Green chemistry tools to influence a medicinal chemistry and research chemistry based organisation†
Kim
Alfonsi
a,
Juan
Colberg
b,
Peter J.
Dunn
*c,
Thomas
Fevig
d,
Sandra
Jennings
a,
Timothy A.
Johnson
b,
H. Peter
Kleine
d,
Craig
Knight
c,
Mark A.
Nagy
d,
David A.
Perry
*b and
Mark
Stefaniak
c
aPfizer Global Research and Development, Ann Arbor, Michigan, MI-48105, USA
bPfizer Global Research and Development, Groton, Connecticut, CT-06340, USA
cPfizer Global Research and Development, Sandwich, Kent, CT139NJ, UK
dPfizer Global Research and Development, Chesterfield, Missouri, MO63017, USA
First published on 16th November 2007
Abstract
Influencing and improving the environmental performance of a large multi-national pharmaceutical company can be achieved with the help of electronic education tools, backed up by site champions and strong site teams. This paper describes the development of two of those education tools.
Introduction
The success of the pharmaceutical industry is, in large part, built on the towering achievements of organic chemistry, a mature science which emerged as a distinct discipline well over 150 years ago. This long history is both a blessing and a curse. Many of our most reliable strategies for assembling target molecules employ reactions which are fifty to one hundred years old and often named in honour of their discoverer. During these early years, the chronic toxicological properties of chemicals were often completely unknown and many unwittingly became indispensable tools of the trade. Infamously, benzene was widely employed as a solvent, a hand-cleaner and even as an aftershave, decades before its carcinogenicity became appreciated.1 Today chemists are still taught the efficacy of chromium, osmium and lead compounds as oxidants, the virtues of chlorinated solvents and the use of atom-inefficient methodologies, while the curricula in most undergraduate chemistry programs provide little or no training in toxicology,2 environmental science3 or sustainable technology.4Early pioneers in green chemistry included Trost (who developed the atom economy principle)5 and Sheldon (who developed the E-Factor).6 These measures were introduced to encourage the use of more sustainable chemistry and provide some benchmarking data to encourage scientists to aspire to more benign synthesis. Later, green chemistry became formalised by the publication by Warner and Anastas7 of a holistic set of principles designed to raise awareness of the safe, environmentally sensitive and sustainable practice of chemistry. While many of these principles were second nature to process development chemists and their manufacturing colleagues in the wake of the pollution control legislation over the last 30 years, the same cannot be said of their medicinal chemistry colleagues. The modern practice of drug discovery relies heavily on speed of execution, which in turn relies on robust methodologies emphasising reliability rather than environmental impact. While the scale of the reactions conducted at the early stages of a program is usually small, the cumulative footprint generated by tens or hundreds of laboratories in a pharmaceutical company becomes significant. Moreover, the delay that may be incurred by the necessity to reengineer a ‘discovery route’ to achieve a scaleable process impacts the development timeline, as well as its cost. This paper describes ongoing initiatives in Pfizer to equip its medicinal chemists with a working knowledge of the principles of green chemistry, favouring restraint over constraint, and providing access to tools which guide the selection of greener solvents and reagents. We believe the success of these initiatives will reduce our environmental impact, improve worker safety and reduce the time taken to deliver important new medicines addressing major unmet medical needs.
Development of the solvent selection tool
A number of companies have previously published solvent selection guides,8 more recently Fischer et al.9 published a detailed and comprehensive approach to the environmental selection of solvents, though in our view this assessment is too generous to volatile solvents. Volatile solvents have more potential for environmental release and may also have more flammability issues (e.g., pentane or diethyl ether). In reviewing previous work, we felt that because of the challenges and time pressures associated with the medicinal chemistry job, any tool needed to be extremely simple for the end user scientist. However, this does not mean that the information behind the tool is simple. The work to produce a tool was initiated in our environment, health and safety (EHS) group, and solvents were assessed in a thorough and systematic way in three general areas.(i) Worker safety10– including carcinogencity, mutagenicity, reprotoxicity, skin absorption/sensitisation, and toxicity
(ii) Process safety – including flammability, potential for high emissions through high vapour pressure, static charge, potential for peroxide formation and odour issues.
