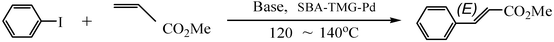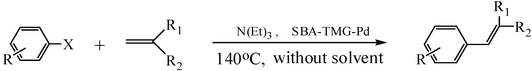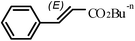Solvent-free Heck reaction catalyzed by a recyclable Pd catalyst supported on SBA-15 via an ionic liquid
Xiumin
Ma
,
Yinxi
Zhou
,
Jicheng
Zhang
,
Anlian
Zhu
,
Tao
Jiang
* and
Buxing
Han
*
Beijing National Laboratory for Molecular Sciences, Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100080, China. E-mail: Jiangt@iccas.ac.cn; Hanbx@iccas.ac.cn; Fax: +86-10-62562821
First published on 19th October 2007
Abstract
Heck arylation of olefins with aryl halides was carried out in solvent-free conditions with a Pd catalyst supported on 1,1,3,3-tetramethylguanidinium (TMG)-modified molecular sieve SBA-15 (designated as SBA-TMG-Pd). SBA-TMG-Pd was much more active and stable than a Pd catalyst supported on pristine SBA-15 (designated as SBA-Pd). The catalysts were characterized by Fourier transform infrared spectroscopy (FT-IR), X-ray photoelectron spectroscopy (XPS), and transmission electron microscopy (TEM), and the reasons for the excellent performance of catalyst SBA-TMG-Pd were also discussed.
Introduction
The palladium-catalyzed coupling of olefins with aryl or vinyl halides, known as the Heck reaction, is one of the most powerful methods to form a new carbon–carbon (Csp2–Csp2) bond in modern synthetic chemistry. The Heck reaction provides a direct route to assemble important arylated and vinylated olefins, and its wide functional group tolerance on both reactants allows the convenient application at a late stage of total synthesis without protecting groups. Since its discovery1 by Heck and Mizoroki et al. in the late 1960s, the reaction has been extensively studied2 and applied to a wide variety of fields, such as natural product synthesis,3 material science,4 and bioorganic chemistry.5 A major transformation in the Heck reaction is the synthesis of cinnamic acid and its derivatives, which are important intermediates in the synthesis of medical products and commonly used as flavor substances and UV absorbents. The reaction is usually catalyzed by palladium complexes in a homogeneous mode of operation and shares common drawbacks, i.e., catalyst recovery and recycling is difficult. In addition, expensive, generally unstable and toxic ligands, such as phosphine, are required to activate and stabilize Pd against agglomeration and formation of Pd black.6 Therefore, cheaper and environmentally benign heterogeneous catalytic systems are attractive, and notable progress has been made in this area. Palladium supported on a diverse array of organic and inorganic materials, such as polymers,7carbon,8 metal oxides,9 clay,10 ordered11 or amorphous silicates,12 and zeolites,13 have been developed and used to catalyze the Heck reaction.Mesoporous SiO2 are attractive supports due to their advantageous properties, such as excellent chemical and thermal stability, high porosity, large surface area, and high surface concentration of silanols. Ying et al.11d reported Pd-grafted molecular sieves MCM-41 that effectively catalyzed heterogeneous Heck reactions. More recently, Crudden et al.11c demonstrated that thiol-modified mesoporous materials (SBA-15-SH) are excellent scavengers for Pd and the Mizoroki–Heck reaction was successfully catalyzed without leaching Pd into the solution.
