Assessment of metal contamination using X-ray fluorescence spectrometry and the toxicity characteristic leaching procedure (TCLP) during remediation of a waste disposal site in Antarctica†
Scott C.
Stark
*a,
Ian
Snape
a,
Nicholas J.
Graham
a,
John C.
Brennan
b and
Damian B.
Gore
c
aEnvironmental Protection and Change—Environmental Risk and Remediation, Australian Antarctic Division, Department of Environment and Water Resources, Channel Hwy, Kingston, TAS 7050, Australia. E-mail: scott.stark@aad.gov.au
bVeolia Environmental Services, 75 Mornington Rd, Mornington, TAS 7018, Australia
cEnvironmental and Life Sciences, Macquarie University, NSW 2109, Australia
First published on 9th November 2007
Abstract
The remediation of the Thala Valley landfill, Casey Station, East Antarctica, is part of efforts to clean-up contaminated sites associated with the Australian Antarctic Program. These sites, ranging from abandoned rubbish dumps to fuel spills, are contaminated principally with metals and petroleum hydrocarbons. Remediation success depends on accurate, cost-effective and timely—fit-for-purpose—chemical analysis of soil and water samples from the site, which is required to guide excavation, the in situ or off-site treatment and disposal of contaminated material, and to validate satisfactory remediation. Owing to the remote location of Antarctica, it is necessary to carry out chemical analyses on-site. Waste and soil contaminated with Pb, Zn, Cd, and Cu were excavated from Thala Valley for removal to Australia, treatment and disposal. Analysis of total metal concentrations in soil was performed at Casey Station with a transportable energy dispersive X-ray fluorescence (EDXRF) spectrometer. Soil samples were prepared using a simple size-fractionation method to expedite sample throughput. A method for assessing contaminant mobility in solid waste (toxicity characteristic leaching procedure, TCLP) was also used to characterise soil. Although this was more labour-intensive and time-consuming than the total metals analysis, it was of great utility because leachable metals were often significant determinants in the assessment of contaminated soil. The combined data helped managers during remediation, directing excavation and allowing waste to be classified for treatment and disposal before its return to Australia.
Introduction
Successful remediation of Antarctic, and other remote contaminated sites, requires efficient use of time and resources under significant operational constraints. In Antarctica, transportation is expensive, lengthy and infrequent, and work on-site is restricted by season and inclement weather. Contaminated sites in Antarctica are located mainly on the coast in ice-free areas, where human occupation is concentrated and the legacy of past waste management practices, with rubbish disposed of in landfills or the sea, is greatest. This unique coastal region, representing only 0.05% of the continent, is also where much of the activity of Antarctic ecosystems occurs, and consequently the environmental impact of contaminants within them is potentially very serious.1 The Protocol on Environmental Protection to the Antarctic Treaty established an international obligation to clean-up or contain abandoned waste and prevent further contamination of Antarctica. This has motivated participating nations to develop procedures and technologies for the management of contaminated sites in Antarctica.1–3Thala Valley, at Casey Station in East Antarctica, contained a waste disposal site (landfill or ‘tip’) that covered 0.15–0.30 ha and was located at the foot of the valley, directly adjacent to Brown Bay (Fig. 1). The site was used for the disposal of waste generated at the former Casey station from ca. 1964 through to the 1980s. Waste ranged from domestic rubbish to building materials and was sometimes partially burnt at the landfill or dumped into the bay via the sea-ice. Prior to remediation, the volume of contaminated material in the landfill was 1600–2500 m3. The main contaminants polluting Thala Valley are Cu, Pb, Zn, Cr, Ni and Cd, derived from discarded machinery, building panels, batteries, insulation flashing and pipes.2,4 Following site disturbance on partial excavation of rubbish in 1996, metal concentrations in the soil were significantly greater than environmental investigation guideline (EIG) values.4 The transport of ~8–10 m3 of contaminated soil by meltwater into Brown Bay each summer has had a significant impact on the local marine ecosystem.5–8
