The methylation of benzyl-type alcohols with dimethyl carbonate in the presence of Y- and X-faujasites: selective synthesis of methyl ethers
Maurizio
Selva
*,
Enrico
Militello
and
Massimo
Fabris
Dipartimento di Scienze Ambientali dell'Università Ca' Foscari, Calle Larga S. Marta, 2137, Venezia, 30123, Italy. E-mail: selva@unive.it; Fax: +39 041 2348 584; Tel: +39 041 2348 687
First published on 9th November 2007
Abstract
At 165–200 °C, in the presence of sodium-exchanged faujasites (NaX or NaY) as catalysts, the reaction of dimethyl carbonate with benzyl-, o- and p-methoxybenzyl-, p-hydroxybenzyl-, diphenylmethyl-, and triphenylmethyl-alcohols (1a, 2a,b, 3a, 4a, and 4c, respectively), produces the corresponding methyl ethers in up to 98% yields. A peculiar chemoselectivity is observed for hydroxybenzyl alcohols (compounds 3a and 3b, para- and ortho-isomers) whose etherification takes place without affecting the OH aromatic groups. Acid–base interactions of alcohols and DMC over the faujasite surface offer a plausible explanation for the catalytic effect of zeolites NaY and NaX, as well as for the trend of reactivity shown by the different alcohols (primary > secondary > tertiary). However, in the case of substrates with mobile protons in the β-position (i.e.1-phenylethanol and 1,1-diphenylethanol), the dehydration reaction to olefins is the major, if not the exclusive, process.
Introduction
Conventional techniques for the synthesis of benzyl methyl ethers are mainly based on the classical base-promoted Williamson reaction of benzyl-type alcohols with methyl halides, dimethyl sulfate or diazomethane.1 Though efficient, these procedures pose a great concern from both safety and environmental standpoints: they use highly noxious methylating agents, often in the presence of toxic solvents (THF, hexane, benzene), and they consume over-stoichiometric amounts of strong bases which imply work-ups with aqueous solutions and the co-generation of by-products and polluted effluents to be disposed of. A viable alternative is the methanolysis of alcohols which has been reported over a variety of Brønsted and Lewis acidic compounds such as HCl,2 H2SO4,3 p-MeC6H4SO3H,4 CF3SO3H,5CAN [(NH4)2Ce(NO3)6],6 FeX3 (X = Cl, NO3),7RE(OTf)3 (RE = Yb, Sc),8 and NaHSO4/SiO2.9 These methods, however, are limited to secondary and tertiary substrates,2–7 or to primary benzyl alcohols only if activated by OH and ORpara-substituents.8,9Safer and selective methylation protocols can be conceived with the non-toxic dimethyl carbonate (MeOCO2Me, DMC).10 In the presence of weak bases or alkali metal-exchanged Y-faujasites (FAU) as catalysts,11 a number of O-, S-, C- and N-nucleophiles (e.g. phenols, thiols, CH2-active compounds, and primary amines) react with DMC to produce the corresponding methyl derivatives [Scheme 1, paths (a)–(d)] in very high yields (85–95%).12 Of particular note are the cases of CH2-active substrates and anilines whose reactions proceed with unprecedented high mono-C- and mono-N-methyl selectivity (up to 99%) towards ArCH(Me)X, and ArNHMe products [paths (c) and (d)].
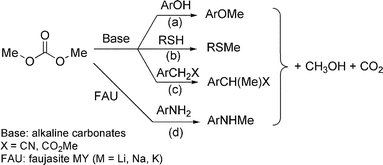 | ||
| Scheme 1 Methylation reactions mediated by DMC. | ||
Under basic catalysis, however, the reaction of alcohols with DMC goes through an exclusive transesterification process (BAc2 mechanism) to yield methyl alkyl carbonates as sole products [ROCO2Me; Scheme 2(a)].13 A selective synthesis of methyl ethers from DMC has been recently reported only for a few substrates, in the presence of alumina and hydrotalcite promoters:14 final products (ROMe) are obtained in two steps via an initial transesterification reaction followed by an in situ decarboxylation process [Scheme 2(b)].
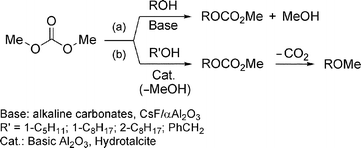 | ||
| Scheme 2 Reactions of DMC with alcohols. | ||
Based on our previous results on DMC-mediated methylation reactions catalyzed by zeolites,12,15 we decided to explore both Y- and X-faujasites as catalysts for the preparation of methyl ethers of primary, secondary and tertiary benzyl-type alcohols (Scheme 3).
 | ||
| Scheme 3 | ||
We wish to report herein that the combined use of DMC and Y- or X-faujasites offers an excellent tool to set up an innovative etherification protocol: at 165–200 °C, six out of nine of the tested alcohols (1a, 2a,b, 3a, 4a and 4c) undergo a clean O-methylation to produce methyl ethers in up to 98% isolated yields. Compound 3b instead gives the corresponding ether in a 46% yield, and alcohols 1b and 4b, are preferably dehydrated to the corresponding olefins. To further elucidate the scope and limitations of the method, commercial NaY and NaX zeolites are also compared to a conventional basic catalyst (K2CO3) used for methylations promoted by DMC.