In recent years, ionic liquids (ILs) have attracted much attention due to their special properties, such as negligible vapor pressure, wide liquid range, excellent chemical stability, high thermal stability, and the strong solvent power for a wide range of organic, inorganic, and polymeric molecules. Many reactions have been carried out in ILs,14 including the Heck reaction in 1,1,3,3-tetramethylguanidinium IL, without using additional base.14c Recently, ILs immobilized onto solid supports have been used to prepare catalysts. For example, 1-butyl-3-methylimidazolium hexafluorophosphate ([bmim][PF6]) and 1-butyl-3-methylimidazolium tetrafluoroborate ([bmim][BF4]) dispersed on silica gel can provide a solvent environment for a Rh complex, resulting in the excellent catalytic activity and stability for hydrogenation.15 Some functional ILs exhibit a strong ability to stabilize nanoparticles. By immobilizing Pd and Ru nanoparticles on different supports with the assistance of 1,1,3,3-tetramethylguanidinium-based IL, different catalysts have been prepared, which also showed outstanding catalytic performance for the hydrogenation of benzene and olefins.16 More recently, a series of very effective supported catalysts for different organic reactions have been prepared using different ILs and solid supports.17
The Heck reaction can be performed in organic solvents, ionic liquids, and CO2. However, less attention has been paid on the Heck reaction in a solvent-free condition,18 although it is environmentally benign and economically profitable. Based on the consideration of minimizing the amount of ILs used, avoiding the use of organic solvents, easy recovery of catalyst, in this work, Pd nanoparticles were immobilized on molecular sieves SBA-15 using IL 1,1,3,3-tetramethylguanidinium lactate (TMGL). The Heck reaction was performed in solvent-free conditions with the immobilized Pd catalyst (designated as SBA-TMG-Pd). It is shown that the catalyst has an excellent activity and reusability for the Heck reaction.
Results and discussion
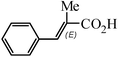
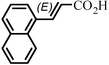
The coupling reaction of iodobenzene with methyl acrylate was used as a model reaction to investigate the catalytic performance of catalyst SBA-TMG-Pd. The influences of bases, solvents, reaction temperature and the catalyst loading on the yield were first investigated. The results are summarized in Table 1. Among the bases examined, Et3N (Table 1, entry 1) showed the best coupling with the catalyst. Other bases, such as CH3COONa (Table 1, entry 2), Na2CO3 (Table 1, entry 3) and NaOH (Table 1, entry 4) gave moderate yields, probably due to the poor solubility of these solid bases in the reaction system. When NaOH (Table 1, entry 4) was used, cinnamic acid was formed. The possible reason is that the product methyl cinnamate reacted with a trace of H2O in the reaction system in the presence of the strong base NaOH. The influence of solvents on the reaction was also investigated (Table 1, entries 5 and 6). It can be seen from Table 1 that the solvent-free condition gave the best result. However, the reaction in N-methylpyrrolidone (NMP), which is one of the commonly used solvents for the Heck reaction, had a lower yield. When H2O was used as the solvent, a high total yield was achieved. However, the main product was cinnamic acid, probably due to the reaction of methyl cinnamate with the reaction medium. Results obtained at other conditions also demonstrated that the catalyst is highly efficient for the Heck reaction. The model reaction proceeded smoothly and the yield of (E)-methyl cinnamate was 93%, even when the amount of Pd was 0.001 mol% (Table 1, entry 7). As expected, when the reaction temperature was reduced from 140 °C to 120 °C, the reaction time was prolonged from 65 min to 3 h to achieve a high yield (Table 1, entry 8).|
|
||||||
|---|---|---|---|---|---|---|
| Entry | Bases | Solvents | Temperature/°C | Pd (mol%) | Reaction time | Isolated yields (%) |
| a The molar ratio of methyl cinnamate to cinnamic acid is 60 : 40; b The molar ratio of methyl cinnamate to cinnamic acid is 38 : 62. | ||||||
| 1 | Et3N | — | 140 | 0.01 | 65 min | 91 |
| 2 | CH3COONa | — | 140 | 0.01 | 65 min | 64 |
| 3 | Na2CO3 | — | 140 | 0.01 | 65 min | 60 |
| 4a | NaOH | — | 140 | 0.01 | 65 min | 68 |
| 5 | Et3N | NMP | 140 | 0.01 | 65 min | 82 |
| 6b | Et3N | H2O | 140 | 0.01 | 65 min | 92 |
| 7 | Et3N | — | 140 | 0.001 | 2 h | 93 |
| 8 | Et3N | — | 120 | 0.01 | 3 h | 92 |
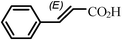
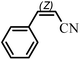
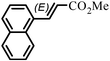
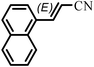
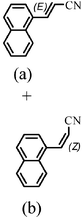
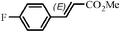
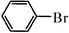
The Heck arylation reaction of a variety of vinylic substrates with different functional groups was also investigated using SBA-TMG-Pd as the catalyst in solvent-free conditions, and the results are illustrated in Table 2. It is known that the catalyst is very effective for the reactions with iodobenzene as the arylating agent. For all the olefins examined, moderate to excellent yields were achieved. When monosubstituted vinylic substrates, such as acrylic acid and different acrylate esters, were employed (Table 2, entries 1, 2, 3 and 4), high yields and only E-isomers were obtained, which was confirmed by 3J (H–H) = 16 Hz. However, when styrene and acrylonitrile were used, except for the main product, a little of 1,1-diphenyl ethylene and Z-isomer were detected, respectively (Table 2, entries 5 and 9). As for the bulky substrates, such as disubstituted olefins (Table 2, entries 6, 7 and 8), the yields were moderate. When 1-iodonaphthalene and 1-fluoro-4-iodobenzene were used as arylating agents (Table 2, entries 10, 11, 12, 13 and 14), the reaction could also proceed smoothly with high yields. For aryl bromides, the catalyst showed lower activity (Table 2, entries 15 and 16).
