Toxicity of imidazolium ionic liquids to freshwater algae
Konrad J.
Kulacki
* and
Gary A.
Lamberti
Department of Biological Sciences, University of Notre Dame, Notre Dame, IN 46556, USA. E-mail: kkulacki@nd.edu
First published on 20th November 2007
Abstract
Room-temperature ionic liquids (ILs) are a class of novel green chemicals being designed to replace traditional volatile organic solvents in industrial processes. The potential effects of ILs on aquatic ecosystems have been poorly studied, despite the possibility of unintentional discharge into rivers and lakes, and their intentional disposal in wastewater treatment plants. We studied the effects of three imidazolium ionic liquids, 1-butyl-, 1-hexyl- and 1-octyl-3-methylimidazolium bromide, on the growth rates of two freshwater algae, Scenedesmus quadricauda and Chlamydomonas reinhardtii, in 96 h standard toxicity bioassays. Increases in alkyl chain length increased the toxicity of these ionic liquids to both S. quadricauda (EC50 values of 0.005–13.23 mg L–1) and C. reinhardtii (EC50 values of 4.07–2138 mg L–1). Bioassays were performed in both nutrient-amended media and low-nutrient groundwater to evaluate if test conditions altered IL toxicity. EC50 values for S. quadricauda were similar between nutrient media and groundwater for all ILs tested, while the presence of nutrient media appeared to partially mitigate the toxicity of ILs to C. reinhardtii (groundwater EC50 < media EC50). Overall, S. quadricauda was much more sensitive than C. reinhardtii to all ILs tested, perhaps reflecting differences in cell wall structure. EC50 values suggest that ILs are more, or just as, toxic to algae than many of the solvents they are intended to replace. Results of this study show that ionic liquids can elicit a range of algal responses, suggesting that a diversity of target organisms be tested in order to predict the effects of ILs in natural environments.
Introduction
New chemicals are constantly being designed to meet the needs of industry and society, both to improve existing processes and to facilitate new ones. Increasingly, these chemicals are being designed specifically to reduce toxicity, enhance biodegradability, and prevent waste at its source, all of which are fundamental principles of green chemistry.1 While these ‘green’ chemicals are often intended to be more efficient and/or safer, this is not always the case.2 Thorough chemical and toxicological research is needed to ensure that the chemicals that are, or may some day be, in widespread use are indeed safe for humans and the environment. Such proactive research is currently underway with a novel class of industrial solvents: room-temperature ionic liquids.3Room-temperature ionic liquids (ILs) are non-volatile organic solvents being designed to replace traditional volatile organic solvents (VOS),4 such as benzene and toluene.5–7 Ionic liquids typically consist of a bulky cation, often an imidazolium or pyridinium ring; side chains that vary in length, number, and position; and a variable anion (Fig. 1). They are often referred to as ‘designer’ chemicals, since the cation and anion can be easily manipulated to change the solvent's properties and thus tailor it to a specific industrial process.8 Ionic liquids could be used, for example, as lubricants, battery electrolytes, catalysts, and in the manufacture of nanomaterials.3,9 However, recent studies have shown that the toxicity of many ionic liquids can be similar to those of the industrial solvents they may replace.10–13 While ILs pose little threat of airborne toxicity, a growing body of evidence suggests that they can be toxic to aquatic organisms, including bacteria, plants, invertebrates, and fish.3,12,14–16 However, varying the anion and length of the side chains of ionic liquids can modify their toxicity to organisms17,18 and thus provide one way to design ionic liquids in which function is balanced by low toxicity should ILs ever be released into the environment.
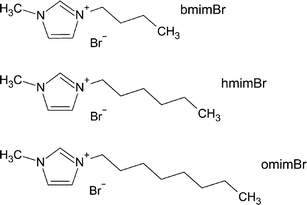 | ||
| Fig. 1 Structures of the imidazolium ionic liquids used in this study: 1-butyl-3-methylimidazolium bromide (bmimBr), 1-hexyl-3-methylimidazolium bromide (hmimBr), 1-octyl-3-methylimidazolium bromide (omimBr). | ||
If ILs were released, either intentionally after industrial waste processing or accidentally during a spill, they would likely enter aquatic ecosystems ranging from groundwater to surface water. Because many ILs are water soluble,19,20 they are likely to move readily through the environment and contact the biota. Primary producers, such as freshwater algae, are essential to aquatic food webs, serving roles in nutrient cycling and conversion of carbon dioxide into biomass,21 and could be vulnerable to chemical stressors due to their ubiquity, small size, large population numbers, and short life cycles.22 To date, few studies have examined the effects of ILs on algal primary producers.12,16,23 Latała et al.23 studied two marine algae, and found that the two species differed dramatically in their ability to recover from IL exposure, and that IL toxicity declined with increasing salinity. Wells and Coombe12 studied one freshwater alga, Pseudokirchneriella subcapitata, in 48 h bioassays and found that longer alkyl chains on the ILs increased toxicity, a trend not evident in the work of Latała et al.23 Matzke et al.16 studied Scenedesmus vacuolatus in rapid (24 h) screening assays, and found it to be sensitive to several different ILs. To better assess the potential effects of ILs on aquatic primary producers, studies of different species at the same trophic level are needed to begin to generalize responses to higher taxonomic groups.
