Human K10 epithelial keratin is the most abundant protein in airborne dust of both occupied and unoccupied school rooms
Karen
Fox
a,
Elisangela
Castanha
a,
Alvin
Fox
*a,
Charles
Feigley
b and
Deborah
Salzberg
b
aDepartment of Pathology, Microbiology and Immunology, School of Medicine, University of South Carolina, SC 29208, Columbia, USA. E-mail: afox@med.sc.edu; Fax: +1 803 733 3192; Tel: +1 803 733 3288
bDepartment of Environmental Health Sciences, Arnold School of Public Health, University of South Carolina, SC 29208, Columbia, USA
First published on 9th November 2007
Abstract
Previously it was demonstrated that the levels of large particles (>2 micron) and associated bacterial cell envelope markers increase greatly on occupation in schools; it was hypothesized that the source of both was shed human skin. In the current work to test this hypothesis, room air cleaners were used to collect airborne dust (>50–100 mg) from occupied and unoccupied school rooms which was then subjected to proteomic analysis. Proteins were extracted from the dust and separated using two dimensional gel electrophoresis (2D GE). In situdigestion of protein spots with trypsin released peptides, which were subsequently analyzed by matrix assisted laser desorption/deionization, time-of-flight mass spectrometry (MALDI-TOF-MS) and tandem mass spectrometry (MALDI-TOF-MS-MS). In Coomassie blue stained gels, a single spot generally dominated the 2D gels; this protein was identified by tandem mass spectrometry as K10 epithelial keratin. The results experimentally confirm previous anecdotal reports that human skin is readily shed into air and suggest that increased levels of microbial markers and large particles observed in occupied rooms are also derived from skin.
Introduction
Children spend as much as one-third of their lives in schools, many of which have poor indoor air quality (IAQ) due to crowding, poor maintenance, and less than optimal ventilation. These conditions may contribute to the development and exacerbation of chronic diseases, such as childhood asthma, which has become increasingly prevalent in the United States during the last two decades.1 Bacterial endotoxin may also be involved in some of the health effects associated with poor indoor air quality.2 In addition, some studies have shown that poor school IAQ is associated with a decreased ability to learn.1 It has also been hypothesized that airborne dust has a significant role in the sick building phenomenon.3For a number of years, we have studied school rooms in the presence and absence of children; thus, only one parameter is varied and changes can be readily ascribed to the building occupants. It has been shown that there is a dramatic elevation in cell wall peptidoglycan (PG) and lipopolysaccharide (LPS) in school room air upon occupation, as determined by gas chromatography-tandem mass spectrometry (GC-MS-MS). The levels of these bacterial biomarkers correlate with increased CO2 concentration from the building occupants.4–6 This was found in all classrooms surveyed among different schools, suggesting that exposure to high concentrations of microbes is extremely widespread in school room air. This also suggests that the bacterial flora in buildings is likely to be from two independent sources: the environment and the skin micro-flora of the building occupants.
The most notable change in marker levels was the elevation of muramic acid derived from bacterial cell walls and the increase of large particles (>2 micron) with occupation. Muramic acid is present at much higher levels in many Gram positive bacteria than Gram negative bacteria.7 Staphylococci, which are Gram positive bacteria, are among the most common organisms found on human skin,8,9 and epithelial shedding with adherent bacteria might explain both chemical and physical observations. Others have also found increased microbial levels,10,11 or particles12–16 associated with occupation. In the current report, the first proteomic studies are described, to delineate human and environmental sources of dust present in indoor air.
Materials and methods
Dust collection
Samples were collected in occupied rooms in the presence of children during school days (occupied sampling) and in the same rooms during evenings and weekends (unoccupied). Two room air cleaners (Ionic Breeze™, GP, Professional series, Sharper Image, Little Rock, AR, USA) were employed, each connected to a pre-set timer, allowing unattended but controlled dust collection. Generally, 2–3 days was sufficient to collect adequate quantities of dust for analysis during occupied periods; however, much longer time periods (several weeks) were necessary to collect adequate quantities of dust when the room was unoccupied.One advantage of the Ionic Breeze for sampling in occupied rooms is that it is very quiet. Because this air cleaner uses an electrostatic principle for collecting dust, its production of ozone was evaluated. Operating in a 19.2 m3 test room (with an air exchange rate of 0.61 m3 min–1), the ozone in the exhaust duct was sampled with a diffusive sampler (Product Number 586, Assay Technology, Pleasanton, CA, USA) and the sample was analyzed in an accredited commercial laboratory. The ozone emission rate was found to be 3.7 mg h–1. Because sampling was not performed during outdoor ozone season, the ozone concentration in the classroom was anticipated to be well below the 8 h Environmental Protection Agency’s National Ambient Air Quality Standard for ozone.