 | ||
| Fig. 1 (a, b) Map of Casey Station and the Thala Valley landfill in the Windmill Islands (WI), Antarctica. (c) Ortho photo of the site. | ||
A staged remediation of Thala Valley landfill was carried out from October 2003–February 2004, in tandem with an ongoing monitoring program designed to measure the effects of clean-up on the local marine ecosystem.3 The specific aim was to remediate the site to as clean as practicable—ideally to background levels—and to leave it in a state where dispersal of residual contaminants is unlikely to cause environmental harm. Contaminated material was excavated and placed into shipping containers for transport to Australia, and soil samples for analysis were collected from the containers and the site. Chemical assessment of soil was carried out to determine contamination levels before (January–February 2003) and during remediation, to validate satisfactory remediation of the site, and classify excavated soil for treatment and disposal on its removal to Australia, according to Australian waste regulations. This involved measurement of total and leachable contaminant metal concentrations in soil samples from Thala Valley, the latter providing a means to estimate the potential mobility of contaminants in the environment.9,10
An important aspect of the analytical program was the capacity to provide information rapidly to site managers. To achieve fast turnaround between sampling and reporting, analyses were performed on-site, and a compromise made in favour of practicality and a larger sample data set at the expense of measurement accuracy and precision. For these reasons, portable, rapid, reliable and robust analytical techniques requiring minimal sample preparation provided attractive options for the remote contaminated site.11,12 The method-of-choice for determining total metals in soil was energy dispersive X-ray fluorescence spectrometry (EDXRF). To assess the leachable metal fraction, the toxicity characteristic leaching procedure (TCLP)10,13 was performed and flame atomic absorption spectrometry (FAAS) used to analyse soil extracts.
This paper describes and evaluates the performance of the EDXRF and TCLP employed to analyse samples in the remediation of Thala Valley, since there are many other contaminated sites in Antarctica and other remote regions that require remediation, and our experience may guide other analysts. We also discuss the role of the chemical data in the remedial operation, some correlations between contaminants, and outline future developments aimed at improving the analytical program, particularly for assessment of leachable metals.
Methods
Soil guidelines and analytical methods
Guideline values relevant to the remediation of the Thala Valley landfill are in Table 1, along with typical data for contaminated soil from the site. The ideal goal for remediation was provided by background soil concentrations at Casey Station.2,4 Recognizing that this goal might not be readily achievable because of operational constraints (e.g. problems in excavating frozen ground, inundation of the site by seawater or meltwater), and in the absence of specific environmental contaminant guidelines for Antarctica, a minimum acceptable remediation level was also set. This was ‘soil suitable for disposal as fill material’, as defined by the Tasmanian State Government of Australia.14 Total element concentrations for fill material are very similar to the interim urban ecological investigation levels (EILs) of the National Environment Protection (Assessment of Site Contamination) Measure 1999.9 Currently, these are the lowest contaminant guidelines for site assessment in Australia, although for most elements, they are still significantly greater than Casey background soil values.| Guideline or analytical valuea | As | Cr | Mn | Ni | Fe | Cd | Cu | Pb | Zn |
|---|---|---|---|---|---|---|---|---|---|