The objective of our study was to determine the effects of imidazolium-based ionic liquids on two contrasting freshwater algae, Scenedesmus quadricauda and Chlamydomonas reinhardtii, using standard toxicity bioassays. Scenedesmus and Chlamydomonas are two common genera of freshwater algae, are easily cultured in the laboratory, and have been used as model organisms in toxicology studies.24–26 Scenedesmus (Order: Chlorococcales) are non-motile green algae, often forming colonies of four, eight, or sixteen ellipsoidal cells joined laterally.27 By contrast, Chlamydomonas (Order: Volvocales) are mobile, possessing two flagella of equal length, and occur as individual, spherical cells.28 Our hypothesis was that the two algae would respond similarly to ILs if toxicity can be generalized over broad taxonomic groups. However, morphological and physiological differences between species could result in differential responses. Furthermore, we hypothesized that the nutrient environment of the algae could influence toxicity. We predicted that the presence of replete nutrients, as provided by typical algal media, would mitigate IL toxicity to some extent.
Results
Scenedesmus quadricauda
The ionic liquid most toxic to S. quadricauda was omimBr, followed by hmimBr, and then bmimBr (Table 1); however, even bmimBr exhibited high toxicity to this alga (96 h EC50 = 13.2 mg L–1). Growth inhibition increased with increasing IL concentration in all cases (Fig. 2A,B). Growth rates declined rapidly with increasing IL concentration until zero or negative growth was exhibited. Algae exposed to omimBr, even in algal media, not only stopped growing, but also died during the experiment, as reflected in negative 96 h growth rates (Fig. 2B). S. quadricaudagrowth rates were more variable in groundwater than in the algal media. However, the addition of media did not significantly affect the toxicity of these ILs to S. quadricauda.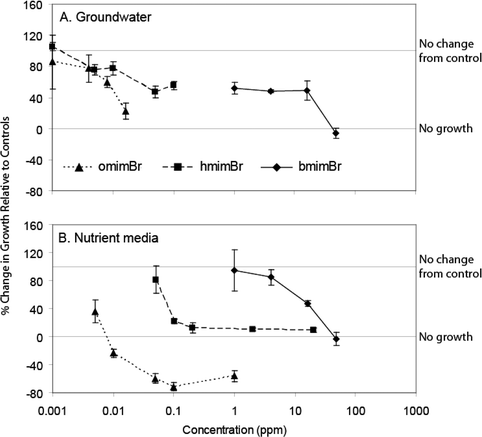 | ||
| Fig. 2 Effects of bmimBr, hmimBr, and omimBr on the growth of S. quadricauda in groundwater (A) and nutrient media (B). All results were normalized to controls for each test. 100% response indicates no difference between treatment and control; 0% response indicates zero growth. Bars represent ± 1 SE for each treatment mean. | ||
| Algae | Media | Ionic liquid | EC50/mg L–1 | 95% confidence interval |
|---|---|---|---|---|
| a n.c. = not computable. | ||||
| S. quadricauda | Groundwater | bmimBr | 4.76 | (n.c.)a |
| Groundwater | hmimBr | 0.078 | (0.045–0.191) | |
| Groundwater | omimBr | 0.005 | (0.0003–0.057) | |
| Enriched | bmimBr | 13.2 | (4.48–29.9) | |
| Enriched | hmimBr | 0.052 | (0.006–0.126) | |
| Enriched | omimBr | 0.005 | (n.c.)a | |
| C . reinhardtii | Groundwater | bmimBr | 1070 | (1020–1110) |
| Groundwater | hmimBr | 260 | (232–284) | |
| Groundwater | omimBr | 4.07 | (2.43–6.44) | |
| Enriched | bmimBr | 2140 | (1180–2640) | |
| Enriched | hmimBr | 851 | (401–14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600) 600) |
|
| Enriched | omimBr | 50.7 | (36.6–65.3) | |
Chlamydomonas reinhardtii
The ionic liquid most toxic to C. reinhardtii was omimBr, followed by hmimBr, and then bmimBr (96 h EC50 = 2140 mg L–1; Table 1). Growth inhibition increased with increasing IL concentration (Fig. 3A,B). EC50 values for the ILs tested were 103–105 higher for C. reinhardtii than for S. quadricauda. Algae exposed to 400 mg L–1bmimBr in groundwater exhibited slight hormesis (i.e. increased growth at low concentrations of otherwise toxic substances), although growth rates were not significantly different from controls. The presence of algal media increased the EC50 of omimBr to C. reinhardtii by an order of magnitude, and had similar effects for bmimBr and hmimBr (Table 1). At 64 mg L–1 in groundwater, omimBr halted the growth of C. reinhardtii and killed cells (Fig. 3A).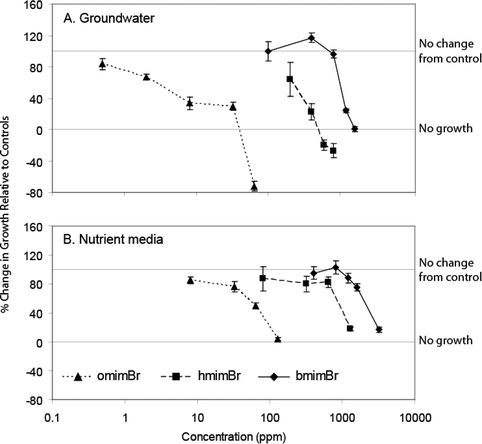 | ||
