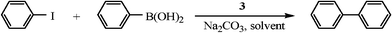Highly effective silica gel-supported N-heterocyclic carbene–Pd catalyst for Suzuki–Miyaura coupling reaction
Huili
Qiu
,
Shaheen M.
Sarkar
,
Dong-Hwan
Lee
and
Myung-Jong
Jin
*
School of Chemical Science and Engineering, Inha University, Incheon 402-751, South Korea. E-mail: mjjin@inha.ac.kr; Fax: +82 32-872-0959; Tel: +82 32-860-7469
First published on 2nd November 2007
Abstract
The immobilized N-heterocyclic carbene–Pd complex was readily prepared by reaction of silica gel-supported imidazolium chloride with Pd(OAc)2. The Pd complex exhibited excellent catalytic activity in the coupling reaction of aryl halides with arylboronic acid. The heterogeneous Pd catalyst was reusable as well as air-stable to allow easy use.
Introduction
Metal-catalyzed coupling reactions have been recognized as convenient one-step methods for assembling complex structures.1 Among them, Suzuki–Miyaura coupling reaction of aryl halides with arylboronic acids is one of the most powerful tools for the synthesis of biaryl derivatives.2Palladium catalysts are known to possess high activity for the coupling reaction.3 However, the traditional homogeneous catalysis causes major problems in purification of product and separation of expensive palladium catalyst that leads to toxic wastes. These problems are of environmental and economic concern in large scale-synthesis. Immobilized Pd catalysts have been developed in order to overcome these problems facing green chemistry.4,5 The search for an effective and recyclable catalyst is still a major challenge. Recently, N-heterocyclic carbene (NHC)–Pd complexes were found to act as efficient catalysts in coupling reactions.6,7 Our interest in this area led us to explore the NHC–Pd complex immobilized onto the surface of amorphous silica gel, which can be easily separated from the reaction mixture. Silica gel as an inorganic support has some advantageous properties such as excellent stability, good accessibility, and the fact that organic groups can be robustly anchored to the surface.8 Herein, we wish to describe the synthesis of reusable silica-supported NHC–Pd complex 3 and its catalytic activity in the Suzuki–Miyaura coupling reaction.Experimental
Immobilization of ionic liquid 1 onto the surface of silica gel
To a solution of triethoxysilylpropylimidazolium chloride ionic liquid 1 (0.70 g, 2.2 mmol) in toluene was added commercially available silica gel (2.0 g, Merck® 9385, particle size 230–400 mesh, surface area 550 m2 g–1). The mixture was stirred at 105 °C for 12 h. After cooling, the reaction mixture was filtered and washed with CH2Cl2 (10 mL × 3), and dried at 60 °C under vacuum to yield silica-supported ionic liquid 2 (2.39 g). Elemental analysis and weight gain showed that 0.89 mmol of ionic liquid 1 was anchored on 1.0 g of 2.Preparation of silica-supported NHC–Pd complex 3
To a solution of silica-supported ionic liquid 2 (1.0 g, 0.89 mmol g–1) in DMSO (4.5 mL) was added Pd(OAc)2 (101 mg, 0.45 mmol). The mixture was stirred for 4 h at 60 °C. The reaction was then allowed to proceed for an additional 30 min at 100 °C. Silica-supported NHC–Pd complex 3 was collected on a glass frit and washed with CH2Cl2 (10 mL × 3) to remove unreacted Pd(OAc)2, and dried at 50 °C under vacuum. ICP analysis (atomic %): Pd 3.65.The ICP analysis showed that 0.34 mmol of Pd was anchored on 1.0 g of 3. The IR spectrum of 3 showed an absorption band at 1575 cm–1 which is attributed to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibration of the imidazolium group.
C stretching vibration of the imidazolium group.