(iii) Environmental and regulatory considerations11 - including ecotoxicity and ground water contamination, potential EHS regulatory restrictions, ozone depletion potential, photoreactive potential. Of course compliance with regulations and company guidelines provide the baseline of Pfizer's environmental policy.
This detailed assessment was then translated into a simple 1 page guide which is shown in Fig. 1.12
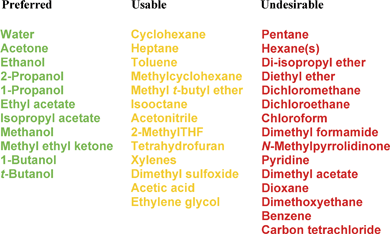 | ||
| Fig. 1 Pfizer solvent selection guide for medicinal chemistry. | ||
A summary of why each solvent is placed in the red category is provided in Table 1.
| Red solvent | Flash point | Reason |
|---|---|---|
| Pentane | –49 °C | Very low flash point, good alternative available. |
| Hexane(s) | –23 °C | More toxic than the alternative heptane, classified as a hazardous airborne pollutant (HAP) in the US. |
| Diisopropyl ether | –12 °C | Very powerful peroxide former, good alternative ethers available. |
| Diether ether | –40 °C | Very low flash point, good alternative ethers available. |
| Chloroform | N/A | Carcinogen, classified as a HAP in the US. |
| Dichloroethane | 15 °C | Carcinogen, classified as a HAP in the US. |
| Dimethyl formamide | 57 °C | Toxicity, strongly regulated by EU Solvent Directive, classified as a HAP in the US. |
| Dimethyl acetamide | 70 °C | Toxicity, strongly regulated by EU Solvent Directive. |
| N-Methyl pyrrolidinone | 86 °C | Toxicity, strongly regulated by EU Solvent Directive. |
| Pyridine | 20 °C | Carinogenic/mutagenic/reprotoxic (CMR) category 3 carcinogen, toxicity, very low threshold limit value TLV for worker exposures. |
| Dioxane | 12 °C | CMR category 3 carcinogen, classified as HAP in US. |
| Dichloromethane | N/A | High volume use, regulated by EU solvent directive, classified as HAP in the US. |
| Dimethoxyethane | 0 °C | CMR category 2 carcinogen, toxicity. |
| Benzene | –11 °C | Avoid use : CMR category 1 carcinogen, toxic to humans and environment, very low TLV (0.5 ppm), strongly regulated in the EU and the US (HAP). |
| Carbon tetrachloride | N/A | Avoid use : CMR category 3 carcinogen, toxic, ozone depleter, banned under the Montreal protocol, not available for large-scale use, strongly regulated in the EU and US (HAP). |
The list of solvents covered in Fig. 1 is not extensive but covers solvents commonly used in medicinal chemistry. Solvents, such as benzene and carbon tetrachloride, were included to reinforce the avoidance of their use.
In addition, the scientists in our green chemistry teams produced a simple solvent replacement table for each of the solvents in the red/undesirable category, with the philosophy of adopting a “use this instead” policy rather than a “don't use” policy. This replacement table is shown in Table 2. The replacements are either chemically similar (e.g., heptane as a replacement for the high flammable pentane) or functionally equivalent (e.g., ethyl acetate, methyl tert-butyl ether (MTBE) or 2-methyltetrahydrofuran (2-MeTHF) as alternative extraction solvents to dichloromethane).
| Undesirable solvents | Alternative |
|---|---|
| Pentane | Heptane |
| Hexane(s) | Heptane |
| Di-isopropyl ether or diethyl ether | 2-MeTHF or tert-butyl methyl ether |
| Dioxane or dimethoxyethane | 2-MeTHF or tert-butyl methyl ether |
| Chloroform, dichloroethane or carbon tetrachloride | Dichloromethane |
| Dimethyl formamide, dimethyl acetamide or N-methylpyrrolidinone | Acetonitrile |
| Pyridine | Et3N (if pyridine used as base) |
| Dichloromethane (extractions) | EtOAc, MTBE, toluene, 2-MeTHF |
| Dichloromethane (chromatography) | EtOAc/heptane |
| Benzene | Toluene |
There are a number of points that need further comment. Many of our scientists are surprised that dichloromethane is the recommended alternative to other chlorinated solvents, such as chloroform. All that Table 2 is indicating is that if a chlorinated solvent needs to be used, dichloromethane is the best choice out of the four.