| Fig. 3 Effects of bmimBr, hmimBr, and omimBr on the growth of C. reinhardtii in groundwater (A) and nutrient media (B). All results were normalized to controls for each test. 100% response indicates no difference between treatment and control; 0% response indicates zero growth. Bars represent ± 1 SE for each treatment mean. | ||
Discussion
Traditionally, phytotoxicity data have not played an important role in the regulation of hazardous chemicals, based on the perception that plants and algae were less sensitive to most chemicals than were invertebrates and fish.29 Only recently has this assumption been re-examined, as many algal species have been found to be just as sensitive, if not more, as animals to a wide array of chemicals, including surfactants.22 We reviewed the published literature for data on the toxicity of ILs to any freshwater or marine alga (Table 2), and also compared those values to the toxicity of common industrial solvents (Table 3). Ionic liquids have been shown to be as toxic, or more so, as their volatile counterparts to freshwater algae (Table 3).| Ionic liquid | Algae | Duration/h | EC50/mg L–1 | Ref. |
|---|---|---|---|---|
| a 1-Ethyl-3-methylimidazolium tetrafluoroborate. b 1-Ethyl-3-methylimidazolium bis(1,2-benzenediolato)borate. c p.w. = present work. d Four anions were used: chloride, tetrafluoroborate, octylsulfate, and bis(trifluoromethylsulfonyl)imide. e The ILs used in this study had alkyl chains of 12, 14, and 16 carbons. | ||||
| emimBF4a | Oocystis submarina | 72 | 0.99 < EC50 < 9.9 | 23 |
| emimBF4a | Cyclotella meneghiniana | 96 | 9.9 < EC50 < 99 | 23 |
| emim(2-OPhO)2B b | Scenedesmus vacuolatus | 24 | 9.15 | 16 |
| bmimBF4 | O . submarina | 72 | >113 | 23 |
| bmimBF4 | C . meneghiniana | 96 | >113 | 23 |
| bmimPF6 | Pseudokirchneriella subcapitata | 48 | 45 | 12 |
| bmimCl | P. subcapitata | 48 | 38.5 | 12 |
| bmimBr | S. quadricauda | 96 | 13.23 (media); 4.76 (water) | p.w.c |
| bmimBr | Chlamydomonas reinhardtii | 96 | 2138 (media); 1066 (water) | p.w.c |
| bmim(CF3)2N | S. vacuolatus | 24 | 244.4 | 16 |
| bmimX–d | S. vacuolatus | 24 | 20.9–29.4 | 16 |
| hmimBF4 | O . submarina | 72 | 12.7 < EC50 < 127 | 23 |
| hmimBF4 | C. meneghiniana | 96 | 12.7 < EC50 < 127 | 23 |
| hmimBr | S. quadricauda | 96 | 0.052 (media); 0.078 (water) | p.w.c |
| hmimBr | C. reinhardtii | 96 | 850.51 (media); 259.95 (water) | p.w.c |
| omimBr | S. quadricauda | 96 | 0.005 (media); 0.006 (water) | p.w.c |
| omimBr | C. reinhardtii | 96 | 50.69 (media); 4.07 (water) | p.w.c |
| omimBF4 | S. vacuolatus | 24 | 0.001 | 16 |
| C12mimCe | P. subcapitata | 48 | 0.0011 | 12 |
| C14mimCle | P. subcapitata | 48 | 0.0041 | 12 |
| C16mimCle | P. subcapitata | 48 | 0.0129 | 12 |
| Chemical | Species | Endpoint reported | Duration/h | Concentration/mg L–1 | Ref. |
|---|---|---|---|---|---|
| a LOEC: Lowest Observed Effect Concentration. b NOEC: No Observed Effect Concentration. c n/r = not reported. d p.w. = present work. | |||||
| Acetone | S. quadricauda | Growth LOEC a | n/rc | 7500 | 49 |
| S. pannonicus | Growth NOEC b | 48 | 4740 | 50 | |
| Benzene | S. quadricauda | Growth LOEC a | n/rc | >1400 | 49 |
| S. abundans | Growth EC50 | 96 | >1360 | 51 | |
| Toluene | S. quadricauda | Growth LOEC a | n/rc | >400 | 49 |