General procedure for heterogeneous Suzuki reaction
Aryl halide (1.0 mmol), phenylboronic acid (134 mg, 1.1 mmol), Na2CO3 (212 mg, 2.0 mmol), dodecane (40 mg, internal standard) and catalyst 3 (3 mg, 0.34 mmol g–1, 0.1 mol%) were mixed in DMF–H2O (3.6 mL, 1 : 1, v/v). The mixture was stirred at 65 °C in an air atmosphere. The samples were withdrawn periodically and analysed by GC/GC-MS. The reaction mixture was filtered and washed with H2O and Et2O. The organic phase was separated and dried over MgSO4, and the solvent was evaporated under reduced pressure. The product was isolated by column chromatography on silica gel. GC/GC-MS analyses were performed on an Agilent 6890N GC (He carrier gas, HP-5MS column, 30 m × 0.25 m × 0.25 µm) coupled to an Agilent 5975 Network Mass Selective Detector (electron impact ionization at 70 eV). The initial temperature of the column was set at 80 °C for 5 min, and ramped at 10 °C min–1 to a final temperature of 240 °C. The carrier gas was set at a constant velocity of 40 cm s–1.The reuse of silica-supported Pd catalyst 3
In the recycling experiment, the reaction was performed by using a mixture of iodobenzene or 1-bromo-4-nitrobenzene (4.0 mmol), phenylboronic acid (4.4 mmol), Na2CO3 (8.0 mmol), and catalyst 3 (0.2 mol%) at 65 °C. After completion of the reaction, the reaction mixture was worked up as described above. The filtered catalyst was successively reused in the same reaction.Results and discussion
As shown in Scheme 1, the immobilization of the NHC–Pd complex onto the surface of amorphous silica gel was attempted through two methods: (A) immobilization of triethoxysilylated ionic liquid 19 onto silica gel, followed by reaction with Pd(OAC)2 and (B) preparation of triethoxysilylated NHC–Pd complex 4, followed by the immobilization onto the silica gel. Method A was chosen as a better strategy for the immobilization of the NHC–Pd complex because it is difficult to immobilize complex 4 which has very low solubility in most organic solvents. A similar procedure was previously used in the conversion of 2 to 3.10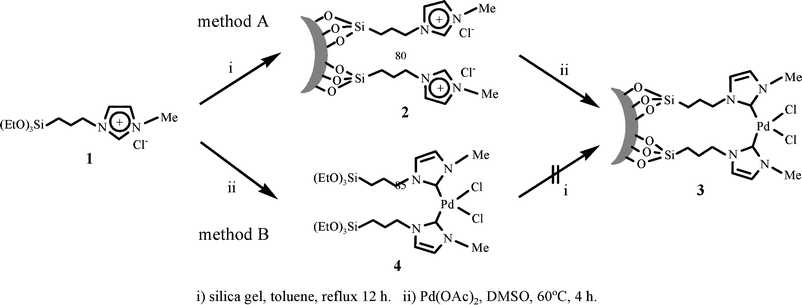 | ||
| Scheme 1 | ||
With the heterogeneous catalyst 3 in hand, we first tested Suzuki coupling of iodobenzene with phenylboronic acid in DMF–H2O (1 : 1, v/v) as a model reaction. The reaction was initially performed in the presence of 1 mol% of 3 and Na2CO3 as a base. High conversion was obtained at 50–65 °C within 0.5 h (entries 1 and 2). It is noteworthy that the catalyst 3 shows outstanding performance even at a low temperature of 40 °C (entry 3). When the loading of 3 was decreased from 1 mol% to 0.01 mol%, reactivity was slightly influenced (entries 4–7). Surprisingly, high conversion could be still maintained at 0.01 mol% of very low catalyst loading, in which high TOF of 9100 h–1 was obtained for the coupling (entry 7). The results are summarized in Table 1. Further optimization of the reaction conditions was not attempted to obtain higher TOF. Solvent effect on the activity of 3 was surveyed with different polar solvents. When the reaction was conducted in aqueous DMSO, DMA, ethanol and methanol instead of aqueous DMF, similar results were obtained under the same conditions (entries 8–18). It is interesting that the reactions in aqueous ethanol and methanol gave excellent results. Aqueous solvent appears to be necessary for mild conditions because of low solubility of Na2CO3 in organic solvents. The complex is very stable to oxygen and moisture. Less change of its activity was observed when the Pd complex was exposed to air and water in the Suzuki reaction.