Results
Benzyl alcohol, 1a
Initially, the model reaction of benzyl alcohol with DMC was investigated. A solution of compound 1a (0.2 g, 1.9 mmol) in DMC (6 × 10–2 M, 30 mL; DMC serving both as a reagent and the solvent) was set to react at 165–200 °C in a stainless steel autoclave (90 mL), in the presence of different faujasites NaY and NaX [weight ratio zeolite : substrate (Q) = 3]. All reactions were carried out under a N2 atmosphere and they were monitored by GC–MS.At 165 °C, the same procedure was also used to perform experiments with K2CO3 (weight ratio K2CO3 : substrate of 3), in place of faujasites. Table 1 reports the results.
| Entry | Catalyst | Q b (wt : wt) | t/h | T/°C | Conv. (%)c | Products (%, by GC)c | Y (%)d | ||
|---|---|---|---|---|---|---|---|---|---|
| PhCH2OMe | PhCH2OCO2Me | (PhCH2)2O | |||||||
| a All reactions were carried out using a solution of 1a (0.2 g, 1.9 mmol) in DMC (6 × 10–2 M, 30 mL). b Q was the weight ratio of catalyst : substrate. c Both conversion and % amounts of BME, BMC, and DBE were determined by GC analyses. d Y: Isolated yield of benzyl methyl ether. e Also, dibenzyl carbonate (7%) was detected. | |||||||||
| 1 | None | 3 | 180 | — | — | — | — | ||
| 2 | K2CO3 | 3 | 4 | 165 | 100 | 100 | |||
| 3 | K2CO3 | 3 | 4 | 180 | 100e | 93 | |||
| 4 | NaY | 3 | 7 | 165 | 35 | 10 | 23 | ||
| 5 | NaY | 3 | 7 | 180 | 80 | 53 | 19 | 6 | |
| 6 | NaY | 3 | 5 | 200 | 100 | 93 | 3 | 4 | 92 |
| 7 | NaX | 3 | 3 | 200 | 100 | 99 | 96 | ||
In the absence of K2CO3 or of faujasites, benzyl alcohol was recovered unreacted after 3 h at 180 °C (entry 1). Otherwise, different products were observed: benzyl methyl ether (BME), benzyl methyl carbonate (BMC), dibenzyl carbonate (DBC), and dibenzyl ether (DBE), respectively (Scheme 4).
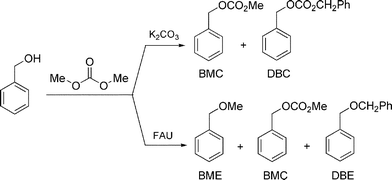 | ||
| Scheme 4 | ||
The nature of the catalyst controlled the relative amounts of these products.
In particular, at 165–180 °C, in the presence of K2CO3, the transesterification of dimethyl carbonate was the exclusive process, and BMC or a mixture of BMC and DBC was obtained (entries 2, 3).
The use of faujasites instead allowed simultaneous methylation and carboxymethylation processes (entries 4, 5). Minor amounts of dibenzyl ether were also observed, plausibly due to the dehydration of benzyl alcohol. However, when the reaction was carried out at 200 °C, BME could be isolated in up to 96% yield (entries 6–7).16 The high temperature, in fact, promoted the quantitative decarboxylation of the transesterification product (BMC). Good evidence for this behavior was gathered from separate experiments, in which solutions (0.2 M, 30 mL) of BMC (0.3 g, 1.9 mmol) in either cyclohexane or dimethoxyethane were heated up to 200 °C in the presence of both NaX and NaY solids (weight ratio for zeolite : BMC of 1.5). In all cases, after 3 h, benzyl methyl ether was the sole product (Scheme 5).
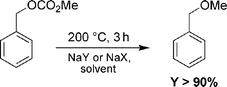 | ||
| Scheme 5 | ||
The excellent selectivity towards BME prompted us to investigate possible effects associated with the character and the amount of the two zeolites. Under the conditions of entry 6 in Table 1 (200 °C; 6 × 10–2 M solution of 1a in DMC, 30 mL), a set of experiments was carried out using different quantities of both NaY and NaX catalysts. The Q ratio (weight ratio cat. : 1a) was ranged from 0.2 to 3, and for each test the reaction was monitored until a complete conversion of benzyl alcohol was obtained.
Results are described in Fig. 1, in which the reaction time necessary to reach a quantitative substrate conversion was plotted against Q (left to right). The grey and black profiles refer to NaY and NaX zeolites, respectively. To complete the picture, the figure also reports the selectivity towards the formation of BME (right to left).
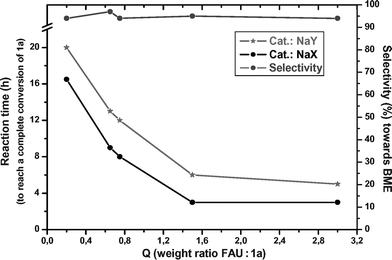 | ||
| Fig. 1 Synthesis of BME over NaY and NaX catalysts. | ||
Four major aspects emerged from this analysis. (i) Both faujasites (NaY and NaX) acted as genuine catalysts: after 16–20 h (left ordinate), benzyl alcohol was totally converted even using Q as low as 0.2. (ii) When Q was increased from 0.2 to 1.5, reaction times dropped considerably: the etherification could be accomplished up to 4–5 times faster (3–5 h, Q = 1.5). However, the rate was not substantially improved by a further increasing of the amount of the zeolite (Q from 1.5 to 3). (iii) The change of Q (0.2–3) never appreciably affected the selectivity towards BME, which remained always very high (93–97%: right ordinate).17 (iv) The comparison of the two zeolites showed that reactions catalyzed by the NaX faujasite took place quicker than those carried out over the NaY one. This difference was particularly pronounced at Q ![[greater than or equal, slant]](https://www.rsc.org/images/entities/char_2a7e.gif) 1.5: under these conditions, reaction times were nearly halved (3 h) when NaX was used.