| S. subspicatus | Growth EC50 | 48 | 125 | 52 | |
| Phenol | S. quadricauda | Growth LOEC a | n/rc | 7.5 | 49 |
| Ionic liquids | S. vacuolatus | Growth EC50 | 24 | 0.001–244 | 16 |
| S. quadricauda | Growth EC50 | 96 | 0.005–13.23 | p.w.d | |
| Chloroform | C. angulosa | Photosynthesis EC50 | 3 | 382.1 | 53 |
| Toluene | C. reinhardtii | Fluorescence EC10 | 2 | 13 | 54 |
| C. angulosa | Photosynthesis EC50 | 3 | 0.13 | 53 | |
| Hexane | C. angulosa | Photosynthesis EC50 | 3 | 8.1 | 53 |
| Benzene | C. angulosa | Photosynthesis EC50 | 3 | 0.46 | 53 |
| Ionic liquids | C. reinhardtii | Growth EC50 | 96 | 4.07–2138 | p.w.d |
In our study, we focused on two algal genera that have been used in several previous toxicological studies. For S. quadricauda and C. reinhardtii, we observed several orders of magnitude difference between their EC50 values for the same IL. In previous studies, the relative sensitivities of these two genera have ranged from Chlamydomonas being the more sensitive of the two,30 to no differences,31 to Scenedesmus being more sensitive (present study). Compared with previous studies12,16,23 of the same imidazolium ILs, but with different anions, Scenedesmus is the most sensitive algal genus tested, with S. quadricauda responding similarly to S. vacuolatus. By contrast, Chlamydomonas appears to be less sensitive to ILs than any other algal genus studied (Table 2). While the differences among these studies are noteworthy, it should be kept in mind that the anion of the ionic liquid can have a limited effect on its toxicity,16,18 at least compared to the cation, and that the duration of these tests differed among studies. The differences seen here between algal taxa and in comparison to previous studies may be explained by addressing the mechanism of IL toxicity.
While the specific mechanism of IL toxicity remains unclear, the structures of many ILs are similar to those of surfactants,32 for which the mode of toxic action is likely narcosis viamembrane disruption.33 Ionic liquids can disrupt synthetic membranes,34 but the concentrations used in that study were several orders of magnitude higher than those shown to have toxic effects in the present study, suggesting a more subtle mechanism of IL toxicity to freshwater algae. One clue to this mechanism may be the very different cell wall structures of the two species we studied. The cell wall of S. quadricauda is composed primarily of cellulose,27 while that of C. reinhardtii is composed primarily of glycoprotein.25,35 Latała et al.23 also proposed that the differences they saw in algal sensitivity were due to cell wall structure differences, with their diatom (silica-based cell wall) being more sensitive than their green alga (cellulose cell wall). The cell wall of algae is known to play a critical role in the transport of materials in and out of the cell, including toxins.36 Thus, differences in the cell wall structure could affect the abilities of cells to resist or repair membrane disruption. Taken together, our results and those of Latała et al.23 suggest that glycoprotein cell walls may be more resistant to IL disruption than cellulose cell walls, which in turn may be more resistant than silica-based walls. However, more studies are needed to fully investigate these cell wall structural differences with ionic liquids in particular. If differences in cell walls are shown to affect susceptibility to ionic liquids, we should be able to scale the effects of ILs across organisms more accurately and improve our ability to predict their effects in natural environments.