|
|
|||||
|---|---|---|---|---|---|
| Entry | Solvent (1 : 1) | 3 (mol%) | Temp./°C | Time/h | Yieldb (%) |
| a Molar ratio: iodobenzene (1.0 equiv.), phenylboronic acid (1.1 equiv.), Pd complex 3 (1–0.01 mol%. Pd loading ratio = 0.34 mmol g–1), and Na2CO3 (2.0 equiv.). b GC yield determined using n-dodecane as an internal standard and based on the amount of iodobenzene employed. Isolated yield is given in parenthesis. | |||||
| 1 | DMF–H2O | 1 | 65 | 0.3 | 100 |
| 2 | DMF–H2O | 1 | 50 | 0.5 | 93 |
| 3 | DMF–H2O | 1 | 40 | 1.0 | 90 |
| 4 | DMF–H2O | 0.5 | 65 | 0.4 | 100 |
| 5 | DMF–H2O | 0.1 | 65 | 0.5 | 100 (96) |
| 6 | DMF–H2O | 0.05 | 75 | 0.5 | 95 (90) |
| 7 | DMF–H2O | 0.01 | 85 | 1.0 | 91 |
| 8 | DMA–H2O | 1 | 65 | 0.3 | 100 |
| 9 | DMA–H2O | 0.5 | 65 | 0.3 | 100 |
| 10 | DMA–H2O | 0.1 | 65 | 0.4 | 100 |
| 11 | DMA–H2O | 0.05 | 75 | 0.5 | 96 |
| 12 | DMSO–H2O | 1 | 65 | 0.3 | 100 |
| 13 | DMSO–H2O | 0.5 | 65 | 0.4 | 99 |
| 14 | DMSO–H2O | 0.1 | 65 | 0.5 | 99 |
| 15 | EtOH–H2O | 1 | 65 | 0.4 | 98 |
| 16 | EtOH–H2O | 0.5 | 65 | 0.5 | 96 |
| 17 | MeOH–H2O | 1 | 65 | 0.4 | 98 |
| 18 | MeOH–H2O | 0.5 | 65 | 0.5 | 97 |
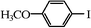
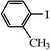
We next examined the catalytic activity of 3 for reaction of aryl halides with arylboronic acid. As shown in Table 2, high catalytic activity was observed in the coupling of deactivated aryl iodides such as 2-iodoanisole, 4-iodo-anisole, 2-iodotoluene and 4-iodophenol (entries 1–4) as well as activated 1-iodo-4-nitrobenzene and 1-iodo-3-nitrobenzene (entries 5 and 6). Deactivated aryl iodides possessing an electron-donating group showed a slight drop in reactivity compared to those possessing an electron-withdrawing group. However, only a little longer reaction time was required to reach almost quantitative conversion. In order to investigate the scope on aryl halides in the coupling with phenylboronic acid, different aryl bromides were employed in the reaction (entries 7–13). Electron-rich, electron-neutral, and electron-poor aryl bromides were readily coupled in the presence of catalyst 3. The catalytic system was further extended to the coupling reactions with arylboronic acids containing electron-donating and electron-withdrawing substituents(entries 14–19). Most of the reactions could be also conducted with high yields. However, catalyst 3 showed low activity in the reaction of deactivated 2-bromoanisole with electron-rich 2-methoxyphenylboronic acid (entry 20). A trace amount of biphenyl was detected as a by-product in the reactions of aryl iodides and aryl bromides.
|
|
||||
|---|---|---|---|---|
| Substrate | ArB(OH)2 | Time/h | Yieldb (%) | |
| a Molar ratio: aryl halide (1.0 equiv.), arylboronic acid (1.1 equiv.), Pd complex 3 (0.1 mol%. Pd loading ratio = 0.34 mmol g–1), and Na2CO3 (2.0 equiv.). b GC yield determined using n-dodecane as an internal standard and based on the amount of iodobenzene employed. Isolated yield is given in parenthesis. c The GC yield of biphenyl. Entries 21–23: reactions were performed in the presence of 1 mol% of catalyst 3 at 85 °C. | ||||
| 1 |
|
|
0.6 | 92 |
| 2 |
|
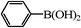
|
0.5 | 95 (91) |
| 3 |
|
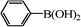
|
0.8 | 90 |
| 4 |
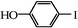
|
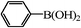
|
0.4 | 100 |
| 5 |
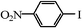
|
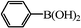
|
0.3 | 100 |
| 6 |
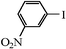
|
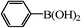
|
0.3 | 100 |
| 7 |
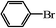
|
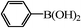
|
0.5 | 95 (90) |
| 8 |
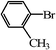
|
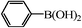
|
0.8 | 91 |
| 9 |
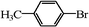
|
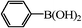
|
0.7 | 92 |
| 10 |
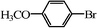
|
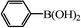
|
0.6 | 95 |
| 11 |
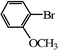
|
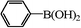
|
1.0 | 96 |
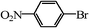