1.5: under these conditions, reaction times were nearly halved (3 h) when NaX was used.
Additional experiments were performed to scale up the reaction. At 200 °C, in the presence of NaY (Q = 0.2), benzyl alcohol (2.5 g, 23.1 mmol) was set to react with different amounts of DMC (10, 40, 58, and 117 mL). The corresponding range of the DMC : 1a molar ratio (W) was from 5 to 60. Each test was carried out for 24 h. Table 2 reports the results.
| Entry | NaY (Q)a | DMC : 1a (W)b | t/h | T/°C | Conv. (%)c | Products (%, by GC)c | |||
|---|---|---|---|---|---|---|---|---|---|
| PhCH2OMe | PhCH2OCO2Me | (PhCH2)2O | Othersd | ||||||
| a Q was the weight ratio of catalyst : substrate. b W was the DMC : 1a molar ratio. c Both conversion and % amounts of BME, BMC, and DBE were determined by GC analyses. d Total amount of other unidentified products. | |||||||||
| 1 | 0.2 | 5 | 24 | 200 | 82 | 42 | 1 | 34 | 5 |
| 2 | 0.2 | 20 | 24 | 200 | 98 | 76 | 1 | 19 | 2 |
| 3 | 0.2 | 30 | 24 | 200 | 100 | 90 | — | 8 | 2 |
| 4 | 0.2 | 60 | 24 | 200 | 100 | 94 | 3 | 3 | |
The reaction selectivity was dramatically affected by the W ratio. At the lowest W (5: entry 1), the dehydration of benzyl alcohol took place to a large extent: at a conversion of 82%, dibenzyl ether was produced at 34%. However, as the DMC : 1a molar ratio was increased up to 30, a quantitative reaction was observed and the amount of DBE decreased markedly to 10% (entries 2 and 3). Finally, at W = 60, the BME yield was of 94% (by GC, entry 4). This last result substantially matched that reported in Fig. 1 (at Q = 0.2). In other words, a selective synthesis of BME was possible only if a large-to-moderate excess of DMC was used. This reagent/solvent, however, could be quantitatively recovered by distillation, and recycled several times.18
Other benzyl-type alcohols (1b, 2a,b, 3a,b, 4a–c)
According to the above-described procedure for 1a, solutions of primary, secondary, and tertiary benzyl-type alcohols 1b, 2a,b, 3a,b, and 4a–c in DMC (6 × 10–2 M, 30 mL) were made to react at 165–200 °C, in the presence of both faujasites NaY and NaX. If not otherwise specified, the weight ratio of zeolite : substrate (Q) was set to 1.5. Table 3 reports the results.| Entry | Substrate XC6H4CR(R′)OH | Cat. (Q)b | t/h | T/°C | Conv. (%)c | Products (%, by GC)c | Y (%) d |
|---|---|---|---|---|---|---|---|
| a All reactions were carried out using a solution of the substrate (1.9 mmol) in DMC (6 × 10–2 M, 30 mL). b Q was the weight ratio of faujasite : substrate. c Both conversion and % amounts of different products were determined by GC analyses. d Y: Isolated yield of methyl ethers. | |||||||
| 1 | 2a: X = p-MeO; R = R′ = H | NaY (1.5) | 6 | 200 | 100 | p-MeOC6H4CH2OMe (94) | 98 |
| 2 | 2a: X = p-MeO; R = R′ = H | NaX (1.5) | 4 | 200 | 100 | p-MeOC6H4CH2OMe (98) | |
| 3 | 2b: X = o-MeO; R = R′ = H | NaY (1.5) | 6.5 | 200 | 100 | o-MeOC6H4CH2OMe (98) | 97 |
| 4 | 2b: X = o-MeO; R = R′ = H | NaX (1.5) | 4 | 200 | 100 | o-MeOC6H4CH2OMe (99) | |
| 5 | 3a: X = p-OH; R = R′ = H | NaY (3) | 5 | 165 | 100 | p-(HO)C6H4CH2OMe (100) | 85 |
| 6 | 4a: X = R = H; R′ = C6H5 | NaY (1.5) | 9 | 200 | 96 | (C6H5)2CHOMe (89) | 90 |
| (C6H5)2CHOCO2Me (7) | |||||||
| 7 | 4a: X = R = H; R′ = C6H5 | NaX (1.5) | 5 | 200 | 100 | (C6H5)2CHOMe (99) | 96 |
| 8 | 4c: X = H; R = R′ = C6H5 | NaY (1.5) | 11 | 200 | 88 | (C6H5)3COMe (74) | 72 |
| (C6H5)3CH (14) |
At 200 °C, alcohols 2a,b and 4a gave the corresponding methyl ethers [Scheme 6(a)] in >95% purity (by GC–MS, entries 1–4 and 6–7). These products were isolated in 90–98% yields by simple filtration of the zeolite and removal of DMC under vacuum.