Nutrient media is commonly used to maintain laboratory cultures of algae, including during experimentation.37 We found that the presence of nutrient media had a mitigating effect on the toxicity of these ionic liquids to C. reinhardtii, but not to S. quadricauda, in comparison to low-nutrient groundwater. Similarly, the presence of nutrient media has been shown to have little effect on the toxicity of another surfactant, FFD-6, to Scenedesmus obliquus.38 Since the conductivities of the Bold's Basal media and the groundwater used in our tests were similar (666 and 742 µS, respectively), the observed differences in response to ILs between test media types were unlikely to be due to an ionic strength effect, as was seen by Latała et al.23 when altering test media salinity. The fact that we did not see a difference in IL effects on S. quadricauda between the modified COMBO media and the groundwater, though the conductivities were different (373 and 742 µS, respectively), supports this point. Established protocols22 for testing the (eco)toxicity of chemicals to freshwater algae do not consider the issue of nutrient conditions, and from the literature it is common practice to use whichever media is appropriate for a given taxon, with no consideration of the potential interactions between the media and toxicity. More broadly, aquatic ecologists are well aware of the influence that nutrient concentrations have on the community composition of pelagic algal communities.21 The differences we observed could reflect the higher nutrient concentrations in Bold's Basal media used with C. reinhardtii relative to the modified COMBO media used with S. quadricauda.37,39 This difference in nutrient levels might enable a taxon to cope better with the higher stress levels associated with a particular ionic liquid. In general, organisms that are stressed by other factors, such as starvation or disease, are more sensitive to toxins.33 Clearly, information about the abiotic conditions under which an organism will likely be exposed to a toxic substance is needed to properly predict the potential responses. Furthermore, we suggest some standardization across ecotoxicology protocols, such as those of the USEPA, to include nutrient media for algal toxicity tests. However, many examples currently in the literature and in databases lack consistency, often for practical reasons. Such inconsistencies severely limit our ability to make informed generalizations from single-species bioassays about the impacts of hazardous chemicals on organisms, including ionic liquids.
Single-species bioassays are considered a rapid, cost-effective way to determine the toxicity of chemicals to target organisms.40 These tests are often based on several assumptions, including that the test organism: (1) is the most sensitive of all the possible choices; (2) will be the most sensitive to a wider array of chemicals represented by the test chemical; and (3) will exhibit responses representative of those observed for other organisms in similar environments. In other words, results should be scalable across similar organisms, conditions, and chemicals.41 While these assumptions may not always hold true, we have addressed some deficiencies of single-species bioassays by studying two different organisms and contrasting environment conditions. In the published literature, Scenedesmus is the most sensitive algal genera to IL toxicity to date. With additional testing, other algal taxa may be shown to be equally or more sensitive to the ILs tested here. Indeed, such marked differences between two genera of freshwater algae suggests that more taxa should be considered, especially given that more than 770 genera of freshwater algae are known to exist in North America alone.42 Laboratory tests, such as the present study, provide a straightforward comparison of LC50 values across compounds (be it individual ILs or more broadly) and organisms. Such tests are a critical step to determining the potential effects of chemicals in the environment, and provide important background information for future multi-species testing, especially given the complexity of response to just two variables (species, nutrient media) apparent from this study.
The wide range of toxicity values seen here and in other studies12,16,23 illustrates the difficulty of extrapolating the results of laboratory bioassays across taxa. Furthermore, the abiotic conditions used in these tests do not span the range of conditions found in natural environments. We feel that this presents a challenge and opportunity for the field of ecotoxicology. Standard protocols typically require a defined test media, a strict light–dark regime, and a constant temperature,43 conditions rarely found in nature. To meet the challenge of making realistic predictions about the potential effects of chemicals in the environment, tests should be performed across a range of conditions in order to (1) determine how abiotic factors affect the toxicity of a chemical, and (2) enhance applicability to field conditions. While this study showed that nutrient environment can affect the toxicity of ILs to algae, further studies are needed to determine if changes in other abiotic factors, such as light and temperature, can modify toxicity. This could be especially relevant to ionic liquids, due to their countless possible structures and subsequent possible interactions with abiotic factors.
Ionic liquids can be produced in a myriad of different chemical forms, estimated to include up to 1018 possible compounds.44 One way in which ILs can be modified is by changing the length of the alkyl chain, such as from butyl to hexyl to octyl. We observed an increase in toxicity with an increase in the length of the alkyl chain, a result consistent with that of several other studies.15,17 Accordingly, in order to minimize the potential for environmental harm, the development of ionic liquids should focus on compounds with shorter alkyl chains (e.g.butyl, propyl, ethyl). We have also shown that even at low concentrations (<0.005 mg L–1), ILs can have strong negative effects on freshwater algae. The broader ecological consequences of IL toxicity to algae are substantial, given that algae represent the base of the food web of most aquatic ecosystems. The design of ionic liquids to minimize the risk to algae and other primary producers will help to protect aquatic ecosystems from unintended harm.