Protein extraction
Proteins were extracted from 50–100 mg of dust. Dust samples were rehydrated with 100 µL lysis buffer (30 mM tris, 2 M thiourea, 7 M urea, 4% CHAPS, 50 mM DTT, 25 mM Pefabloc SC and 2 mM Pefabloc protector [Roche] pH 8.5) and an equal volume of 0.5 mm sirconia/silica beads (Biospec Products, Bartlesville, OK, USA). The samples were placed in a Mini-BeadBeater-1™ for 5 min total (1 min beating with 1 min on ice, alternating). The samples were then centrifuged in a micro-centrifuge for 5 min at 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm and the supernatant removed. Protein concentration was calculated using the Pierce BCA Protein Assay (Rockford, IL, USA).
000 rpm and the supernatant removed. Protein concentration was calculated using the Pierce BCA Protein Assay (Rockford, IL, USA).
For 2D GE analysis, a total of 10–150 µg of protein solution was mixed with isoelectric focusing solution (IEF, 8 M urea, 1% pH 3–10 Pharmalyte™, 2% DTT, 4% CHAPS) placed on a BioRad immobilized pH gradient (IPG) strip (pH 3–10) and run on a BioRad isoelectric focusing (IEF) cell (Hercules, CA, USA). For the second dimension, the focused IPG strip was placed in SDS equilibration buffer 1 (50 mM TrisCl pH 8.8, 6 M urea, 30% glycerol, 2% SDS, 0.5% (w/v) DTT) for 10 min, followed by SDS equilibration buffer 2 (50 mM TrisCl, pH 8.8, 6 M urea, 30% glycerol, 2% SDS, 4.5% iodoacetamide) for 10 min, and SDS running buffer (192 mM glycine, 25 mM TrisCl, 0.1% SDS) for 10 min. Then, the strip was placed on a Ready Gel 4–15% pre-cast gradient gel in a BioRad IPG SDS-PAGE vertical gel for electrophoretic separation. Proteins were then stained with Coomassie blue or, for greater sensitivity, with the fluorescent dye Krypton (Pierce Rockford, IL, USA).
Mass spectrometry
For MS and MS-MS analyses, spots were excised from Coomassie blue stained gels and destained with 50% acetonitrile : 25 mM ammonium bicarbonate. The gel was then dehydrated by the addition of enough 100% acetonitrile to cover each gel piece (5 min). The gel was rehydrated with 100 µL of 100 mM ammonium bicarbonate (10 min). Then the protein spot was dehydrated with 100% acetonitrile (5 min), the acetonitrile was removed, and the sample was dried in a Speed-Vac for 2–3 min. The sample was rehydrated with 10 mM DTT and incubated for 30 min at room temp; then it was alkylated with 50 mM iodoacetamide for 30 min at room temp and then washed in 100 µL of 100 mM ammonium bicarbonate. Once the iodacetamide was removed, the sample went through 3 rounds of dehydration–rehydration (100% acetonitrile–100 mM ammonium bicarbonate). After the final dehydration, the sample was dried in a Speed-Vac. A solution of trypsin (Sigma) was prepared using 100 µL of 1 mM HCl and 900 µL of 40 mM NH4CO3 in 9% acetonitrile, yielding a final concentration of trypsin of 20 µL mL–1. The gel spots were rehydrated with 30 µL of the solution of trypsin and kept on ice for 15 min. The excess of trypsin solution was removed and replaced with 50 mM ammonium bicarbonate; the tube was wrapped in parafilm to seal the lid and prevent evaporation, then placed at 37 °C for 15 h.The next morning, the trypsin digestion was stopped by the addition of 2 µL of 5% formic acid. One hundred µL of sterile distilled de-ionized water was added and the tube was vortexed gently to mix. The sample was allowed to sit at room temp for 10 min then centrifuged in a micro-centrifuge for 5 min at 4000 