| a All data are total concentrations (mg kg–1) on a dry matter basis (Fe is wt%), except for TCLP values (mg L–1); data in brackets are standard deviations of means; ‘–’ indicates not analysed or no value set. Guideline values are: Casey background soil (including mean XRF data, n = 9, for Casey quarry soil); the NEPM (ASC) 1999 interim urban EIL; Tasmania DPIWE values for the classification of contaminated soil for disposal (maximum allowable concentrations). Typical Thala Valley contaminated soils are represented by the composite sample (aqua regia/ICP-OES) and the tip fines standard (TV-tf). TV-tf data determined at two independent labs or at Casey (XRF, n = 43; TCLP, FAAS, n = 6 for Cd, Cu, Pb, Zn), 2003–2004. | |||||||||
| Casey background soil4 | <4 | <5 | — | <10 | — | <1 | 20 | <10 | 32 |
| Casey background soil2 | 1.0 (0.1) | 31 (4) | 350 (50) | 38 (9) | 2.9% (0.3) | 0.20 (0.02) | 33 (2) | 7.5 (1.1) | 74 (5) |
| Casey background soil (quarry), 2003 | <20 | <100 | 770 (110) | 49 (7) | 3.6% (0.2) | — | 61 (19) | 63 (10) | 89 (10) |
| NEPM (ASC) 1999 interim urban EIL | 20 | — | 500 | 60 | — | 3 | 100 | 600 | 200 |
| DPIWE level 1–total concentration | 20 | 50 | — | 60 | — | 3 | 100 | 300 | 200 |
| DPIWE level 2–total concentration | 200 | 500 | — | 600 | — | 40 | 2000 | 1200 | 14000 |
| DPIWE level 2–TCLP concentration | 0.5 | 0.5 | — | 1 | — | 0.1 | 1 | 0.5 | 25 |
| DPIWE level 3–total concentration | 750 | 5000 | — | 3000 | — | 400 | 7500 | 3000 | 50000 |
| DPIWE level 3–TCLP concentration | 5 | 5 | — | 8 | — | 0.5 | 10 | 5 | 250 |
| Composite tip soil2 | 12 (1) | 60 (9) | 1200 (100) | 94 (9) | 11.1% (0.4) | 24 (1) | 2200 (90) | 6700 (300) | 4600 (200) |
| Thala Valley tip fines (TV-tf), XRF, lab#1 | 9.4 (0.8) | 215 (16) | 2160 (100) | 88 (14) | 11% (0.5) | 20 (3) | 1670 (160) | 5300 (600) | 4070 (230) |
| TV-tf, HF digest/ICP-MS, lab#1 | 9.8 (0.5) | 105 (18) | 2500 (90) | 95 (29) | — | 17 (4) | 1200 (180) | 6400 (1600) | 3400 (240) |
| TV-tf, aqua regia digest/ICP-OES, lab#2 | 8.2 (0.4) | 50 (3) | 1190 (50) | 69 (9) | 9.5% (0.3) | 22 (10) | 1870 (400) | 6100 (500) | 3200 (120) |
The Tasmanian State Government guidelines14 for total and leachable contaminants were also used to classify soil excavated from Thala Valley into one of four categories for treatment and disposal in Tasmania: Level 1 (L1) fill material, L2 low-level contaminated soil, L3 contaminated soil, and L4 contaminated soil for remediation. High concentration of a single member of the prescribed list of inorganic or organic contaminants is sufficient to classify soil as L3 or L4 waste, invoking strict and potentially costly disposal requirements (e.g. chemical fixation prior to deep burial). Generally, leachable takes precedence over total concentrations, and can be used as the sole determinant of classification.
Analytical methods suitable for the Thala Valley remediation were those capable of meeting both data quality objectives (DQOs) and practical requirements, the latter determined by resource (e.g. personnel, equipment, laboratory space) and time constraints. To achieve the DQOs, a method had to be capable of providing data that were of sufficient accuracy and precision for reliable comparison with guidelines, subsequently enabling management decisions regarding site remediation or waste treatment. To meet the practical requirements—primarily the need for fast analytical turnaround—the most important criterion was minimal sample preparation. To simplify analytical procedure and facilitate fast turnaround during remediation, usually only major contaminants are monitored, a strategy further justified if the concentrations of anthropogenic elements are positively correlated. For Thala Valley, major contaminants were defined as those potentially present at total or leachable concentrations classifying soil as L3 or L4 waste—from the earlier site assessments: total and leachable Cu, Pb and Zn, and leachable Cd. Minor contaminants were typically present at concentrations that would alone classify soil as L1 or L2 waste, and included Sb, As, Cr, Hg, Ni, Ag and Sn. Iron and Mn were prevalent in soil from the landfill because of the dominance of metalliferous waste, but not at levels affecting classification. Because it was a straightforward task by XRF, quantitative measurements of some minor contaminants and geogenic elements were made to provide a more detailed characterisation of soil from the site.