Conclusions
Room-temperature ionic liquids are considered to be an improvement over conventional industrial solvents due to their flexibility in design and non-volatility.4 However, these compounds have been shown to be as toxic, or more so, as their volatile counterparts to aquatic organisms, including freshwater algae. We observed considerable variation in toxicity between chemicals and organisms. To ensure the safety of natural ecosystems, additional testing should be performed on other IL structures and algal taxa using standard toxicity tests, as well as with more ecologically complex systems. Ideally, such systems would mimic the natural systems that chemicals might enter, in terms of species diversity and abiotic conditions. The ‘perfect solvent’ that has all the desirable chemical properties with none of the toxicity is unlikely to exist. However, ecologists and toxicologists can guide the development of environmentally safe ionic liquids by providing direct feedback to chemical engineers about the ecotoxicology of the compounds they generate.Experimental
Test chemicals
The three ILs used in this study were different forms of 1-alkyl-3-methylimidazolium bromide, where the alkyl chain was either four (butyl; bmimBr), six (hexyl; hmimBr), or eight (octyl; omimBr) carbons long (Fig. 1). The Kow values for these and other similar ILs range from 0.0033 to 11.1,20 suggesting that they are unlikely to bioaccumulate. The ILs used in these bioassays were synthesized in the Department of Chemical and Biomolecular Engineering, University of Notre Dame, Notre Dame, IN, USA using established synthesis procedures.45,46 These specific ILs were chosen because imidazolium ionic liquids are already commercially available, and are beginning to be used in industrial processes (Sigma Aldrich, St. Louis, MO, USA).Test organisms
S. quadricauda was obtained from the University of Texas Culture Collection (UTCC #76), and C. reinhardtii from the University of Toronto Culture Collection (UTCC #243). Both algae were batch-cultured in nutrient media at a constant temperature of 24 °C on a 12 h : 12 h light–dark cycle. Culture flasks were decanted and refilled with fresh nutrient media weekly to maintain exponential population growth. S. quadricauda was cultured and tested in modified COMBO media.39 Media was modified to include higher concentrations of nitrogen and phosphorus (7 mg L–1P, as KH2PO4–; 0.7 mg L–1N, as NaNO3) to promote algal growth. C. reinhardtii was cultured and tested in Bold's Basal Media (BBM).37 Different media were used because the two algae have different nutrient requirements.37Groundwater used in tests was low in all major nutrients (<1 mg L–1 DOC, <15 µg L–1N, <10 µg L–1P), and was not chlorinated or fluorinated. The specific conductivities of the COMBO, BBM, and groundwater were 373, 666, and 742 µS, respectively.
Test methods
All bioassays were performed according to standard protocols.47 Tests were performed in 500 mL Erlenmeyer flasks containing the appropriate alga, test media, and a range of concentrations of bmimBr, hmimBr, or omimBr. Each algae/media/IL combination was tested separately. Range-finding tests were performed initially to establish concentrations for definitive testing. All test concentrations were replicated four or five times. Experimental flasks were completely randomized and placed on a rotary shaker table (140 rpm) under a full spectrum light source (20 µM photons m–2 s–1) set for a 12 h photoperiod. All experiments were performed at a constant temperature of 24 °C. Samples for chlorophyll a (chl a) analysis were taken daily for five days (96 h) and analyzed on a fluorometer (TD-700, Turner Designs) using a methanol extraction method.43Algal growth rates [Δchl a (µg L–1 d–1)] were determined for each flask from changes in chl a over 96 h and then averaged across replicates. Growth rates for each IL concentration were then compared to the controls for that experiment to determine percent response relative to controls.Statistical analyses
EC50 values and associated 95% confidence intervals for the algal growth rate for each algae/media/IL combination were determined by fitting the dose–response curves to a logistic model using the maximum likelihood method.48 In some cases, 95% confidence intervals could not be calculated because of high variability in algal response. All analyses were performed using SAS® (Version 9.1) statistical software (SAS, Cary, NC, USA).Acknowledgements
We would like to thank Nicole Gifford and Michael Brueseke for laboratory assistance. Discussions with Randall Bernot led to the development of this study, and Dominic Chaloner provided useful comments on previous versions of the manuscript. This study was supported by grants from the US Department of Education's GAANN Program (Graduate Assistance in Areas of National Need) and the US National Oceanic and Atmospheric Administration (NOAA Awards No. NA04OAR4600076 and NA05OAR4601153).References
-
P. T. Anastas and J. C. Warner, Green Chemistry: Theory and Practice, Oxford University Press, New York, 1998 Search PubMed
.