rpm. The supernatant was removed and placed in a clean tube with 5 µl of 50% acetonitrile and 5% formic acid. The gel spot was extracted 3 more times with 50 µL water and allowed to sit for 15 min after each extraction. The extracts were combined and placed in a Speed-Vac and evaporated down to 20 µL. The peptide solution was passed through a C18 Zip-Tip to remove metal ions. The Zip-Tip was washed 3 times with 100% acetonitrile, 3 times with 60% acetonitrile : 0.1% TFA and 3 times with 0.1% TFA in water. The peptide solution was aspirated into the Zip-Tip 6 times. The Zip-Tip was then washed 3 times with 0.1% TFA in water and the peptides eluted with 5 cycles of eluent (50% acetonitrile : 0.1% TFA in water). The sample was then ready for MS or MS-MS analysis.17
For mass spectrometric analysis, a matrix consisting of 1 µL of α-cyano-4-hydroxy-cinnamic acid (α-CHCA, 10 mg mL–1) (Sigma Chemical Co., St. Louis, MO, USA) was added to the MALDI plate, and allowed to dry. Peptide extracts (1 µL of the above gel extract) were added on the spots containing the matrix and dried at room temp. For external calibration in the protein mass range, a bovine insulin standard was used. MALDI-TOF-MS and TOF-TOF-MS-MS analysis were performed initially using a Bruker Ultraflex II.
Identification of peptide sequences directly from a product ion spectrum employs “virtual spectra,” which are generated for peptide sequences of the appropriate PI and MW (predicted from the DNA code of sequenced genomes). The measured “peptide spectra” are then compared to these “virtual peptide” spectra and the best matches are provided with computer-assistance. The program, Mascot (http://www.matrixscience.com), was utilized in these identifications.
Results and discussion
In this study, large amounts of airborne dust (approximately 50–100 mg) were sampled for proteomic analysis from 6 occupied and 5 unoccupied school rooms, using a room air cleaner. All 11 samples were analyzed by 2D GE and the identity of the most abundant protein identified by MS and MS-MS. Ionic Breeze air cleaners have been shown to generate ozone and thus commercial devices are required to possess a device to remove this chemical (“Ozone-Guard”). Thus, for additional safety reasons, prior to placing the devices in school rooms, the levels of ozone generation were measured in an experimental laboratory chamber and were shown to be well below the US National Ambient Air Quality Standard.The rate of dust collection in occupied rooms was one order of magnitude higher than for unoccupied rooms (see Table 1). These results were consistent with previous work indicating that the concentrations of bacterial markers (muramic acid and hydroxy fatty acids) and large particles were also much higher when rooms were occupied.6
| Sample time/h | Weight collected/mg | Rate/mg h–1 |
|---|---|---|
| Occupied | ||
| 57.5 | 110 | 1.91 |
| 80.8 | 176 | 2.18 |
| 70.1 | 191 | 2.72 |
| 73.7 | 276 | 3.74 |
| 61.5 | 265 | 4.31 |
| 57.3 | 241 | 4.20 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Unoccupied | ||
| 355.2 | 74 | 0.21 |
| 882.2 | 213 | 0.24 |
| 793.0 | 157 | 0.20 |
| 573.0 | 150 | 0.26 |
| 301.6 | 80 | 0.27 |
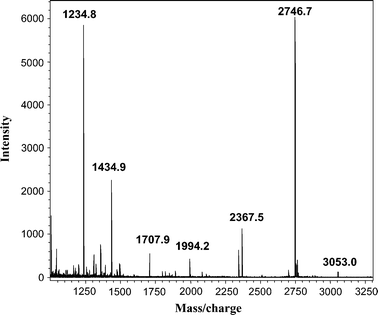
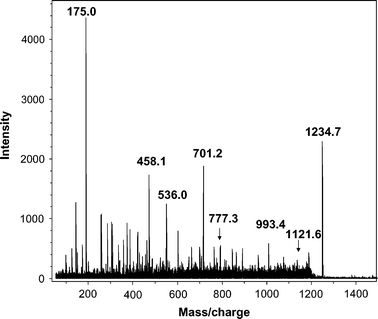
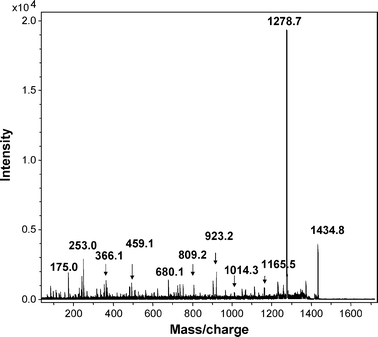