|
|
|||||
|---|---|---|---|---|---|
| Entry | Aryl halides | Alkenes | Products | Reaction time | Isolated yields (%) (a/b) |
| a Reaction conditions: ArI (2 mmol), alkene (2.2 mmol), Et3N (2.4 mmol), the amount of Pd is (0.01 mol%); b ArI (2 mmol), acrylic acid (2.2 mmol), Et3N (4.4 mmol); c ArI (2 mmol), methacrylic acid (2.2 mmol), Et3N (4.4 mmol); d The amount of Pd is 0.05 mol%; e The amount of Pd is 0.05 mol%. | |||||
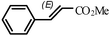
| 1 |
|
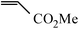
|
|
65 min | 91 |
| 2 |
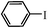
|
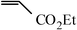
|
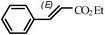
|
1.5 h | 92 |
| 3 |
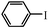
|
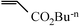
|
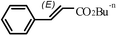
|
2 h | 93 |
| 4b |
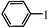
|
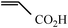
|
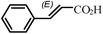
|
45 min | 94 |
| 5 |
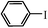
|
|
|
4 h | 90 (95/5) |
| 6c |
|
|
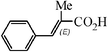
|
1.5 h | 67 |
| 7 |
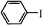
|
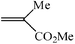
|
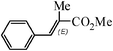
|
3 h | 72 |
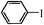
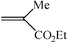
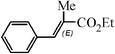
| 8 |
|
|
|
4.5 h | 60 |
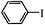
| 9 |
|
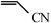
|
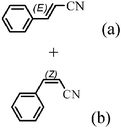
|
1.5 h | 89 (88/12) |
| 10 |
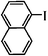
|
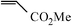
|
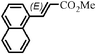
|
3.5 h | 88 |
| 11 |
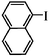
|
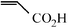
|
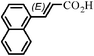
|
1.5 h | 90 |
| 12 |
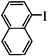
|
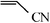
|
|
4.5 h | 85 (80/20) |
| 13 |
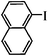
|
|
|
7 h | 92 |
| 14 |
|
|
|
60 min | 92 |
| 15d |
|
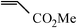
|
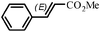
|
15 h | 33 |
| 16e |
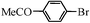
|
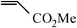
|
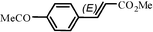
|
8 h | 54 |
Experiments were also conducted to examine the recyclability of SBA-TMG-Pd. As can be seen from Fig. 1, after six repeated catalytic coupling reactions of iodobenzene with methyl acrylate, no obvious deactivation of the catalyst was observed, and the catalyst still remained as a grey powder. For comparison, we examined the reusability of the Pd catalyst supported on SBA-15 in the absence of the IL (designated as SBA-Pd). The results in Fig. 1 indicated that the deactivation of SBA-Pd was obvious. The catalyst was observed to become a white powder after 2 recycles. In other words, SBA-TMG-Pd had a much higher stability, although the catalytic activity of the two catalysts was similar in the first run.