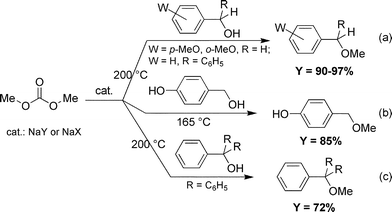 | ||
| Scheme 6 | ||
A similar result was obtained also for alcohol 3a on the condition that a lower temperature was used (entry 5: 165 °C; 85% yield) [Scheme 6(b)].19 In this case, the high chemoselectivity was a further added value of the procedure: a clean O-methylation of the alcohol group took place, the aromatic OH function being fully preserved from any possible methylation or carboxymethylation process (see also Scheme 1). It should be noted that only a very few methods are available in the literature, for a straightforward high-yield synthesis of methyl p-hydroxybenzyl ether.6c,9
In the case of triphenylcarbinol (4c), at 200 °C, the reaction still proceeded with a high conversion (88%, entry 8); however, it required a longer time than for previous compounds, and the etherification selectivity was not as excellent as before. Methyl triphenylmethyl ether was isolated in 72% yield [Scheme 6(c)].20
The reactivity of alcohols 2–4 reflected some aspects already observed for benzyl alcohol: (i) the NaX faujasite generally allowed faster reactions with respect to NaY (compare entries 1–2, 3–4, and 6–7); (ii) etherification reactions were truly catalytic processes which could be simply scaled up to 1–2 g. For instance, in separate experiments, when compounds 2a (1.5 g, 10.9 mmol) and 4a (1.5 g, 8.2 mmol) were set to react at 200 °C in the presence of DMC (70 mL) and NaY (Q = 0.2), the corresponding methyl ethers were obtained in amounts of 97 and 91%, respectively, after 11 and 21 h. Under the same conditions, the reaction of 4c (1.5 g, 5.8 mmol) showed a conversion of only 52% (methyl ether: 44%), after 26 h; (iii) if zeolites were replaced with K2CO3, the synthesis of methyl ethers of alcohols 2–4 was never possible. At 165–200 °C, the reaction of 2a and 4a, with DMC and K2CO3 (molar ratio substrate : DMC : base of 1 : 60 : 1.5, respectively) gave transesterification products exclusively (ROCO2Me, R = p-hydroxybenzyl and 1,1-diphenylmethyl).
By contrast to the good result obtained for p-hydroxybenzyl alcohol (3a), both NaY and NaX catalysts offered a poor selectivity in the reaction of the ortho-isomer 3b. Under the conditions of entry 5 in Table 3 (NaY, 165 °C, 5 h, Q = 3), the conversion of 3b was quantitative, but its methyl ether [o-(HO)C6H4CH2OMe] was detected in only a 47% amount (by GC–MS), other products being o-cresol [o-(HO)C6H4CH3, 28%] and o-hydroxybenzaldehyde [o-(HO)C6H4CHO, 27%].21 Yet, no methylation of the aromatic OH group took place.
Faujasites did not succeed in the reaction of DMC with alcohols 1b and 4b. In these cases, methyl ethers were minor products or they were not obtained at all. For example, under the conditions of entry 5 in Table 3 (NaY, 165 °C, 5 h, Q = 3), 1-phenylethanol (1b) was converted into a mixture of methyl 1-phenylethyl ether (9%) and styrene (56%), whereas 1,1-diphenylethanol (4b) yielded 1,1-diphenylethylene as the sole product.
Discussion
The role of the NaX and NaY zeolites
Alkali metal-exchanged faujasites are often reported as amphoteric solids.11c,22 Accordingly, the interactions of these zeolites with alcohols, particularly of the benzyl type, and with DMC as well, are basically described through H-bonds and acid–base reactions occurring at the surface of the solid catalyst.23,24 Scheme 7 offers a plausible mechanistic pattern for the synthesis of benzyl methyl ether promoted by DMC, over both NaY and NaX catalysts.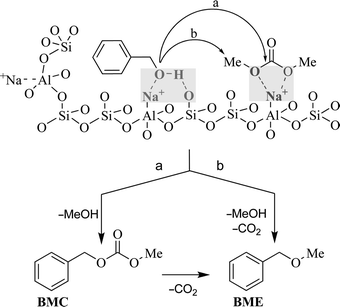 | ||
| Scheme 7 Pictorial view of the reaction of benzyl alcohol and DMC over a faujasite. | ||
At the catalyst surface, benzyl alcohol and DMC undergo a nucleophilic (shaded section, left) and an electrophilic (shaded section, right) activation, respectively.25 The so-formed alcoholate-like species attacks methyl and carbonyl carbons of DMC to produce both BMC and BME (paths a and b), through tetrahedral and SN2 mechanisms. Then, at a high temperature (preferably over 180 °C), the decarboxylation of BMC takes place to yield BME as the sole product (see Scheme 5). This last reaction of BMC is reported also over basic alumina or hydrotalcite solids in the presence of dimethyl carbonate.14
Data of Tables 1 and 3, and of Fig. 1, suggest that NaX and NaY faujasites exhibit a different activity: in particular, according to the acid–base scale proposed by Barthomeuf (Scheme 6),11c,22 one would conclude that the more basic NaX has a better performance than NaY. This is consistent with our previous findings on the reactions of indolyl carboxylic acids with DMC, promoted by zeolites.15b In the present case, however, Fig. 1 also shows that the difference in reaction times observed for the two faujasites is rather constant (3–4 h), regardless of the amount of catalyst used (compare black and grey lines with Q). This trend might imply the occurrence of an induction period for the less active NaY faujasite. At the moment, this behavior has no clear reasons.
Traces of water (and Brønsted acidity) in the catalysts may also explain the dehydration of benzyl alcohol to form dibenzyl ether (Tables 1 and 2):26 not surprisingly, the process becomes significant when a high concentration of the alcohol is available at the zeolite surface (entries 1 and 2, Table 2). Notwithstanding, the onset of this side-reaction can be substantially prevented if a large-to-moderate excess of DMC is used.