All of the solvents have good replacements, with the exception of one group, which is the dipolar aprotic solvents dimethyl formamide, dimethyl acetamide and N-methylpyrrolidinone. For this group of solvents, acetonitrile is a relatively poor substitute, especially for reactions involving a strong base. Due to the lack of good alternatives, Pfizer, with a group of other pharmaceutical companies, has identified finding replacements for these solvents as a key target in green chemistry research.13
The guide and replacement table seem almost ridiculously simple but when used by our enthusiastic site teams they led to amazing results, including a 50% reduction in chlorinated solvent use across the whole of our research division (more than 1600 lab based synthetic organic chemists, and four scale-up facilities) during the time period 2004–2006. Even sites that had an increase in the number of chemists during that period were able to report a 50% reduction in chlorinated solvent use. In addition, we were able to reduce the use of an undesirable ether by 97% over the same two year period and substantially promote the use of heptane compared with hexane (more toxic) and pentane (much more flammable).
The development of a reagent guide
This was much more challenging than the solvent guide because of the wide variety of reagents and by the fact that reagents by their very nature are designed to be reactive (whereas solvents are ideally inert), potentially causing additional safety and environmental issues. To our knowledge, no other company has tried to develop a guide of this nature. We wanted the guide to achieve three purposes.• To provide a balanced assessment of chemical methodologies, taking into account the many constraints that scientists have to take into account when making decisions in their work. To our mind the ideal reagent would have three ideal characteristics:
• (i) The ability to work in good yield in a wide variety of “drug like molecules” —this is a characteristic highly valued by medicinal chemists.
• (ii) The ability of a reagent to be used for scale-up to prepare multi-kilogram batches—a characteristic valued by our Chemical R and D, Kilo Lab and Pilot Plant chemists and engineers.
• (iii) To be as green as possible. Our green chemistry teams would like to introduce the greenest possible reagent as early as possible in the discovery/development process. The assessment of greenness included worker safety, ecotoxicity and atom economy.
• To provide easy access to the chemical literature or procedures for reagents that score well in the assessment. In the on-line Pfizer version of the guide, reagents that score well are linked directly through electronic links to key literature papers, internal procedures or both.
• To raise awareness of newer emerging green methodologies.
We decided to map the reagents onto a series of grids (or Venn diagrams), with each grid representing a commonly used chemical transformation. Each Venn diagram indicates which of the three ideal characteristics each reagent met. A breakdown of the grids and a discussion of the zones in the grid are shown in Fig. 2.
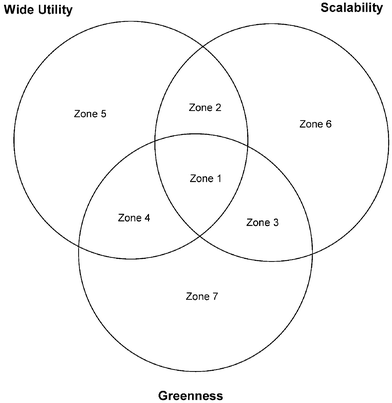 | ||
| Fig. 2 The zones in the Venn diagram (or grid) that form the basis of the reagent guide. | ||
Zone 1: reagents in this zone have all three desirable characteristics. These are reagents we would like our scientists in medicinal chemistry and chemical research and development to try first.