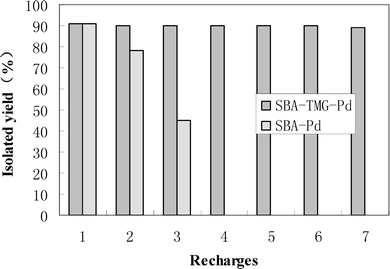 | ||
| Fig. 1 Recycling of SBA-TMG-Pd and SBA-Pd for the Heck reaction of iodobenzene with methyl acrylate at 140 °C with a reaction time of 50 min each time (the amount of Pd is 0.05 mol%). | ||
The catalysts were characterized by X-ray photoelectron spectroscopy (XPS). Fig. 2 shows the Pd 3d spectrum of the catalyst. It can be seen that the deconvoluted spectrum showed a doublet for two chemically different Pd entities, with peak binding energies of 335.0 eV (Pd 3d5/2) and 340.25 eV (Pd 3d3/2), which confirmed the presence of Pd0 in the catalyst. Moreover, the peak at 336.1 eV suggests the presence of a Pd–O component, which probably resulted from the oxidation of the Pd nanoparticles upon exposure to air.
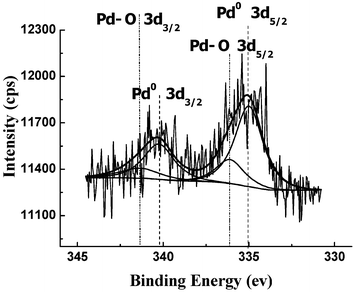 | ||
| Fig. 2 XPS spectrum of the Pd 3d edge of the SBA-TMG-Pd sample. The vertical lines indicate the peak positions of the binding energies of Pd–O, and Pd. | ||
Fig. 3 shows the transmission electron microscope (TEM) images of the two catalysts. For SBA-TMG-Pd (Fig. 3a), most of Pd nanoparticles existed in the channels of SBA-15, and the diameters of Pd nanoparticles were in the range of 3–6 nm before use. However, for the catalyst SBA-Pd (Fig. 3b), the diameters of most Pd nanoparticles were in the range of 9–12 nm, which is bigger than the pore diameter of SBA-15, resulting in their existence on the outside surface of SBA-15. After six recycles, most Pd nanoparticles on SBA-TMG-Pd still existed in the channel of SBA-15, although some bigger Pd particles were formed (Fig. 3c). For SBA-Pd, after 2 recycles, most of Pd disappeared and few Pd nanoparticles existed on the surface of the support, as can be seen from the micrograph (Fig. 3d). The change of color of the two catalysts further confirmed this. The catalyst SBA-TMG-Pd still remained as a grey powder after six recycles. However, SBA-Pd became white powder after 2 recycles.
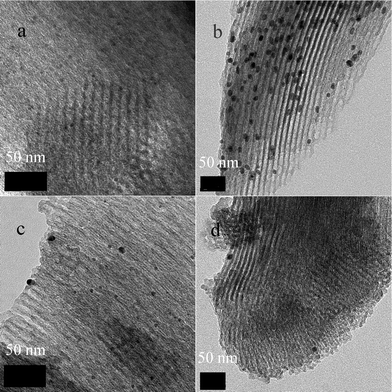 | ||
| Fig. 3 TEM images of the catalysts (a) SBA-TMG-Pd; (b) SBA-Pd; (c) SBA-TMG-Pd after 6 recycles; (d) SBA-Pd after 2 recycles. | ||
The difference of the two catalysts was that ionic liquid TMGL was utilized when preparing SBA-TMG-Pd. The presence of the cation of the ionic liquid in the catalyst SBA-TMG-Pd is supported by the fact that the catalyst contained 10 wt% organic compound, as determined by TGA. The existence of the TMG cations was also supported by the FT-IR spectrum (Fig. 4). The characteristic peaks of CH3group at 1412 cm–1, 1455 cm–1, and 2944 cm–1, and the peak of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond at 1612 cm–1 can be observed in the spectrum of SBA-TMG-Pd. TMG group played an important role in stabilizing the catalyst SBA-TMG-Pd. It is known that guanidine has considerable coordination ability.19 The reason for the better reusability of SBA-TMG-Pd than SBA-Pd was that TMG-modified SBA-15 was a better scavenger for Pd than the pristine SBA-15. After the aryl halides were completely consumed, palladium redeposited on the support with the help of TMG. But for SBA-Pd, most Pd was lost in the reaction solution.
N bond at 1612 cm–1 can be observed in the spectrum of SBA-TMG-Pd. TMG group played an important role in stabilizing the catalyst SBA-TMG-Pd. It is known that guanidine has considerable coordination ability.19 The reason for the better reusability of SBA-TMG-Pd than SBA-Pd was that TMG-modified SBA-15 was a better scavenger for Pd than the pristine SBA-15. After the aryl halides were completely consumed, palladium redeposited on the support with the help of TMG. But for SBA-Pd, most Pd was lost in the reaction solution.