- W. Hartley, A. Englande, Jr. and D. Harrington, Water Sci. Technol., 1999, 39, 305–310 CrossRef CAS
.
- B. Jastorff, K. Mölter, P. Behrend, U. Bottin-Weber, J. Filser, A. Heimers, B. Ondruschka, J. Ranke, M. Schaefer, H. Schröder, A. Stark, P. Stepnowski, F. Stock, R. Störmann, S. Stolte, U. Welz-Biermann, S. Ziegert and J. Thöming, Green Chem., 2005, 7, 362–372 RSC
.
- J. F. Brennecke and E. J. Maginn, AIChE J., 2001, 47, 2384–2389 CAS
.
- Y. Chauvin and H. Olivier-Bourbigou, Chemtech, 1995, 25, 26–30 CAS
.
-
R. D. Rogers and K. R. Seddon, Ionic Liquids: Industrial Applications for Green Chemistry, Oxford University Press, San Diego, CA, 2002 Search PubMed
.
-
R. D. Rogers and K. R. Seddon, Ionic Liquids IIIA: Fundamentals, Progress, Challenges, and Opportunities: Properties and Structure, Oxford University Press, San Diego, CA, 2005 Search PubMed
.
- K. N. Marsh, A. Deev, A. C. T. Wu, E. Tran and A. Klamt, Korean J. Chem. Eng., 2002, 19, 357–362 Search PubMed
.
- P. L. Short, Chem. Eng. News, 2006, 84, 15–21
.
- J. Ranke, S. Stolte, J. Störmann, J. Arning and B. Jastorff, Chem. Rev., 2007, 107, 2183–2206 CrossRef CAS
.
- R. J. Bernot, M. A. Brueseke, M. A. Evans-White and G. A. Lamberti, Environ. Toxicol. Chem., 2005, 24, 87–92 CrossRef
.
- A. S. Wells and V. T. Coombe, Org. Process Res. Dev., 2006, 10, 794–798 Search PubMed
.
- K. M. Docherty and C. F. Kulpa, Jr., Green Chem., 2005, 7, 185–189 RSC
.
- C. Pretti, C. Chiappe, D. Pieraccini, M. Gregori, F. Abramo, G. Monni and L. Intorre, Green Chem., 2006, 8, 238–240 RSC
.
- R. J. Bernot, E. E. Kennedy and G. A. Lamberti, Environ. Toxicol. Chem., 2005, 24, 1759–1765 CrossRef CAS
.
- M. Matzke, S. Stolte, K. Thiele, T. Juffernholz, J. Arning, J. Ranke, U. Welz-Biermann and B. Jastorff, Green Chem., 2007, 9, 1198–1207 RSC
.
- D. J. Couling, R. J. Bernot, K. M. Docherty, J. K. Dixon and E. J. Maginn, Green Chem., 2006, 8, 82–90 RSC
.
- S. Stolte, J. Arning, U. Bottin-Weber, M. Matzke, F. Stock, K. Thiele, M. Uerdingen, U. Welz-Biermann, B. Jastorff and J. Ranke, Green Chem., 2006, 8, 621–629 RSC
.
- J. L. Anthony, E. J. Maginn and J. F. Brennecke, J. Phys. Chem., 2001, 105, 10942–10949 Search PubMed
.
- L. Ropel, L. S. Belvèze, S. N. V. K. Aki, M. A. Stadtherr and J. F. Brennecke, Green Chem., 2005, 7, 83–90 RSC
.
-
R. G. Wetzel, Limnology, Academic Press, San Diego, CA, 2001 Search PubMed
.
-
M. A. Lewis, in Fundamentals of Aquatic Toxicology, ed. G. M. Rand, Taylor & Francis, London, 2003, pp. 135–169 Search PubMed
.
- A. Latała, P. Stepnowski, M. Nędzi and W. Mrozik, Aquat. Toxicol., 2005, 73, 91–98 CrossRef CAS
.
- A. E. Girling, D. Pascoe, C. R. Janssen, A. Peither, A. Wenzel, H. Schäfer, B. Neumeier, G. C. Mitchell, E. J. Taylor, S. J. Maund, J. P. Lay, I. Jüttner, N. O. Crossland, R. R. Stephenson and G. Personne, Ecotoxicol. Environ. Safety, 2000, 45, 148–176 CrossRef CAS
.