The different reactivity of alcohols
A reactivity scale can be highlighted for the different alcohols. In particular, primary substrates react more rapidly than secondary and tertiary ones (Tables 1 and 3). This result is likely ascribed to the modes of surface interactions illustrated in Scheme 7: the higher the steric crowding around the OH group, the worse the adsorption and the contact of reagents, the poorer the catalysis is. Aromatic substituents also modify the reactivity of alcohols 2 and 3, though a general tendency cannot be inferred. For instance, compounds 3a,b bearing an electron-donating OH aryl substituent undergo the etherification reaction at a relatively low temperature (165 °C). Methoxybenzyl alcohols would then be expected to be more reactive than benzyl alcohol, but they are not: at 200 °C, the reaction rate for compounds 2a,b is comparable (if not lower) to that of 1a (Fig. 1 and Table 3). A rationale for this incongruity is perhaps on the nature and the geometry of adsorption of reagents over the catalyst: since OH aryl substituents are able to interact directly with the zeolite surface,11c,22,27 it is plausible that hydroxybenzyl alcohols (3a,b) are adsorbed more strongly than other compounds, such that the resulting catalysis is favored.In the case of 1-phenyl- and 1,1-diphenyl-ethanol (compounds 1b and 4b, respectively), the co-presence of mobile methyl protons and of traces of Brønsted acidity in the catalysts allows the dehydration reaction to olefins, to be the major, if not the exclusive, process.
Conclusions
The combination of dimethyl carbonate and alkali metal-exchanged Y- and X-faujasites offers unique possibilities to accomplish selective methylations of a variety of N- and S-nucleophiles. This work discloses a further important application of the same system: in the presence of both NaY and NaX zeolites, a highly selective and straightforward etherification of benzyl-type alcohols is possible using dimethyl carbonate as a reagent and a solvent. Typically, methyl ethers of primary, secondary and tertiary substrates (1–4) can be isolated in 72–98% yields. In the case of hydroxybenzyl alcohols, the reaction is also very chemoselective: for instance, at 200 °C, methyl p-hydroxybenzyl ether is obtained in 85% yield, the aromatic OH-substituent being fully preserved from methylation and/or methoxycarbonylation side-reactions.Under the same reaction conditions, if zeolites are replaced with K2CO3 (a conventional basic catalyst for DMC-mediated reactions), alcohols undergo an exclusive transesterification process to produce the corresponding methyl alkyl carbonates (ROCO2Me).
The possible reaction mechanism involves the initial activation of both DMC and the reactant alcohols over the zeolite surface. This occurs through the formation of H-bonds and acid–base interactions with basic oxygen atoms and weakly acidic cations, which belong to the zeolite framework. Then, a sequence of tetrahedral and SN2-type processes followed by a decarboxylation reaction takes place to yield methyl ethers as the final products. The overall reactivity of compounds 1–4 is sensitive to the steric crowding around the alcoholic function: primary substrates are more reactive than secondary and tertiary ones. The reaction rate is also modified by aromatic substituents whose presence plausibly alters the adsorption of reagents on the catalysts.
Although the reported protocol is rather energy intensive, several green features can be recognized: (i) DMC is used as a non-toxic reagent and solvent; (ii) commercially available sodium-exchanged faujasites (NaY and NaX) are eco-safe materials which can be easily separated by filtration, reactivated, and recycled without any loss of activity and/or selectivity;15 (iii) except for MeOH and CO2, no organic/inorganic by-products are observed; and (iv) thanks to the excellent O-methylation selectivity, high-quality methyl ethers are obtained with simple and cheap purification methods.
Experimental
Compounds 1a,b, 2a,b, 3a,b, 4a–c and DMC were ACS grade and were employed without further purification. Zeolites NaY and NaX were from Aldrich (art. # 334448 and 283592, respectively): before each reaction, these solids were dried under vacuum (65 °C; 8 mbar) overnight.MS (EI, 70 eV) analyses were run using a HP5/MS capillary column (30 m). 1H NMR spectra were recorded on a 300 MHz spectrometer, using CDCl3 as solvent. Chemical shifts were reported in δ values downfield from TMS.
Reactions carried out in autoclave. General procedure
A stainless-steel autoclave (150 mL of internal volume) was charged with a solution (6 × 10–2 M; 30 mL) of the chosen substrate (1a,b, 2a,b, 3a,b, and 4a–c; 1.9 mmol), dimethyl carbonate (0.36 mol) and NaY or NaX [the weight ratio (Q) of faujasite : substrate was in the range of 0.2–3; see Tables 1 and 2 and Fig. 1 for details]. At room temperature and before the reaction, air was carefully removed by a purging valve with a N2 stream. The autoclave was then electrically heated, while the mixture was kept under magnetic stirring throughout the reaction. A thermocouple fixed onto the autoclave head checked the temperature (165–200 °C). After different time intervals (3–20 h), the autoclave was cooled to rt, purged from CO2, and finally, opened. The reaction mixture was analysed by GC–MS.The same procedure was also used for the following:
(i) scale up of the reaction to the gram level. In this case, compounds 1a (1.5 g, 13.9 mmol), 2a (1.5 g, 10.9 mmol), 4a (1.5 g, 8.2 mmol), and 4c (1.5 g, 5.8 mmol) were set to react at 200 °C in the presence of DMC (70 mL) and NaY (Q = 0.2).
(ii) carrying out experiments with K2CO3 as a catalyst. In this case, the reaction temperature was set to 165–180 °C, and the molar ratio of K2CO3 : substrate was in the range of 1.5–2.3.