| 12 |
|
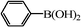
|
0.4 | 100 |
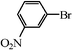
| 13 |
|
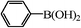
|
0.4 | 100 |
| 14 |
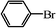
|
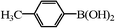
|
1.0 | 95 |
| 15 |
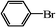
|
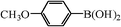
|
0.7 | 92 |
| 16 |
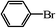
|
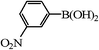
|
3 | 83 |
| 17 |
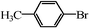
|
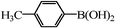
|
3 | 91 |
| 18 |
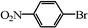
|
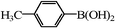
|
0.7 | 100 |
| 19 |
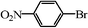
|
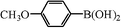
|
0.8 | 99 |
| 20 |
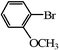
|
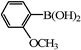
|
3 | 46 |
| 21 |
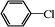
|
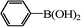
|
12 | 99 (92) |
| 22 |
|
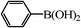
|
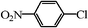
12 | 66/23c |
| 23 |
|
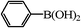
|
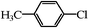
20 | 50/29c |
Encouraged by these results, we tested the coupling of several aryl chlorides in the presence of 1 mol% of 3 at 85 °C. The reaction of chlorobenzene with phenylboronic acid proceeded smoothly to afford biphenyl product with high yield (entry 21). However, low yields were observed in the coupling of substituted aryl chlorides (entries 22 and 23). In addition, biphenyl by-product from homocoupling of phenylboronic acid was remarkably formed with the increase of temperature and the prolongation of reaction time.
The recycling of the catalyst is an important issue in the heterogeneous reaction. We turn our attention to reusability of our Pd catalyst. As shown in Table 3, the catalyst 3 was recycled in the reactions of iodobenzene and 1-bromo-4-nitrobenzene with phenylboronic acid. We have observed that the catalyst could be reused six times without significant loss of activity. Furthermore, analysis of the solution by atomic absortion indicated that no Pd species leached into the reaction solution. This excellent reusability and high stability of the catalyst would be explained by strong binding of the NHC to palladium and site isolation, that is, the absence of interactions between catalytic sites, which are followed by aggregation of the Pd complex and formation of less active Pd catalyst.
| Aryl halide | Cycle | Yieldb (%) | Cycle | Yieldb (%) |
|---|---|---|---|---|
| a Molar ratio: aryl halide (1.0 equiv.), phenylboronic acid (1.1 equiv.), 3 (0.2 mol%.), and Na2CO3 (2.0 equiv.). b GC yield determined using n-dodecane as an internal standard and based on the amount of aryl halide employed. | ||||
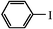
|
1 | 100 | 4 | 100 |
| 2 | 100 | 5 | 98 | |
| 3 | 100 | 6 | 96 | |
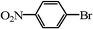
|
1 | 100 | 4 | 100 |
| 2 | 100 | 5 | 99 | |
| 3 | 100 | 6 | 98 |
Conclusions
In conclusion, we have easily prepared a silica-supported NHC–Pd complex 3 from triethoxysilylated ionic liquid 1. The heterogeneous complex showed high catalytic activity for Suzuki coupling reaction in an aqueous medium. Furthermore, this catalyst could be simply recovered and reused without a significant loss of catalytic activity. Further studies of other coupling reactions catalyzed by this system are currently in progress.Notes and references
- For reviews of metal-catalyzed cross-couplings, see: (a) F. Diederich and P. J. Stang, Metal-Catalyzed Cross-Coupling Reactions, Wiley-VCH, New York, 1998; (b) M. Beller and C. Bolm, Transition Metals for Organic Synthesis, Wiley-VCH, Weinheim, 2nd edn, 2004.
- For reviews, see: (a) N. Miyaura and A. Suzuki, Chem. Rev., 1995, 95, 24572483; (b) A. F. Littke and G. C. Fu, Angew. Chem., Int. Ed., 2002, 41, 4176–4211 CrossRef CAS; (c) S. Kotha, K. Lahiri and D. Kashinath, Tetrahedron, 2002, 58, 9633–9695 CrossRef CAS; (d) L. A. Agrofoglio, I. Gillaizeau and Y. Saito, Chem. Rev., 2003, 103, 1875–1916 CrossRef CAS.