One spot dominated the 2D gels of dust extracts collected from both occupied and unoccupied rooms, as determined by MALDI-MS-MS (spot X, Fig. 1). The intensity of spots was typically much lower for 2D gels, of proteins from unoccupied rooms. The major spot was identified from mass profiles of the mass of tryptic peptides using MALDI-TOF (Fig. 2) and by sequence of selected peptides using MALDI-TOF-TOF (Fig. 3 and 4) as the human epithelial keratin K10. Probability scores for MS data for two typical samples were 115, 78, 57 and 146, 93, 82 (human, canine and bovine K10, respectively). MS-MS was performed on two parent ions (m/z 1234.71 [LKYENEVALR from mass profile and sequence data] and m/z 1434.81 [IRLENEIQTYR from mass profile and sequence data]). These peptides are both derived from the conserved portion of K10, so the probability scores for the top 3 hits were the same 89 (rat, canine, human) and 73 (bovine, canine, human), respectively. K10 is an acidic keratin (PI 5.1, MW 59 kDa) which also correlates with its migration position on the gel.
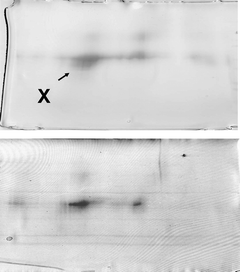 | ||
| Fig. 1 2D gel electrophoresis of proteins extracted from airborne dust and stained with Coomassie blue: (A) occupied; (B) unoccupied room. Spot X – human keratin K10. | ||
It is well known to mass spectrometrists, from anecdotal discussions, that keratin is often found as a contaminant in proteomic samples and is believed to derive from shed human skin. However, to our knowledge this is the first time experimental data has been generated to directly identify keratin derived from airborne dust using tandem mass spectrometry. Keratin can be introduced artefactually into proteomic samples. However, other proteomic samples, (e.g.proteins extracted from heart or blood vessels) taken through the entire procedure did not contain detectable levels of keratins, so contamination from sample processing was clearly not the explanation for the presence of keratins in airborne dust.
Conclusions
In work reported here, we introduced the use of a room air cleaner to collect large amounts of airborne dust (>50–100 mg) from occupied and unoccupied school rooms, in order to perform proteomic analysis using 2D GE separation, followed by MS-MS analysis. In addition to the ability to collect large dust samples much more readily than conventional impactors or impingers, another advantage of using an Ionic Breeze or similar air cleaner for sample collection is that these devices are extremely quiet. This is particularly important when sampling an occupied school room.In the past, for GC-MS-MS analyses, we employed a standard air sampling pump, drawing air at 30–40 L min–1 through a cassette containing a Teflon filter, which possessed pores small enough to trap large particles. With extended use, the filters had a tendency to clog and the maximum amount that could be collected was 3–5 mg. This system was quite noisy, so we used sound-dampening boxes, which proved to be expensive, inconvenient, and only moderately effective. Other commercially available impactors and impingers were also found to be inadequate for the collection of large amounts of dust. We evaluated an impinger marketed primarily for military use, which sampled at a rate of about 1000 L min–1 of air in a liquid medium, which was intended for culture-based analysis. Unfortunately, the high flow rate caused the liquid used for collection purposes to rapidly evaporate. Furthermore, on extended sampling periods, microbial growth would be likely in a liquid medium, whereas room air cleaners collect dust in a dry form.