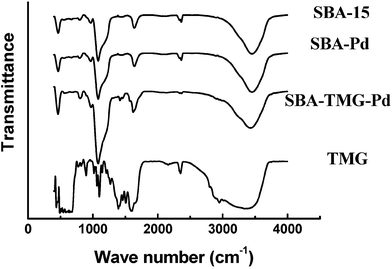 | ||
| Fig. 4 The FT-IR of SBA-15, 1,1,3,3-tetramethylguanidine (TMG) and the as prepared catalysts. | ||
Recently, the mechanism of the catalytic processes of the Heck reaction employing a supported palladium source as the catalyst has been intensely disputed in literature. Some researchers deduced that the catalyst was working in a heterogeneous manner,11b,11c,11d,20 while others reported that the support acted merely as a reservoir for a more active soluble form of Pd.9,21 In order to study the behavior of the Pd in our catalytic system, a filtration test was carried out using Pd-TMG-SBA as the catalyst in this work. After 25 min (the reaction was completed in 65 min), the reaction mixture was taken out from the autoclave. To ensure that all solid catalyst was separated, the reaction mixture was centrifuged at 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 30 min. Then, the solid-free filtrate was allowed to continue to react under the same conditions for another 1 h. The results indicated that the reaction continued and iodobenzene was completely consumed. This suggests that the leaching of active palladium species from the solid support occurred during the reaction. However, Pd redeposited back onto the support after the completion of the reaction.9,20 This argument was supported by the AAS analysis of the reaction samples. After reaction, SBA-TMG-Pd was separated from the ethanol solution of the reaction mixture, and the solution was collected and analysed by AAS method for Pd metal. Only less than 0.3% Pd of the initially added catalyst was detected. From the whole catalytic process, the catalyst apparently behaved just like a heterogeneous catalyst, which could be recovered and reused facilely.
000 rpm for 30 min. Then, the solid-free filtrate was allowed to continue to react under the same conditions for another 1 h. The results indicated that the reaction continued and iodobenzene was completely consumed. This suggests that the leaching of active palladium species from the solid support occurred during the reaction. However, Pd redeposited back onto the support after the completion of the reaction.9,20 This argument was supported by the AAS analysis of the reaction samples. After reaction, SBA-TMG-Pd was separated from the ethanol solution of the reaction mixture, and the solution was collected and analysed by AAS method for Pd metal. Only less than 0.3% Pd of the initially added catalyst was detected. From the whole catalytic process, the catalyst apparently behaved just like a heterogeneous catalyst, which could be recovered and reused facilely.
Conclusion
The Pd catalyst immobilized onto SBA-15 by the cations of IL TMGL is a very active and stable catalyst for the Heck coupling reaction. The Heck reaction can be carried out smoothly in solvent-free conditions using the supported Pd catalyst. The yields for the arylated olefins, with respect to reaction time and amount of catalyst, show that the catalyst has excellent activity. It is easy to separate the supported catalyst from the reaction mixture and this catalyst can be reused at least 6 times without considerable deactivation. The cations of the IL is necessary for the excellent stability of the catalyst.Experimental
Chemicals
1,1,3,3-tetramethylguanidine, lactic acid, tetraethyl orthosilicate, methyl acrylate, ethyl acrylate, n-butyl acrylate, acrylic acid, methacrylic acid, methyl methacrylate, ethyl methacrylate, acrylonitrile, styrene, bromobenzene, 1-iodonaphalene, ethyl ether, petroleum ether (with a boiling point range of 60–90 °C) were supplied by Beijing Chemical Reagent Factory. Pluronic P123, iodobenzene, 4′-bromoacetophenone, 1-fluoro-4-iodobenzene were purchased from ACROS organics. 1,1,3,3-tetramethylguanidine was distilled before use. All other chemicals (A. R. grade) were used without further purification.Apparatus