Zone 2: the reagents in this zone meet the wide applicability and scalability criteria but do not meet our greenness criterion. Reagents in this zone are still fully acceptable for use in late discovery/early development. Note that reagents with gross environmental issues, such as a thallium or tin reagent, would not be in this zone as they would fail the scalability criterion, but reagents with a slightly higher molecular weight and poor atom economy, such as EDCI for amide coupling, would make this zone.
Zone 3: this zone retains the positive attributes of scalability and greenness and reagents in this zone are good for our chemical research and development groups.
Zone 4: this zone has positive attributes for greenness and wide applicability but fails the scalability criterion, an example might be an electrolysis reaction where the company does not have access to large-scale electrolysis equipment.
Reagents in zones 5, 6 and 7 only meet one positive attribute and are less favoured. In the Pfizer electronic version of the guide, only reagents that fall in zones one to four are hypertext linked to in-house procedures or key references.
Two sample grids are shown to illustrate the reagent guide with a further two available in the electronic supplementary information.†
Fig. 3 14,15 shows the grid for the oxidation of alcohols to aldehydes.
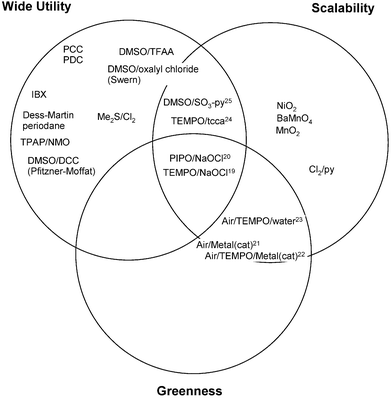 | ||
| Fig. 3 Oxidation of primary alcohol to aldehyde. | ||
The three most common oxidants used by Pfizer's medicinal chemists for this transformation are Dess–Martin periodinane16 or its precursor IBX, tetrapropylammonium perruthenate (TPAP)17 and the Swern oxidation.18 All of these methods have significant scale-up issues, for example Dess–Martin periodinane is a high energy molecule14 that has poor atom economy and is prohibitively expensive for use on a multi-kilogram scale. The use of stoichiometric TPAP again has very poor atom economy and is also prohibitively expensive for large-scale use. A review of large-scale oxidations since 1980 revealed only one large-scale use of TPAP to catalyse an oxidation with a co-oxidant and no examples of stoichiometric use.15 The Swern oxidation is used at Pilot Plant scale but generates toxic by-products and the stench of dimethylsulfide. Hence, the purpose of the reagent guide is to influence the medicinal chemist away from the reliable but environmentally unfriendly methods to more friendly methods, such as the oxidation with bleach (NaOCl) catalysed by nitroxyl radicals, such as TEMPO19 and PIPO.20 In addition, there has been an explosion in the chemical literature of methods that use molecular oxygen as an oxidant, with more than 150 papers in the last 3 years. These methods carry some challenges on scale-up, as the use of molecular oxygen to aerate flammable solvents is a significant safety concern. These concerns can be reduced by using oxygen diluted with large volumes of nitrogen but still these methods21,22 lie on the edge of acceptability when judged against the scalability criteria. An improved safety profile and more acceptable scalability is obtained if the oxidation is performed in water.23 Again, the purpose of the reagent guide is to provide scientists with easy up-to-date access to developments in this exciting area of green oxidation. Other methods shown in Fig. 3 can be found in the following publications.24,25
A similar Venn diagram covering the oxidation of secondary alcohols to ketones can be found in the electronic supplementary information.†
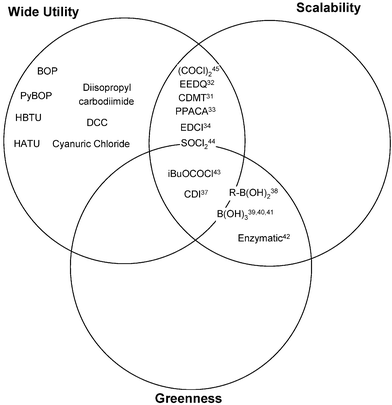
Fig. 4 shows the grid for amide formation from acids (not prone to racemisation) and amines.