It was observed that the most abundant protein present in airborne dust was the epithelial keratin, K10, derived from shed human skin. Fifty-four distinct human keratins, differing in protein sequence, have been identified in the human genome, with some derived from hair and others skin epithelium.18 While it might be expected intuitively, as far as we are aware, this is the first report to identify specific human keratins in airborne dust. These results also go a long way to help explain our earlier observations on the differences in the microbial content and particle size distinctions found between occupied and unoccupied rooms.6
Staphylococci, which cause opportunistic infections in the compromised host, are among the most commonly isolated microbes from airborne dust and are believed to derive from the shed skin of building occupants.8,9 However, all bacteria, whether infectious or not, contain natural endotoxins (PG and LPS). Low-level exposures to endotoxins have been suggested to play a role in asthma attacks and sick building syndrome symptoms.3 The magnitude of the problem with bacterial irritants increases when large quantities of biological matter are introduced into the air, as occurs in barns, swine houses, chicken coops and other agricultural environments. Heavily occupied schools, factories, and offices, which often have limited air-flow due to modern air conditioning systems, represent a lesser but still substantial degree of contamination.10–15
Environmental measurements of exposure to allergens, in dust samples, are routinely performed using individual enzyme immunoassays (ELISA) for each protein of interest. Antibodies are employed that target the most abundant allergens, whose source includes cats, dogs and dust mites.19–21 Human proteins are not among those generally assayed in airborne dust and thus have not been detected previously. Mass spectrometry, to our knowledge, has also not been previously employed to detect other proteins in indoor air.
Biochemical and analytical chemical approaches are being developed to isolate the lower abundance proteins, like staphylococci (derived from the normal flora), infectious agents, such as pneumococcal meningitis and pneumonia,22 or mycobacteria23 (derived from natural sources), and species that could be used in a biological attack, such as the one which occurred using Bacillus anthracis and the US postal service in 2001.24 Knowledge of microbial diversity in indoor air is essential for a comprehensive approach to the evaluation of communicable diseases, as well as respiratory allergy and endotoxemia.
The primary challenge in metaproteomics (characterizing sequences of protein mixtures derived from multiple microbial genomes) is detection sensitivity for low abundance components present in complex matrices. Using liquid chromatography-tandem mass spectrometry (LC-MS-MS) a number of proteins were identified in a microbial biofilm from an iron mine, but they were from the five most abundant bacterial species. Indeed, 67% of the proteins were from the dominant organism, Leptospirillumgroup II.25 This is similar to our experience, that without pre-fractionation, only the most abundant proteins are observed in environmental samples. A few abundant proteins have been detected in other more complex matrices (e.g. marine samples)26 but the analyses are still relatively simple compared to airborne dust, particularly after occupation; since contributions not only come from environmental microbes, but also from the normal flora associated with the skin of building inhabitants.
We hope to explore the diversity of keratins derived from animal sources, particularly cats and dogs, which are known to play an important role in human allergies. Ultimately, proteomics will provide substantial information on assessing indoor air quality and how to make significant improvements. An inventory of proteins derived from microbial and non-microbial allergens present in our daily environment would provide an essential baseline reference for understanding changes associated with health and disease.
The most abundant protein derived from airborne dust of both occupied and unoccupied rooms is the human keratin K10, consistent with the shedding of human skin and its associated bacteria. 2D gel electrophoresis followed by matrix assisted time-of-flight/deionization (MALDI) tandem mass spectrometry was employed in these studies. When the technology has been extended to analysis of lower abundance proteins, this should allow for the determination of the origin of all the biological matter (i.e. human, animal, insect, plant or microbial) present in indoor air.
Acknowledgements
This work was supported by the USC Research Council Small Grants Program.References
- G. Smedje and D. Norback, Int. J. Tuberc. Lung Dis., 2001, 5, 1059 Search PubMed.
- R. Castellan, S. A. Olenchok, J. Hankinson, L. Millner, J. B. Cocke, C. L. Brag, H. Perking and R. R. Jacobs, Ann. Intern. Med., 1984, 101, 157 CAS.
- F. Gyntelberg, P. Suadicani, N. Wolhlfahrt, P. Skov, O. Valbjorn, P. A. Nielsen, T. Schneider, O. Jorensen, P. Wolkoff, C. K. Wilkins, S. Gravesen and S. Norn, Indoor Air, 1994, 4, 223 CrossRef CAS.
- S. Liu, M. Krahmer, A. Fox, C. E. Feigley, A. Featherstone, A. Saraf and L. Larsson, J. Air Waste Manage. Assoc., 2000, 50, 1957.
- A. Fox, W. Harley, C. Feigley, D. Salzberg, A. Sebastian and L. Larsson, J. Environ. Monit., 2003, 5, 1 RSC.