All the experiments were conducted in a 7 mL stainless steel reactor with a magnetic stirrer in a batch mode. A constant temperature air bath was employed to heat the reactor and its temperature was controlled by a PID temperature controller (model SX/A-1, Beijing Tianchen Electronic Company). The temperature fluctuation of the air bath was ±0.1 °C. The reactions were monitored by TLC. 1H NMR spectra were determined in CDCl3 or DMSO-d6 on a Brucker 400 MHz spectrometer at room temperature. The morphology of the catalysts was investigated with a transmission electron microscope (TEM, JEOL, JEM-2010). The X-ray photoelectron spectrum of the catalyst was collected on an ESCALab220i-XL spectrometer. The FT-IR spectrum was determined by a Bruker Tensor 27 infrared spectrometer.Synthesis of ionic liquid TMGL
TMGL was prepared directly by neutralization of 1,1,3,3-tetramethylguanidine with lactic acid at room temperature (Scheme 1).19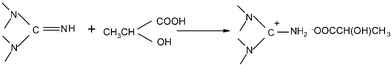 | ||
| Scheme 1 The synthesis of TMGL. | ||
Synthesis of SBA-15
The SBA-15 silica was synthesized using the procedures reported by Zhao et al.22 In the experiment, 4.0 g Pluronic P123 was dissolved in 150 g of 1.6 M HCl solution. Then, 8.50 g of tetraethyl orthosilicate (TEOS) was added. The resulting mixture was stirred for 5 min and kept at 35 °C for 24 h without stirring, and then aged for 48 h at 80 °C. The solid product was filtered, and dried in an oven for 4 h at 140 °C. To completely remove organic materials, the as-synthesized product was subsequently calcined at 550 °C for 6 h in the air. The surface area, pore diameter, and pore volume were 750 m2 g–1, 6.3 nm, and 1.1 cm3 g–1, respectively.Typical procedure to prepare SBA-TMG-Pd or SBA-Pd
The procedures were similar to those for preparing the TMG stabilzed Ru catalyst for hydrogenation of benzene.16c In a typical experiment, 21 mg of Pd(OAc)2 and 0.25 g of TMGL were dissolved in a mixture of THF and methanol (5 mL : 25 mL). Then 1.0 g of SBA-15 was added, and a slurry was obtained. The slurry was dried at 50 °C under vacuum for 2 h to obtain the powder catalytic precursor. The Pd(II) in the powder was reduced by H2 at 180 °C for 2 h and then heated up to 220 °C for 3 h to remove the anion of the IL. The Pd catalyst designed as SBA-TMG-Pd was obtained as gray powders. The preparation of the catalyst designed as SBA-Pd was similar to that of SBA-TMG-Pd. The only difference was that TMGL was not employed to functionalize the SBA-15 silica for SBA-Pd.General procedures for Heck reaction
The Heck reaction was carried out in a 7 mL stainless steel autoclave with a magnetic stirrer. In a typical experiment, aryl halide (2 mmol), vinylic substrate (2.2 mmol), triethylamine (2.4 mmol) and 2 mg of Pd catalyst were added into the stainless steel autoclave. Then the reactor was sealed and placed in the constant temperature air bath under vigorous stirring. After a desired time, the autoclave was cooled by ice water. Workup was accomplished by adding the reaction mixture into 2 N HCl, followed by extraction with ethyl ether and TLC analysis. After drying and removal of the volatiles from the organic layer, the product was purified by column chromatography on silica gel with ethyl ether, petroleum ether or their mixtures as the eluent, depending on the structure of the products.Recycling procedure of SBA-TMG-Pd or SBA-Pd
In the recycling experiment, iodobenzene (10 mmol), methyl acrylate (11 mmol), triethylamine (12 mmol) and 50 mg of Pd catalyst (the mole ratio of Pd to iodobenzene was 0.05%) were added into the reactor. After 50 min, the reaction was completed. The catalyst was separated by filtration and washed with ethanol several times. Then the catalyst was dried at 50 °C under vacuum for 3 h. The catalyst was reused in the next run without any regeneration.The 1H NMR datum of the products are presented below
1(E) - methyl cinnamate (CDCl3, 400 MHz, ppm): 7.70 (d, J = 16.0 Hz, 1H), 7.54–7.52 (m, 2H), 7.40–7.38 (m, 3H), 6.45 (d, J = 16.1 Hz, 1H), 3.81 (s, 3H);
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
Acknowledgements
We sincerely acknowledge the financial supports from National Natural Science Foundation of China (20332030, 20473105) and State Key Laboratory of Coal Conversion (06-905).References
- (a) R. F. Heck, J. Am. Chem. Soc., 1969, 91, 6707–6714 CrossRef CAS; (b) Y. Fujiwara, I. Moritani, S. Danno, R. Asano and S. Teranishi, J. Am. Chem. Soc., 1969, 91, 7166–7169 CrossRef CAS.