For the oxidation grids we were able to set strict criteria for greenness (reaction by-products should be either water or sodium chloride and there should be no major process safety issues). For amide formation, the majority of literature methods had very poor atom economy. We decided to set the greenness criteria for this transformation as the following.
• Side products should have a molecular weight less than 200.
• No major process safety issues.
• No major environmental issues.
The first of these criteria, based on atom economy, might seem overly generous but in fact 50% of the reagents in Fig. 4 fail this criterion.
Uronium salts, such as HATU26 and HBTU,27 have become widely used in research laboratories but have many green chemistry issues. Their by-products have molecular weights of 398 and 397, respectively, for accomplishing a dehydration reaction (removing a molecule of water with a molecular weight of 18). They are both highly energetic molecules and HATU is shock sensitive.28 The phosphorus based reagent BOP29 and PyBOP30 are again energetic molecules and have even worse atom economy. BOP has the further major disadvantage that its manufacture and use involve HMPA (a class 1 carcinogen).
Dicyclohexyl carbodiimide (DCC) and di-isopropyl carbodiimide fail our green criteria because of their very strong sensitisation properties and hence in recent years have become rarely used for scale-up in the pharmaceutical industry. Cyanuric chloride is similarly a very strong sensitiser. Oxalyl chloride does not meet our greenness criteria on account of its poisonous by-product carbon monoxide. 1-Chloro-4,6-dimethoxy-1,3,5-triazine (CDMT) is a sensitiser but has been used by some process groups for scale-up.31 EEDQ,32PPACA,33 and EDCI34 do not meet our greenness criteria on the basis of atom economy but are widely used for scale-up chemistry. Thionyl chloride and chloroformates are the most common reagents for this transformation used by the pharmaceutical industry,35N,N′-carbonyldiimidazole (CDI) is growing in popularity and was used in the commercial synthesis of sildenafil36 and sunitinib.37 We judged that thionyl chloride did not fully meet our greenness criteria because of its worker safety issues but was preferred to oxalyl chloride for acid chloride formation. Although reagents such as CDI and isobutyl chloroformate are described as green, they are not without issue, for example, the synthesis of CDI uses highly poisonous phosgene, our assessment simply says they are greener than some of the alternatives available at this time.
All of the reagents discussed so far are stoichiometric reagents but the real opportunity is in the development of catalytic reagents where the only by-product would be water. The use of boronic acids,38 and in particular boric acid,39 to catalyse amide formation is very exciting and works well in some substrates.40 In reality, boric acid is a poor catalyst for amide formation but it does help drive the reaction of acids and amines that undergo substantial uncatalysed reaction over to completion.41 For these substrates, boric acid catalysis represents a very green methodology. Enzymatic methods are another catalytic method where the only by-product is water.42
The boric acid and enzymatic methodology are active research areas and the regularly updated Pfizer reagent guide gives Pfizer scientists easy access to the latest green advances in these areas. The grid also gives references to other reagents that meet two out of the three criteria.43–45
A Venn diagram covering amide formation from acids, prone to racemisation, and amines can be found in the electronic supplementary information.†
Conclusions
The experience within Pfizer has demonstrated that the medicinal chemistry population is very receptive to changing work habits in response to our green chemistry outreach initiatives. Particularly encouraging has been the remarkable response to two separate solvent reduction campaigns targeting chlorinated solvents and selected ethers. In addition, the replacement of hexane and pentane in our stockrooms with the less toxic and less volatile heptane has been extraordinarily well received. Key to these successes has been the philosophy of encouragement and education rather than obligation and scrutiny. The advantage of this Pfizer solvent tool over previous work is its simplicity, in many ways the replacements given in Table 2 are obvious. Nevertheless, the results are outstanding and we wonder if a similar approach could also work in academic laboratories and make a huge environmental difference. Chemists are highly creative individuals and when provided with the new guidance they have proved willing to adopt or invent new, greener practices. We are now moving forward with a new suite of on-line tools designed to promote greener synthetic reagents. These tools provide simple access to a diverse range of documentation and literature, which can rapidly provide the working chemist with the information they need to try new procedures. We are optimistic that this guide will share the success of our solvent initiatives and will influence our scientists to adopt safer and greener syntheses.Acknowledgements
We would like to thank the following chemistry and EHS colleagues who helped us develop the solvent and reagent tools: Jerry Clark, Robert Dugger, David Ellis, Elizabeth Girardi Schoen, Laurence Harris, Catherine Kostlan, James Long, Frank Mastrocco, Thomas Mannsbart, Andrew Mansfield, Julie Newman, Stuart Rozze, Christopher Smith, Chuck Schwartz, Brian Swierenga, Richard Webster, Christine Visnic and Ji Zhang. We also thank Steve Groombridge and Donna Medley for IT support, especially for the in-house Pfizer version of the reagent guide which provides rapid access to literature and in-house procedures.References
- P. Harremoës, The Precautionary Principle in the 20th Century: late lessons from early warnings, James & James/Earthscan, London, 2002, pp. 35–48 Search PubMed.