The synthesis and the decarboxylation of benzyl methyl carbonate (BMC)
BMC was prepared according to a procedure previously reported by us:13b a mixture of benzyl alcohol (1.0 g, 9.3 mmol), dimethyl carbonate (30 mL) and K2CO3 (2.6 g, 18.5 mmol) was set to react at 90 °C for 15 h. After filtration of the solid base and removal of DMC under vacuum, BMC was isolated in 98% purity (yield: 1.28 g, 83%). The structure of BMC was assigned by GC–MS and by comparison to an authentic sample. The crude product was used as such for the the decarboxylation step of Scheme 5: the reaction was carried out in an autoclave, under conditions similar to those described above (see general procedure). A solution of BMC (0.2 g, 1.2 mmol) in a given solvent (cyclohexane or dimethoxyethane, 30 mL) was charged in an autoclave of 90 mL in the presence of NaY or NaX faujasite (the weight ratio of faujasite : BMC was 1.5). After purging with a N2 stream, the reactor was heated at 200 °C for 3 h, while the mixture was kept under magnetic stirring throughout the reaction. The final product (benzyl methyl ether) was characterised by GC–MS.The isolation and characterisation of methyl ethers
Crude methyl ethers of benzyl, p- and o-methoxybenzyl-, p-hydroxybenzyl-, and diphenylmethyl-alcohols were isolated in 96–98% GC-purity, by simple filtration of the zeolite and removal of DMC under vacuum (35 °C/250 mm). Methyl triphenylmethyl ether was further purified by flash column chromatography on silica gel F60 (eluant : petroleum ether/diethyl ether in 5 : 1 v/v). The products were characterized by GC–MS and 1H NMR. In the case of o-hydroxybenzyl methyl ether and methyl 1-phenylethyl ether, which were not isolated from the reaction mixture, the respective structures were assigned only by GC–MS.Spectroscopic and physical properties of all ethers were in agreement with those reported in the literature.
Benzyl methyl ether
Pale-yellow liquid [lit.1d bp 29–30 °C/0.1 mm] 1H NMR (300 MHz, CDCl3) δ 3.40 (s, 3H), 4.47 (s, 2H), 7.29–7.39 (m, 5H). MS (EI), m/z (relative int.): 122 (M+, 61%), 121 (M+ – H, 61), 92 (24), 91 (M+ – OMe, 100), 77 (28), 65 (16).p-Methoxybenzyl methyl ether
Yellow liquid [lit.1f bp 47–50 °C/1.5 × 10–3 mm]. 1H NMR (300 MHz, CDCl3) δ 3.36 (s, 3H), 3.81 (s, 3H), 4.39 (s, 2H), 6.88 (d, 2H, J = 8.5 Hz), 7.27 (d, 2H, J = 8.7 Hz). MS (EI), m/z (relative int.): 152 (M+, 29 %), 151 (M+ – H, 19), 122 (11), 121 (M+ – OMe, 100), 77 (15).o-Methoxybenzyl methyl ether
Pale-yellow liquid [lit.28 bp 168–169 °C]. 1H NMR (300 MHz, CDCl3) δ 3.42 (s, 3H), 3.84 (s, 3H), 4.50 (s, 2H), 6.84–7.00 (m, 2H), 7.23–7.38 (m, 2H). MS (EI), m/z (relative int.): 152 (M+, 55 %), 151 (M+ – H, 16), 122 (16), 121 (M+ – OMe, 100), 92 (12), 91 (99), 77 (24), 65 (16).p-Hydroxybenzyl methyl ether
Mp 79–82 °C (white solid) [lit.4 mp 80–81 °C]. 1H NMR (300 MHz, CDCl3) δ 3.37 (s, 3H), 4.36 (s, 2H), 6.72 (d, 2H, J = 8.3 Hz), 7.21 (d, 2H, J = 8.6 Hz). MS (EI), m/z (relative int.): 138 (M+, 35 %), 137 (M+ – H, 24), 121 (15), 107 (M+ – OMe, 100), 106 (28), 78 (14), 77 (27), 51 (12).o-Hydroxybenzyl methyl ether
(Not isolated), MS (EI), m/z (relative int.): 138 (M+, 44 %), 107 (M+ – OMe, 19), 106 (65), 78 (100), 77 (34), 51 (12).Diphenylmethyl methyl ether
Viscous oil [lit.2 bp 157–158 °C/20 mm]. 1H NMR (300 MHz, CDCl3) δ 3.34 (s, 3H), 5.21 (s, 1H), 4.50 (s, 2H), 7.17–7.35 (m, 10H), MS (EI), m/z (relative int.): 198 (M+, 66 %), 197 (M+ – H, 18), 168 (16), 167 (M+ – OMe, 100), 166 (22), 165 (55), 152 (25), 121 (M+ – Ph, 99), 105 (21), 91 (17), 77 (49).Methyl triphenylmethyl ether
Mp = 81–82 °C (pale-yellow solid) [lit.6a mp 83–84 °C]. 1H NMR (300 MHz, CDCl3) δ 3.06 (s, 3H), 7.18–7.37 and 7.41–7.49 (m, 15H). MS (EI), m/z (relative int.): 274 (M+, 29 %), 243 (M+ – OMe, 45), 197 (M+ – Ph, 100), 166 (M+ – Ph – OMe, 12), 165 (62), 105 (PhCO+, 73), 77 (53).Methyl 1-phenylethyl ether