- (a) J. P. Wolfe, R. A. Singer, B. H. Yang and S. L. Buchwald, J. Am. Chem. Soc., 1999, 121, 9550–9561 CrossRef CAS; (b) A. D. Littke, C. Dai and G. C. Fu, J. Am. Chem. Soc., 2000, 122, 4020–4028 CrossRef CAS; (c) M. R. Netherton, C. Dai, K. Neuschutz and G. C. Fu, J. Am. Chem. Soc., 2001, 123, 10099–10100 CrossRef CAS; (d) J. Yin, M. P. Rainka, X.-X. Zhang and S. L. Buchwald, J. Am. Chem. Soc., 2002, 24, 1162–1163 CrossRef; (e) R. B. Bedford, C. S. J. Cazin and S. L. Hazelwood, Angew. Chem., Int. Ed., 2002, 41, 4120–4122 CrossRef CAS; (f) F. Churruca, R. SanMartin, B. Inés, I. Tellitu and E. Domínguez, Adv. Synth. Catal., 2006, 348, 1836–1840 CrossRef CAS.
- For reviews, see: (a) N. E. Leadbeater and M. Marco, Chem. Rev., 2002, 102, 3217–3274 CrossRef CAS; (b) L. Yin and J. Liebscher, Chem. Rev., 2007, 107, 133–173 CrossRef CAS.
- (a) L. Djakovitch and K. Koehler, J. Am. Chem. Soc., 2001, 123, 5990–5999 CrossRef CAS; (b) Y. M. A. Yoichi, K. Takeda, H. Takahashi and S. Ikegami, Org. Lett., 2002, 20, 3371–3374; (c) S. Paul and J. H. Clark, Green Chem., 2003, 5, 635–638 RSC; (d) A. Corma, H. Carcia, A. Leiva and A. Primo, Appl. Catal., A, 2003, 247, 41–49 CrossRef CAS; (e) A. Corma, H. Garcia, A. Leiva and A. Primo, Appl. Catal., A, 2004, 257, 77–83 CrossRef CAS; (f) J. J. E. Hardy, S. Hubert, D. J. Macquarrie and A. J. Wilson, Green Chem., 2004, 6, 53–56 RSC.
- For review, see: W. A. Herrmann, Angew. Chem., Int. Ed., 2002, 41, 1290–1390 Search PubMed.
- (a) W. A. Herrmann, C.-P. Reisinger and M. Spiegler, J. Organomet. Chem., 1998, 557, 93–96 CrossRef CAS; (b) W. A. Herrmann, V. P. W. Bohm, C. W. K. Gstottmayr, M. Grosche, C. P. Reisinger and T. J. Wskamp, J. Organomet. Chem., 2001, 617, 616–628 CrossRef; (c) O. Navarro, R. A. III. Kelly and S. P. Nolan, J. Am. Chem. Soc., 2003, 125, 16194–16195 CrossRef CAS; (d) O. Navarro, H. Kaur, P. Mahjoor and S. P. Nolan, J. Org. Chem., 2003, 69, 3173–3180; (e) G. Altenhoff, R. Goddard, C. W. Lehmann and F. Glorius, J. Am. Chem. Soc., 2004, 126, 15195–15201 CrossRef CAS; (f) F. B. Barrett, J. L. Chaytor and M. E. A. Heska, Org. Lett., 2004, 6, 3641–3644 CrossRef CAS; (g) A. E. Wang, J. H. Xie, L. X. Wang and Q. L. Zhou, Tetrahedron, 2005, 61, 259–266 CrossRef CAS.
- (a) M. K. Richmond, S. L. Scott and H. Alper, J. Am. Chem. Soc., 2001, 123, 10521–10525 CrossRef CAS; (b) C. Baleizao, A. Corma, H. Garcia and A. Leyva, Chem. Commun., 2003, 606–607 RSC; (c) P.-H. Li and L. Wang, Adv. Synth. Catal., 2006, 348, 681–685 CrossRef CAS.
- (a) C. P. Mehnert, R. A. Cook, N. C. Dispenziere and M. Afeworki, J. Am. Chem. Soc., 2002, 124, 12932–12933 CrossRef CAS; (b) S. Brenna, T. Posset, J. Furrer and J. Blumel, Chem.–Eur. J., 2006, 12, 2880–2888 CrossRef CAS; (c) B. Gadenne, P. Hesemann and J. J. E. Moreau, Chem. Commun., 2004, 1768–1769 RSC.
- J. Schwarz, V. P. W. Bohm, M. G. Gardiner, M. Grosche, W. A. Herrmann, W. Hieringer and G. R. Sieber, Chem.–Eur. J., 2000, 6, 1773–1780 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2008 |