- J. H. Duffus and H. G. J. Worth, Fundamental Toxicology for Chemists, The Royal Society of Chemistry, London 1996, p. vi Search PubMed.
- Precaution, Environmental Science, and Preventive Public Policy, ed. J. Tickner, Island Press, Washington DC, 2002, p. 297 Search PubMed.
- Beyond the Molecular Frontier: Challenges for Chemistry and Chemical Engineering, National Academy Press, Washington DC, 2003, p. 181 Search PubMed.
- B. Trost, Science, 1991, 254, 1471–1477 CrossRef CAS.
- R. A. Sheldon, Chem. and Ind., 1992, 903–906 Search PubMed.
- Green Chemistry Theory and Practice, ed P. T. Anastas and J. C. Warner, Oxford University Press, Oxford, 1998 Search PubMed.
- A. D. Curzons, D. C. Constable and V. L. Cunningham, Clean Prod. Process., 1999, 1, 82–90; C. Jimenez-Gonzalez, A. D. Curzons, D. J. C. Constable and V. L. Cunningham, Clean Technol. Environ. Pol., 2005, 7, 42–50 Search PubMed.
- C. Capello, U. Fischer and K. Hungerbuhler, Green Chem., 2007, 9, 917–934 Search PubMed.
- The highest priority for the solvent evaluations was given to worker safety issues as handling of large quantities of solvents in manufacturing facilities bears the highest potential health and safety risk to our workforce. Within this group of solvents, the Carcinogenicty, Mutagenicity, Reprotoxicity as indicated from the CMR classification are rated as highest concern due to the potential for chronic effects on human health. Sensitisation and toxicity were also given a very high priority in the evaluation. Skin absorption properties increase the likelihood for sensitisation due to the potential carrier effects of these solvents. Toxicity (mainly assessed through literature LD50 figures) has the potential for acute and direct impact on the health and safety of our workforce.
- Environmental and regulatory considerations were considered next. Regulatory considerations vary globally so this work incorporated both major EU and US classifications such as the EU risk phrases and the US hazardous air pollutant and toxic chemical lists. Solvents with ecotoxic properties such as those designated by the EU R50, R51 and R53 risk phrases, are difficult to treat in wastewater facilities or very expensive to dispose of. There is increased public attention to potential environmental impact of facility operations which is supported by publicly available polluter registers in some countries such as the Toxic Release Inventory in the United States. Some solvents with ozone depleting and photoreactive potential are getting more public and government attention as they are regularly discussed at profession forums and regulated under various country permitting or use restriction regulations. Solvents classified as very toxic and/or classified with CMR properties or as potentially environmentally difficult materials (e.g. with the potential for persistence and bioaccumulation) are subject to increasing regulatory attention. This may include, restricted or prohibited use and/or increased requirements increased requirements to control and report use. Certain regulated compounds such as US HAPs and chemicals subject to EU Integrated Pollution Prevention and Control (IPPC) can trigger expensive and technically challenging control requirements. In summary, the use and handling of such substances is monitored very tightly by the Environmental Protection Agencies worldwide.