(Not isolated), MS (EI), m/z (relative int.): 136 (M+, 2%), 121 (M+ – Me, 100), 105 (M+ – OMe, 24), 91 (12), 77 (25).By-products
The structures of o-cresol, o-hydroxybenzaldehyde, styrene, and 1,1-diphenylethylene were assigned by GC–MS.o-Cresol
MS (EI), m/z (relative int.): 108 (M+, 100 %), 107 (85), 91 (M+ – OH, 10), 90 (22), 79 (36), 77 (36).o-Hydroxybenzaldehyde
MS (EI), m/z (relative int.): 122 (M+, 100 %), 121 (M+ – H, 91), 93 (M+ – CHO, 18), 76 (19), 65 (29).Styrene
MS (EI), m/z (relative int.): 104 (M+, 100 %), 103 (M+ – H, 46), 78 (41), 77 (19).1,1-Diphenylethylene
MS (EI), m/z (relative int.): 180 (M+, 100 %), 179 (M+ – H, 73), 178 (62), 165 (92), 89 (20), 77 (12).Acknowledgements
MIUR (Italian Ministry of University and Research) and Consorzio Interuniversitario ‘La Chimica per l'Ambiente’ are gratefully acknowledged for financial support. We also thank Dr A. Perosa for his helpful comments.References and notes
- (a) G. W. Griffin and A. Manmade, J. Org. Chem., 1972, 37, 2589–2599 CrossRef CAS; (b) B. A. Stoochnoff and L. Benoiton, Tetrahedron Lett., 1973, 21–24 CrossRef CAS; (c) H. Ogawa, Y. Ichimura, T. Chihara, S. Teratami and K. Taya, Bull. Chem. Soc. Jpn., 1986, 59, 2481–2483 CAS; (d) J. Blagg, S. G. Davies, N. J. Holman, C. A. Laughton and B. E. Mobbs, J. Chem. Soc., Perkin Trans. 1, 1986, 1581–1589 RSC; (e) H. Ogawa, T. Hagiwara, T. Chihara, S. Teratami and K. Taya, Bull. Chem. Soc. Jpn., 1987, 60, 627–629 CAS; (f) E. Baciocchi, M. Bietti and M. Mattioli, J. Org. Chem., 1993, 58, 7106–7110 CrossRef CAS; (g) X.-K. Fu and S.-Y. Wen, Synth. Commun., 1995, 25, 2435–2442 CrossRef CAS; (h) H. Surya Prakash Rao, S. P. Senthilkumar, D. Srinivasa Reddy and G. Mehta, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 1999, 38, 260–263; (i) J. J. Gajewski, W. Bocian, N. J. Harris, L. P. Olson and J. P. Gajewski, J. Am. Chem. Soc., 1999, 121, 326–324 CrossRef CAS; (j) R. Shu, J. F. Harrod and A.-M. Lebuis, Can. J. Chem., 2002, 80, 489–495 CrossRef CAS; (k) B. Branchi, M. Bietti, G. Ercolani, M. A. Izquierdo, M. A. Miranda and L. Stella, J. Org. Chem., 2004, 69, 8874–8885 CrossRef CAS.
- G. E. Hartzell and E. S. Huyser, J. Org. Chem., 1964, 29, 3341–3344 CrossRef CAS.
- H. A. Smith and R. J. Smith, J. Am. Chem. Soc., 1948, 70, 2400–2402 CrossRef CAS; E. A. Mayeda, L. L. Miller and J. F. Wolf, J. Am. Chem. Soc., 1972, 94, 6812–6816 CrossRef CAS.
- J. M. Saa, A. Llobera, A. Garcia-Raso, A. Costa and P. M. Deya, J. Org. Chem., 1988, 53, 4263–4273 CrossRef CAS.
- G. Olah and J. Welch, J. Am. Chem. Soc., 1978, 100, 5396–5401 CrossRef CAS.
- (a) N. Iranpoor and E. Mothaghineghad, Tetrahedron, 1994, 50, 1859–1870 CrossRef CAS; (b) J.-M. Chapuzet, S. Beauchemin, B. Daoust and J. Lessard, Tetrahedron, 1996, 52, 4175–4180 CrossRef CAS; (c) B. Das, B. Venkataiah and P. Madhusudhan, J. Chem. Res. (S), 2000, 266–268 Search PubMed.
- P. Salehi, N. Iranpoor and F. K. Behbahani, Tetrahedron, 1998, 54, 943–948 CrossRef CAS; V. V. Namboodiri and R. S. Varma, Tetrahedron Lett., 2002, 43, 4593–4595 CrossRef CAS.
- A. Kawada, K. Yasuda, H. Abe and T. Harayama, Chem. Pharm. Bull., 2002, 50, 380–383 CrossRef CAS.
- R. Ramu, R. Nath, M. R. Reddy and B. Das, Synth. Commun., 2004, 34, 3135–3145 CrossRef CAS.
- A.-A. G. Shaik and S. Sivaram, Chem. Rev., 1996, 96, 951–976 CrossRef CAS; P. Tundo and M. Selva, Acc. Chem. Res., 2002, 35, 706–716 CrossRef CAS.
- Alkali metal-exchanged Y-faujasites are a class of zeolites in which the negative charge of the aluminosilicate framework is counterbalanced by an alkali metal cation. For example, a NaY faujasite possesses the general formula Na56[(AlO2)56(SiO2)136]·250H2O. For morphological details and properties, see: (a) F. Schwochow and L. Puppe, Angew. Chem., Int. Ed. Engl., 1975, 14, 620 CrossRef; (b) G. C. Bond, Heterogeneous Catalysis Principles and Applications, Clarendon Press, Oxford, 2nd edn, 1987 Search PubMed; (c) D. Barthomeuf, J. Phys. Chem., 1984, 88, 42–45 CrossRef CAS.
