitner, U. Welz-Biermann, B. Jastorff and J. Ranke, Green Chem., 2007, 9(8), 760–767 RSC
.
- S. Stolte, M. Matzke, J. Arning, A. Böschen, W. R. Pitner, U. Welz-Biermann, B. Jastorff and J. Ranke, Green Chem., 2007 10.1039/b711119c
.
- S. Stolte, J. Arning, U. Bottin-Weber, M. Matzke, F. Stock, K. Thiele, M. Uerdingen, U. Welz-Biermann, B. Jastorff and J. Ranke, Green Chem., 2006, 8(7), 621–629 RSC
.
- M. H. Fulton and P. B. Key, Environ. Toxicol. Chem., 2001, 20(1), 37–45 CAS
.
- J. Massoulie, L. Pezzementi, S. Bon, E. Krejci and F. M. Vallette, Prog. Neurobiol., 1993, 41(1), 31–91 CrossRef CAS
.
- J. M. Chemnitius, R. Sadowski, H. Winkel and R. Zech, Chem.-Biol. Interact., 1999, 120, 183–192 CrossRef
.
- C. Pope, S. Karanth and J. Liu, Environ. Toxicol. Pharmacol., 2005, 19(3), 433–446 CrossRef CAS
.
- K. J. Eder, C. M. Leutenegger, B. W. Wilson and I. Werner, Mar. Environ. Res., 2004, 58(2–5), 809–813 CrossRef CAS
.
- C. J. Rickwood and T. S. Galloway, Aquat. Toxicol., 2004, 67(1), 45–56 CrossRef CAS
.
- J. Keizer, G. Dagostino, R. Nagel, T. Volpe, P. Gnemi and L. Vittozzi, Sci. Total Environ., 1995, 171(1–3), 213–220 CrossRef CAS
.
- J. Kaur and M. Q. Zhang, Curr. Med. Chem., 2000, 7(3), 273–294 CAS
.
- M. Harel, D. M. Quinn, H. K. Nair, I. Silman and J. L. Sussman, J. Am. Chem. Soc., 1996, 118(10), 2340–2346 CrossRef CAS
.
- M. Harel, J. L. Hyatt, B. Brumshtein, C. L. Morton, R. M. Wadkins, I. Silman, J. L. Sussman and P. M. Potter, Chem.-Biol. Interact., 2005, 157–158, 153–157 CrossRef CAS
.
- J. L. Sussman, M. Harel, F. Frolow, C. Oefner, A. Goldman, L. Toker and I. Silman, Science, 1991, 253(5022), 872–879 CrossRef CAS
.
- F. Stock, J. Hoffmann, J. Ranke, R. Stormann, B. Ondruschka and B. Jastorff, Green Chem., 2004, 6(6), 286–290 RSC
.
- J. Ranke, R News, 2006, 3, 7–12 Search PubMed
.
- J. Ranke, S. Stolte, R. Störmann, J. Arning and B. Jastorff, Chem. Rev., 2007, 107(6), 2183–2206 CrossRef CAS
.
- M. Harel, I. Schalk, L. Ehret-Sabatier, F. Bouet, M. Goeldner, C. Hirth, P. H. Axelsen, I. Silman and J. L. Sussman, Proc. Natl. Acad. Sci. U. S. A., 1993, 90(19), 9031–9035 CAS
.
- Y. Bourne, P. Taylor, Z. Radic and P. Marchot, EMBO J., 2003, 22(1), 1–12 CrossRef
.
- J. P. Colletier, D. Fournier, H. M. Greenblatt, J. Stojan, J. L. Sussman, G. Zaccai, I. Silman and M. Weik, EMBO J., 2006, 25(12), 2746–2756 CrossRef CAS
.
- A. V. Plakhotnyk, L. Ernst and R. Schmutzler, J. Fluorine Chem., 2005, 126(1), 27–31 CrossRef CAS
.
- M. Ponikvar, B. Zemva and J. F. Liebman, J. Fluorine Chem., 2003, 123(2), 217–220 CrossRef CAS
.
- R. P. Swatloski, J. D. Holbrey and R. D. Rogers, Green Chem., 2003, 5(4), 361–363 RSC
.
- E. Heilbron, Acta Chem., Scand., 1965, 19(6), 1333–1346 Search PubMed
.
- R. M. Krupka, Mol. Pharmacol., 1966, 2(6), 558–569 CAS
.
| This journal is © The Royal Society of Chemistry 2008 |