- The detailed information on these 39 solvents and a further 45 solvents are held in a more detailed Pfizer solvent selection tool, called the bundle book (solvents are classified into bundles). This more detailed tool is available to all Pfizer scientists through various internal websites.
- D. J. C. Constable, P. J. Dunn, J. D. Hayler, G. R. Humphrey, J. Leazer, Jr., R. J. Linderman, K. Lorenz, J. Manley, B. A. Pearlman, A. Wells, A. Zaks and T. Y. Zhang, Green Chem., 2007, 9, 411–420 RSC.
- For a review covering the green and sustainable aspects of oxidation reactions see D. Lenoir, Angew. Chem., Int. Ed., 2006, 45, 3206–3210 Search PubMed.
- For a review covering large-scale oxidations in the Pharmaceutical Industry see S. Caron, R. W. Dugger, S. Gut Ruggeri, J. A. Ragan and D. H. B. Ripin, Chem. Rev., 2006, 106, 2943–2989 Search PubMed.
- D. B. Dess and J. C. Martin, J. Org. Chem., 1983, 48, 4155–4156 CrossRef CAS.
- S. V. Ley, J. Norman, W. P. Griffith and S. P. Marsden, Synthesis, 1994, 639–666 CrossRef.
- A. J. Mancuso, S.-L. Huang and D. Swern, J. Org. Chem., 1978, 12, 2480–2482 CrossRef; A. J. Mancuso and D. Swern, Synthesis, 1981, 165–185 CrossRef CAS.
- M. R. Leanna, T. J. Sowin and H. E. Morton, Tetrahedron Lett., 1992, 33, 5029–5032 CrossRef CAS; A. E. J. de Nooy, A. C. Besemer and H. van Bekkum, Synthesis, 1996, 1153–1174 CrossRef CAS.
- A. Dijksman, I. W. C. E. Arends and R. A. Sheldon, Chem. Commun., 2000, 271–272 RSC; A. Dijksman, I. W. C. E. Arends and R. A. Sheldon, Synlett, 2001, 102–104 CAS; R. A. Sheldon, I. W. C. E. Arends, G.-J. ten Brink and A. Dijksman, Acc. Chem. Res., 2002, 35, 774–781 CrossRef CAS; S. Grimaldi, Speciality Chem., 2006, 32–33 Search PubMed.
- For aerobic oxidations catalysed by copper reagents see I. E. Marko, A. Gautier, R. Dumeunier, K. Doda, F. Philippart, S. M. Brown and C. J. Urch, Angew. Chem., Int. Ed., 2004, 43, 1588 Search PubMed and references therein. For aerobic oxidations by palladium reagents see G.-J. ten Brink, I. W. C. E. Arends and R. A. Sheldon, Science,, 2000, 287, 1636–1639 CrossRef CAS and S. S. Stahl, Angew. Chem., Int. Ed., 2004, 43, 3400 Search PubMed and references therein. For aerobic oxidations catalysed by ruthenium reagents see B.-Z. Zhan, M. A. White, T.-K. Sham, J. A. Pincock, R. J. Doucet, K. V. Ramano Rao, K. N. Robertson and T. S. Cameron, J. Am. Chem. Soc., 2003, 124, 2195–2199 Search PubMed and references therein.
- P. Gamez, I. W. C. E. Arends, J. Reedijk and R. A. Sheldon, Chem. Commun., 2003, 2414–2415 RSC.
- R. Liu, C. Dong, X. Liang, X. Wang and X. Hu, J. Org. Chem., 2005, 70, 729–731 CrossRef CAS . Note this reference also gives a very logical breakdown of the recent literature involving air oxidations of alcohols.
- J. De Luca, G. Giacomelli and A. Porcheddu, Org. Lett., 2001, 3, 3041–3043 CrossRef CAS.
- J. R. Parikh and W. v. E. Doering, J. Am. Chem. Soc., 1967, 89, 5505–5507 CrossRef CAS.