- P. Tundo, Continuous Flow Methods in Organic Synthesis, Horwood Publishers, Chichester (UK), 1991 Search PubMed; Z.-H. Fu and Y. Ono, Catal. Lett., 1993, 22, 277–281 Search PubMed; M. Selva, C. A. Marques and P. Tundo, J. Chem. Soc., Perkin Trans. 1, 1994, 1323–1328 CrossRef CAS; Z.-H. Fu and Y. Ono, J. Catal., 1994, 145, 166–170 RSC; M. Selva, A. Bomben and P. Tundo, J. Chem. Soc., Perkin Trans. 1, 1997, 1041–1045 CrossRef CAS; A. Bomben, M. Selva, P. Tundo and L. Valli, Ind. Eng. Chem. Res., 1999, 38, 2075–2079 RSC; M. Selva, P. Tundo, A. Perosa and S. Memoli, J. Org. Chem., 2002, 67, 1071–1077 CrossRef CAS; M. Selva, P. Tundo and A. Perosa, J. Org. Chem., 2003, 68, 7374–7378 CrossRef CAS; M. Selva, P. Tundo and T. Foccardi, J. Org. Chem., 2005, 70, 2476–2485 CAS.
- (a) P. Tundo, F. Trotta and G. Moraglio, Ind. Eng. Chem. Res., 1988, 27, 1565–1571 CrossRef CAS; (b) M. Selva, F. Trotta and P. Tundo, J. Chem. Soc., Perkin Trans. 2, 1992, 519–522 RSC; (c) B. Veldurthy, J.-M. Clacens and F. Figueras, J. Catal., 2005, 229, 237–242 CrossRef CAS.
- P. Tundo, S. Memoli, D. Herault and K. Hill, Green Chem., 2004, 6, 609–612 RSC . Hydrotalcite is a mixed Mg–Al oxide of the general formula Mg0.7Al0.3O1.15.
- (a) M. Selva and P. Tundo, J. Org. Chem., 2006, 71, 1464–1470 CrossRef CAS; (b) M. Selva, P. Tundo, D. Brunelli and A. Perosa, Green Chem., 2007, 9, 463–468 RSC.
- The product was easily separated in >95 % purity (by GC) by filtration of the solid zeolite and removal of DMC under vacuum. To obtain pure samples of BME, air had to be carefully purged with a N2 stream before the reaction. Otherwise, the oxidation of benzyl alcohol took place. Under the conditions of entries 6 and 7 of Table 1, in a single experiment where moisture was not excluded, benzaldehyde was detected in a 10% amount (by GC) at a conversion of 85%.
- For brevity, the selectivity reported in Fig. 1 (uppermost curve) was averaged over the values obtained in the reactions catalyzed by both NaY and NaX faujasites. At complete conversion of 1a, these values did not differ by more than 3% from each other.
- The number of recycling episodes, however, might be limited by the formation of an azeotropic mixture DMC–MeOH (70 : 30 v/v); see: W. Wona, X. Feng and D. Lawless, Sep. Purif. Technol., 2003, 31, 129–140 Search PubMed; M. Fuming, P. Zhi and L. Guangxing, Org. Process Res. Dev., 2004, 8, 372–375 CrossRef . In the reported reaction, this problem was not experienced after three recycling tests.
- At 200 °C, the reaction of p-hydroxybenzyl alcohol (3a) afforded p-cresol as the major product.
- Methyl triphenylmethyl ether was purified by FCC (see Experimental section).
- A similar disproportionation reaction was also observed in the heating (200–400 °C) of benzyl alcohol in the presence of spinels as well as H-ZSM5 zeolites. See: G. R. Dube and V. S. Darshane, J. Chem. Soc., Faraday Trans., 1992, 88, 1299–1304 Search PubMed; I. L. Yatovt, E. I. Kotov and N. R. Bursian, J. Appl. Chem. USSR, 1986, 59, 604–609 RSC (Engl. Transl.).
- B. Su and D. Barthomeuf, Stud. Surf. Sci. Catal., 1995, 94, 598 CAS.
- C. Bezoukhanova and Y. A. Kalkachev, Catal. Rev. Sci. Eng., 1994, 36, 125–143 CrossRef CAS; C. Bezoukhanova, Y. A. Kalkachev, V. Nenova and H. Lechert, J. Mol. Catal., 1991, 68, 295–300 CrossRef.
- T. Beutel, J. Chem. Soc., Faraday Trans., 1998, 94, 985 RSC; F. Bonino, A. Damin, S. Bordiga, M. Selva, P. Tundo and A. Zecchina, Angew. Chem., Int. Ed., 2005, 44, 4774–4777 CrossRef CAS.
- The OH function of benzyl alcohol undergoes a double interaction over the solid surface: the OH-oxygen atom reacts with the weak Lewis acidic sodium cation of the zeolite, while H-bonding takes place between the OH-hydrogen atom and a basic oxygen available in the lattice (see ref. 21). Simultaneously, an acid–base complex is formed between oxygen atoms of DMC and another Na cation.
- G. Öztürk and B. Gümgüm, React. Kinet. Catal. Lett., 2004, 82, 395–399 CrossRef; S. R. Kirumakki, N. Nagaraju and S. Narayanan, Appl. Catal., A, 2004, 273, 1–9 CrossRef CAS.
- The interaction of X and Y zeolites with OH groups of phenols is similar to that reported in Scheme 7; see: T. Beutel, M.-J. Peltre and B. L. Su, Colloids Surf., A, 2001, 187–188, 319–325 Search PubMed.
- P. Wan and B. Chak, J. Chem. Soc., Perkin Trans. 2, 1986, 1751–1756 RSC.
| This journal is © The Royal Society of Chemistry 2008 |