Soil sampling and preparation for analysis
Soil sampling from the site and from containers of excavated soil was carried out following Australian Standard AS 4482.1-199715 and the NEPM.9 Samples of soil (≥100 g dry) were collected at a depth of 0–15 cm using stainless steel implements and stored in polyethylene bags. Judgemental sampling was employed for the site assessment in early 2003. Within areas of obvious contamination, samples were collected from random locations ∼1–2 m apart. For site validation during the clean-up, samples were located on a 5–8 m square grid, allowing identification at 95% confidence of contamination hot-spots, radius 3–5 m.15 For waste-grading during excavation, a composite soil sample, made up of 3–5 discrete samples dug randomly from the surface of the pile, was collected from each 5 m3 waste container. This provided a sampling density five times greater than the minimum requirement for classifying homogeneous, stockpiled soil for disposal.14 Duplicate composites were collected from 5% of the containers to evaluate the uncertainty associated with sampling.A simple three-step preparation provided soil samples that could be analysed satisfactorily within the constraints of the DQOs. Following removal of large objects, samples were dried in aluminium trays at 105 °C overnight, and then size-fractionated using stacked 9.5 mm and 2.0 mm stainless steel Endecotts sieves and a mechanical sieve-shaker (Endecotts EFL2000/2). The resultant <2.0 mm soil fraction was a mixture of fines, mineral sands and rubbish. Analysis of this provided a more conservative estimate of contamination than the <9.5 mm fraction (size limit for the TCLP), but was still representative of chemical concentrations in soil as a whole, not just the fine material.16,17 It was also consistent with assessments of sediment quality carried out in local marine environments, such as Brown Bay.3,5,7
Total metals by EDXRF
Analysis of total metals in soil samples was performed with a PANalytical PW4025 MiniPal2 benchtop EDXRF spectrometer with a 9 W Rh anode tube. Calibration for contaminant and matrix elements Fe, Mn, Ca, K, As, Cr, Cu, Ni, Pb, and Zn, from low mg kg–1 to wt% levels, involved base-metal certified reference materials (CRMs) designed primarily for mining applications (Geostats Pty Ltd, White Gum Valley, Western Australia). The set of 19 finely powdered standards comprised mainly Cu/Pb/Zn oxide and sulfide ores, along with some basalt, laterite and pulverised soil, representing a diverse sample matrix.
XRF analyses were made on loose powders prepared from the <2 mm dried soil fraction. This method was selected following a favourable comparison between XRF data for samples analysed loose and as powders pressed into disks at 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) tonne for 1 min (Herzog TP20 press).18 Pressed powders, although of greater consistency, required considerably more time and effort to prepare, which did not facilitate fast analytical turnaround—grinding soil to a fine powder in a planetary ball mill (Fritsch Pulverisette-6 with ZrO2grinding bowl and 20 mm balls) typically required 1–2 h, and often resilient larger particles remained. Loose powders were placed in polyethylene P1 cups fitted with a 3.6 µm polyethylene film. Samples were measured in air using two operating conditions: (1) 8.00 kV, 90 µA, kapton filter (Ca, Cr, K, Mn – Kα line); (2) 30 kV, 120 µA, Mo filter (As, Cu, Fe, Ni, Pb, Zn – Kα line; Pb – Lβ1 line); measurement time 100 s per condition. Spectra were measured in duplicate and the mean calculated. Analysis time per sample averaged ∼20 min, and was longer than total measurement live-time (400 s), owing to detector dead-time in highly contaminated samples, but this did not result in spectral complications such as peak shifts.19