- (a) A. M. Trzeciak and J. J. Ziółkowski, Coordin. Chem. Rev., 2007, 251, 1281–1293 Search PubMed; (b) A. Biffis, M. Zecca and M. Basato, J. Mol. Catal. A: Chem., 2001, 173, 249–274 CrossRef CAS; (c) I. P. Beletskaya and A. V. Cheprakov, Chem. Rev., 2000, 100, 3009–3066 CrossRef CAS; (d) R. F. Heck, in Comprehensive Organic Synthesis; ed. B. M. Trost, Pergamon, New York, 1991, vol. 4, ch. 4.3, pp. 833–863 Search PubMed.
- (a) T. Mizutani, S. Honzawa, S. Tosaki and M. Shibasaki, Angew. Chem., Int. Ed., 2002, 41, 4680–4682 CrossRef CAS; (b) J. T. Link and L. E. Overman, in Metal-Catalyzed Cross-Coupling Reaction; ed. F. Diederich and P. J. Stang, Wiley-VCH, Weinheim, Germany, 1998, ch. 6, pp. 231–269 Search PubMed.
- (a) L. F. Tietze, G. Kettschau, U. Heuschert and G. Nordmann, Chem. Eur. J., 2001, 7, 368–373 CrossRef CAS; (b) Step-Growth Polymers for High-Performance Materials, ed. J. K. Hedrick and J. W. Labadie, ACS Symposium Series 624, American Chemical Society, Washington, DC, 1996, ch. 1, pp. 29–31; ch. 2, pp. 58–62; ch. 4, pp. 90–91 Search PubMed.
- (a) A. Haberli and C. J. Leumann, Org. Lett., 2001, 3, 489–492 CrossRef CAS; (b) T. R. Burke, Jr., D. G. Liu and Y. Gao, J. Org. Chem., 2000, 65, 6288–6291 CrossRef CAS.
- (a) C. Amatore, B. Godin, A. Jutand and F. Lemaître, Chem. Eur. J., 2007, 13, 2002–2011 CrossRef CAS; (b) A. F. Littke and G. C. Fu, J. Am. Chem. Soc., 2001, 123, 6989–7000 CrossRef CAS.
- (a) N. Panziera, P. Pertici, L. Barazzone, A. M. Caporusso, G. Vitulli, P. Salvadori, S. Borsacchi, M. Geppi, C. A. Veracini, G. Martra and L. Bertinettti, J. Catal., 2007, 246, 351–361 CrossRef CAS; (b) C. A. Lin and F. T. Luo, Tetrahedron Lett., 2003, 44, 7565–7568 CrossRef CAS.
- (a) F. Y. Zhao, M. Shirai, Y. Ikushima and M. Arai, J. Mol. Catal. A: Chem., 2002, 180, 211–219 CrossRef CAS; (b) R. G. Heidenreich, K. Köhler, J. G. E. Krauter and J. Pietsch, Synlett, 2002, 1118–1122 CrossRef CAS; (c) H. Yoon, S. Ko and J. Jang, Chem. Commun., 2007, 1468–1470 RSC.
- S. S. Pröckl, W. Kleist, M. A. Gruber and K. Köhler, Angew. Chem., Int. Ed., 2004, 43, 1881–1882 CrossRef.
- (a) Z. H. Zhang and Z. Y. Wang, J. Org. Chem., 2006, 71, 7485–7487 CrossRef CAS; (b) H. Zhou, G. L. Zhuo and X. Z. Jiang, J. Mol. Catal. A: Chem., 2006, 248, 26–31 CrossRef CAS.
- (a) A. Papp, G. Galbacs and R. Molnar, Tetrahedron Lett., 2005, 46, 7725–7728 CrossRef CAS; (b) L. Li, J. L. Shi and J. N. Yan, Chem. Commun., 2004, 1990–1991 RSC; (c) C. M. Crudden, M. Sateesh and R. Lewis, J. Am. Chem. Soc., 2005, 127, 10045–10050 CrossRef CAS; (d) C. P. Mehnert, D. W. Weaver and J. Y. Ying, J. Am. Chem. Soc., 1998, 120, 12289–12296 CrossRef CAS; (e) M. Z. Cai, Q. H. Xu and J. W. Jiang, J. Mol. Catal. A: Chem., 2006, 260, 190–196 CrossRef CAS.