- A. Fox, W. Harley, C. Feigley, D. Salzberg, C. Toole, A. Sebastian and L. Larsson, J. Environ. Monit., 2005, 7, 450 RSC.
- A. Fox, R. Rosario and L. Larsson, Appl. Environ. Microbiol., 1993, 59, 4354 CAS.
- C. Y. Bitkover, E. Marcusson and U. Ransjo, Ann. Thorac. Surg., 2000, 69, 1110 CrossRef CAS.
- W. Noble, J. Habberna, R. van Furth, I. Smith and C. de Ray, J. Med. Microbiol., 1976, 9, 53 CrossRef CAS.
- S. C. Lee and M. Chang, Indoor Air, 1999, 9, 134–138 CrossRef CAS.
- R. Sessa, S. Di Pietro, G. Schiavoni, L. Santino, A. Altiera, S. Pinelli and M. Del Piano, Microbiologica, 2002, 25, 51 Search PubMed.
- A. M. Anderson, N. Weiss, F. Rainey and M. S. Salkinoja-Salonen, J. Appl. Microbiol., 1999, 156, 622 CrossRef.
- T. Reponen, A. Nevalainen and T. Raunemaa, Environ. Int., 1989, 15, 203 CrossRef CAS.
- N. A. Janssen, N. A. H. Hoek, G. Harssema and H. Brunekreef, Occup. Environ. Med., 1997, 54, 888 CrossRef CAS.
- A. Jansson, T. Pekonen and J. Waher, Proceedings from 10th International Conference on Indoor Air Quality and Climate Beijing, China, 2005, p. 847 Search PubMed.
- E. Kujundzic, M. Hernandez and L. Miller, Indoor Air, 2006, 16, 216 CrossRef CAS.
- M. Wilm, A. Shevchenko, T. Houthaeve, S. Breit, L. Schweiger, T. Fotsis and M. Mann, Nature, 1996, 379, 466 CrossRef CAS.
- J. Schweitzer, P. Bowden, P. Coulombe, L. Langbein, E. Lane, T. Magin, L. Maltais, M. Omary, D. Parry, M. Rogers and M. Wright, J. Cell Biol., 2006, 174, 169 CrossRef.
- C. Earle, E. King, A. Tsay, K. Pittman, B. Saric, L. Vailes, R. Godbout, K. Oliver and M. Chapman, J. Allergy Clin. Immunol., 2007, 119, 428 CrossRef CAS.
- J. C. Gore and C. Schal, Annu. Rev. Entomol., 2007, 52, 439 CrossRef CAS.
- G. Ko and H. A. Burge, Indoor Air, 2004, 14, 434 CrossRef CAS.
- D. J. Ecker, R. Sampath, L. Blyn, M. Eshoo, C. Ivy, J. Ecker, B. Libby, V. Samant, K. Sannes-Lowery, R. Melton, K. Russell, N. Freed, C. Barrozo, J. Wu, K. Rudnick, A. Desai, E. Moradi, J. Knize, D. Robbins, J. Hannis, P. Harrell, C. Massire, T. Hall, Y. Jiang, R. Ranken, J. Drader, N. White, J. A. McNeil, S. Crooke and S. Hofstadler, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 8012 CrossRef CAS.
- S. M. Mastorides, R. L. Oehler, J. N. Greene, J. T. Sinnott, M. Kranik and R. L. Sandin, Chest, 1999, 115, 19 Search PubMed.
- J. A. Higgins, M. Cooper, L. Schroder-Tucker, S. Black, D. Miller, J. Karns, J. Manthry, R. Breeze and M. L. Perdue, Appl. Environ. Microbiol., 2003, 69, 593 CrossRef CAS.
- I. Lo, V. Denef, N. VerBerkmoes, M. Shah, D. Goltsman, G. DiBartolo, G. Tyson, E. Allen, J. Detter, P. Richardson, M. Thelen, R. Hettich and J. Banfield, Nature, 2007, 446, 537 CrossRef CAS.
- J. Kan, T. E. Hanson, J. M. Ginter, K. Wang and F. Chen, Saline Syst., 2005, 1, 7 Search PubMed.
| This journal is © The Royal Society of Chemistry 2008 |