- F. Albericio, M. Cases, J. Alsina, S. A. Triolo, L. A. Carpino and S. A. Yates, Tetrahedron Lett., 1997, 38, 4853–4856 CrossRef CAS.
- R. Knorr, A. Trzeciak, W. Bannwarth and D. Gillessen, Tetrahedron Lett., 1989, 30, 1927–1930 CrossRef CAS; V. Dourtoglou, J.-C. Ziegler and B. Gross, Tetrahedron Lett., 1978, 15, 1269–1272 CrossRef.
- D. J. Dale , unpublished results.
- B. Castro, J. R. Dormoy, G. Evin and C. Selve, Tetrahedron Lett., 1975, 16, 1219–1222 CrossRef.
- W. Weng and J. S. McMurray, Tetrahedron Lett., 1999, 40, 2501–2504 CrossRef.
- C. E. Garrett, X. Jiang, K. Prasad and O. Repic, Tetrahedron Lett, 2002, 43, 4161–4165 CrossRef CAS; C. J. Barnett, T. M. Wilson and M. E. Kobierski, Org. Process Res. Dev., 1999, 3, 184–188 Search PubMed.
- B. Belleau and G. Malek, J. Am. Chem. Soc., 1968, 90, 1651–1652 CrossRef CAS; N. Izumiya and M. Muraoka, J. Am. Chem. Soc., 1969, 91, 2391–2392 CrossRef CAS.
- For an example of the large scale use of PPACA see P. J. Dunn, M. L. Hughes, P. M. Searle and A. S. Wood, Org. Process Res. Dev., 2003, 7, 244–253 Search PubMed.
- For an example of the large scale use of EDCI see ref. 33.
- J. S. Carey, D. Laffan, C. Thomson and M. T. Williams, Org. Biomol. Chem., 2006, 4, 2337–2347 RSC.
- P. J. Dunn, S. Galvin and K. Hettenbach, Green Chem., 2004, 6, 43–48 RSC.
- R. Vaidyanathan, V. G. Kalthod, N. P. Ngo, J. M. Manley and S. P. Lapekas, J. Org. Chem., 2004, 69, 2565–2568 CrossRef CAS.
- K. Ishihara, S. Ohara and H. Yamamoto, J. Org. Chem., 1996, 61, 4196–4197 CrossRef CAS.
- J. E. Anderson, R. Davies, R. N. Fitzgerald and J. M. Haberman, Synth. Commun., 2006, 36, 2129–2133 CrossRef.
- P. Tang, Org. Synth., 2005, 81, 262 CAS.
- K. Arnold, B. Davies, R. L. Giles, C. Grosjean, G. E. Smith and A. Whiting, Adv. Synth. Catal., 2006, 348, 813–820 CrossRef CAS.
- V. Schellenberger and H.-D. Jakubke, Angew. Chem., Int. Ed., 1991, 30, 1437–1449 CrossRef.
- For an example of the large scale use of iBuOCOCl see M. Prashad, D. Har, B. Hu, H.-Y. Kim, M. J. Girgis, A. Chaudhary, O. Repic, T. J. Blacklock and W. Marterer, Org. Process Res. Dev., 2004, 8, 330–340 Search PubMed.
- For an example of the large scale use of thionyl chloride see S. Cai, M. Dimitroff, T. McKennon, M. Reider, L. Robarge, D. Ryckman, X. Shang and J. Therrien, Org. Process Res. Dev., 2004, 8, 353–359 Search PubMed and M. Allegretti, R. Anacardio, M. C. Cesta, R. Curti, M. Mantovanini and G. Nano, Org. Process Res. Dev., 2003, 7, 209–213 Search PubMed.
- For an example of the large scale use of oxalyl chloride see I. H. Smith, A. Alorati, G. Frampton, S. Jones, J. O'Rourke and M. W. Woods, Org. Process Res. Dev., 2003, 7, 1034–1039 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Grid 3–oxidation of secondary alcohols to ketones. Grid 4–amide formation from acids (prone to racemisation) and amines. See DOI: 10.1039/b711717e |
| This journal is © The Royal Society of Chemistry 2008 |