- (a) L. Huang, Z. Wang, T. P. Ang, J. Tan and P. K. Wong, Catal. Lett., 2006, 112, 219–225 CrossRef CAS; (b) K. Okubo, M. Shirai and C. Yokoyama, Tetrahedron Lett., 2002, 43, 7115–7118 CrossRef CAS.
- (a) N. Ren, Y. H. Yang, Y. H. Zhang, Q. R. Wang and Y. Tang, J. Catal., 2007, 246, 215–222 CrossRef CAS; (b) L. Djakovitch and K. Koehler, J. Am. Chem. Soc., 2001, 123, 5990–5999 CrossRef CAS.
- (a) T. Welton, Coordin. Chem. Rev., 2004, 248, 2459–2477 Search PubMed; (b) J. Dupont, R. F. de Souza and P. A. Z. Suarez, Chem. Rev., 2002, 102, 3667–3692 CrossRef CAS; (c) S. Li, Y. Lin, H. Xie, S. Zhang and J. Xu, Org. Lett., 2006, 8(3), 391–394 CrossRef CAS.
- C. P. Mehnert, E. J. Mozeleski and R. A. Cook, Chem. Commun., 2002, 3010–3011 RSC.
- (a) J. Huang, T. Jiang, H. X. Gao, B. X. Han, Z. M. Liu, W. Z. Wu, Y. H. Chang and G. Y. Zhao, Angew. Chem., Int. Ed., 2004, 43, 1397–1399 CrossRef CAS; (b) S. D. Miao, Z. M. Liu, B. X. Han, J. Huang, Z. Y. Sun, J. L. Zhang and T. Jiang, Angew. Chem., Int. Ed., 2006, 45, 266–269 CrossRef CAS; (c) J. Huang, T. Jiang, B. X. Han, W. Z. Wu, Z. M. Liu, Z. L. Xie and J. L. Zhang, Catal. Lett., 2005, 103, 59–62 CrossRef CAS.
- (a) Y. L. Gu, C. Ogawa, J. Kobayashi, Y. Mori and S. Kobayashi, Angew. Chem., Int. Ed., 2006, 45, 7217–7220 CrossRef CAS; (b) M. R. Castillo, L. Fousse, J. M. Fraile, J. I. García and J. A. Mayoral, Chem. Eur. J., 2006, 13, 287–291 CAS.
- (a) N. E. Leadbeater, V. A. Williams, T. M. Barnard and M. J. Collins, Synlett, 2006, 2953–2958 CrossRef CAS; (b) A. DiazOrtiz, P. Prieto and E. Vazquez, Synlett, 1997, 269–270 CAS.
- H. X. Gao, B. X. Han, J. C. Li, T. Jiang, Z. M. Liu, W. Z. Wu, Y. H. Chang and J. M. Zhang, Synth. Commun., 2004, 34, 3083–3089 CrossRef CAS.
- (a) N. T. S. Phan, D. H. Brown, H. Adams, S. E. Spey and P. Styring, Dalton Trans., 2004, 1348–1357 RSC; (b) M. T. Reetz and G. Lohmer, Chem. Commun., 1996, 1921–1922 RSC; (c) A. Papp, K. Miklós, P. Forgo and A. Molnár, J. Mol. Catal. A: Chem., 2005, 229, 107–116 CrossRef CAS.
- (a) M. Weck and C. W. Jones, Inorg. Chem., 2007, 46, 1865–1875 CrossRef CAS; (b) K. Kohler, W. Kleist and S. S. Prockl, Inorg. Chem., 2007, 46, 1876–1883 CrossRef; (c) C. C. Cassol, A. P. Umpierre, G. Machado, S. I. Wolke and J. Dupont, J. Am. Chem. Soc., 2005, 127, 3298–3299 CrossRef CAS.
- D. Y. Zhao, J. L. Feng, Q. S. Huo, N. Melosh, G. H. Fredrickson, B. F. Chmelka and G. D. Stucky, Science, 1998, 278, 548–552 CrossRef.
| This journal is © The Royal Society of Chemistry 2008 |