-
E. H. Harris, The Chlamydomonas Sourcebook, Academic Press, San Diego, CA, 1989 Search PubMed
.
- T. Zbigniew and P. Wojciech, Ecotoxicol. Environ. Safety, 2006, 65, 323 CrossRef CAS
.
-
L. E. Shubert, in Freshwater Algae of North America, ed. J. D. Wehr and R. G. Sheath, Academic Press, Amsterdam, 2003, ch. 7 Search PubMed
.
-
H. Nozaki, in Freshwater Algae of North America, ed. J. D. Wehr and R. G. Sheath, Academic Press, Amsterdam, 2003, ch. 6 Search PubMed
.
- E. E. Kenaga and R. J. Moolenaar, Environ. Sci. Technol., 1979, 13, 1479–1480 CrossRef CAS
.
- J. F. Fairchild, D. S. Ruessler and A. R. Carlson, Environ. Toxicol. Chem., 1998, 17, 1830–1834 CrossRef CAS
.
- R. Rojíčková-Padrtová and B. Maršálek, Chemosphere, 1999, 38, 3329–3338 CrossRef CAS
.
- P. J. Scammells, J. L. Scott and R. D. Singer, Aust. J. Chem., 2005, 58, 155–169 CrossRef CAS
.
-
G. M. Rand, P. G. Wells and L. S. McCarty, in Fundamentals of Aquatic Toxicology, ed. G. M. Rand, Taylor & Francis, London, 2003, pp. 3–67 Search PubMed
.
- K. O. Evans, Colloids Surf., A, 2006, 274, 11–17 CrossRef CAS
.
- J. Voigt, Planta, 1988, 173, 373–384 CrossRef CAS
.
- F. Kasai and S. Hatakeyama, Chemosphere, 1993, 27, 899–904 CrossRef CAS
.
-
R. A. Andersen, Algal Culturing Techniques, Academic Press, New York, 2005 Search PubMed
.
- M. Lurling, Chemosphere, 2006, 62, 1351–1358 CrossRef CAS
.
- S. S. Kilham, D. A. Kreeger, S. G. Lynn, C. E. Goulden and L. Herrera, Hydrobiologia, 1998, 377, 147–159 CrossRef CAS
.
-
G. M. Rand and S. R. Petrocelli, Fundamentals of Aquatic Toxicology, Hemisphere, New York, 1985 Search PubMed
.
- J. Cairns, Jr., BioScience, 1986, 36, 670–672 Search PubMed
.
-
Freshwater Algae of North America, ed. J. D. Wehr and R. G. Sheath, Academic Press, Amsterdam, 2003 Search PubMed
.
- American Public Health Association, Standard Methods for the Examination of Water and Wastewater, Port City Press, Baltimore, 1999 Search PubMed
.
- A. E. Visser, R. P. Swatloski, W. M. Reichert, R. Mayton, S. Sheff, A. Wierzbicki, J. H. Davis and R. D. Rogers, Environ. Sci. Technol., 2002, 36, 2523–2529 CrossRef CAS
.
- L. Cammarata, S. G. Kazarian, P. A. Salter and T. Welton, Phys. Chem. Chem. Phys., 2001, 3, 5192–5200 RSC
.
- P. Bonhote, A. P. Dias, N. Papageorgiou, K. Kalyanasundaram and M. Gratzel, Inorg. Chem., 1996, 35, 1168–1178 CrossRef CAS
.
-
Annual Book of ASTM Standards, E1218–1290, American Society for Testing and Materials, Philadelphia, PA, 1990 Search PubMed
.
-
M. C. Newman, Quantitative Methods in Aquatic Ecotoxicology, Lewis, Boca Raton, FL, 1995 Search PubMed
.
- G. Bringmann and R. Kuhn, Vom Wasser, 1978, 50, 45–60 Search PubMed
.
- W. Sloof, J. H. Canton and J. L. M. Hermens, Aquat. Toxicol., 1983, 4, 113–128 CrossRef
.
- H. Geyer, I. Scheunert and F. Korte, Chemosphere, 1985, 14, 1355–1369 CrossRef CAS
.
- R. Kuhn and M. Pattard, Water Res., 1990, 24, 31–38 CrossRef
.
- T. C. Hutchinson, J. A. Hellebust, D. Tam, D. Mackay, R. A. Mascarenhas and W. Y. Shiu, Environ. Sci. Res., 1980, 16, 577–586 Search PubMed
.
- W. Brack and H. Frank, Ecotoxicol. Environ. Safety, 1998, 40, 34–41 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2008 |