tonne for 1 min (Herzog TP20 press).18 Pressed powders, although of greater consistency, required considerably more time and effort to prepare, which did not facilitate fast analytical turnaround—grinding soil to a fine powder in a planetary ball mill (Fritsch Pulverisette-6 with ZrO2grinding bowl and 20 mm balls) typically required 1–2 h, and often resilient larger particles remained. Loose powders were placed in polyethylene P1 cups fitted with a 3.6 µm polyethylene film. Samples were measured in air using two operating conditions: (1) 8.00 kV, 90 µA, kapton filter (Ca, Cr, K, Mn – Kα line); (2) 30 kV, 120 µA, Mo filter (As, Cu, Fe, Ni, Pb, Zn – Kα line; Pb – Lβ1 line); measurement time 100 s per condition. Spectra were measured in duplicate and the mean calculated. Analysis time per sample averaged ∼20 min, and was longer than total measurement live-time (400 s), owing to detector dead-time in highly contaminated samples, but this did not result in spectral complications such as peak shifts.19
Analytical quality control (QC) was maintained by regular measurement of three of the calibration CRMs (GBM-399-1, -900-2, -902-6: low, medium, high contaminant concentrations, respectively), clean and moderately contaminated marine sediment CRMs (MESS-3 and PACS-2; National Research Council Canada), and two site-specific standards: a Casey background soil, sourced from the local quarry, and a sample of Thala Valley tip fines (TV-tf). The TV-tf was a composite sample (sieved <500 µm) with elemental concentrations typical of highly contaminated landfill soil, characterised by a variety of techniques at independent analytical laboratories in 2003 (Table 1). For comparison with total metals XRF data, a sample subset was analysed at an independent, certified laboratory. This contained 25 soils ranging from clean to contaminated, plus the three GBM QC standards and the TV-tf. A 1–2 g quantity of soil was digested in 5 mL of hot aqua regia for 4 h, diluted to 50 mL with deionized water, filtered if necessary, and analysed by ICP-OES (Varian Vista Pro spectrometer).
Leachable metals by TCLP
The TCLP simulates the leachability of contaminants from solid waste under environmental groundwater conditions.10,13 This was required for the classification of contaminated soil for disposal,14 and can also be used in site assessment or validation to estimate the potential for contaminant mobilisation.9,10,16 However, the TCLP, with a lengthy aqueous extraction and labour-intensive sample preparation requirements, imposes a significant limitation on analytical turnaround time. For the majority of contaminant metals included in soil disposal guidelines, TCLP concentrations differentiating Levels 2–4 waste range from ∼10 to ≤1 mg L–1 (Table 1)—too low for analysis by the MiniPal2 EDXRF spectrometer without preconcentration. A fast and cost-efficient alternative for the small analyte set of the remediation—Cd, Cu, Pb, Zn—was provided by FAAS. Like ICP techniques, FAAS is generally not as suitable as EDXRF for work in remote locations because of the higher operational requirements, (e.g. gas supply, space),11,20 but fortunately a suitable FAAS facility was already at Casey Station.TCLP was performed on the dry <2.0 mm soil fraction with analytical grade reagents and Milli-Q deionized water. The pH of typical water extracts of the soil was 7 ± 1. Consequently, samples (100 ± 10 g) were placed in acid-washed, water-rinsed, 2 L HDPE bottles (Kartell), mixed with 1.9 L of 0.10 M Na acetate (pH 4.71), and extracted for 18 h at 20 ± 1 °C inside a rotary sample tumbler. After filtration of the extraction mixtures through acid-washed glass fibre filters (Whatman GF/F), extracts were acidified to pH <2 with concentrated HNO3, and analysed by FAAS (Varian SpectrAA-400 spectrometer) using typical operating conditions for the analytes (Table 2). Calibration was with matrix-matched, multi-element standards prepared from certified standard solutions (1000 mg L–1 single-element, BDH Spectrosol). Preliminary work demonstrated that acid-digestion changed FAAS data for extracts by only 15% on-average, so measurements were made either directly on filtrates or (usually for Pb and Zn) after 1 : 20 dilution with acidified extractant.
| FAAS parameter | Cd | Cu | Pb | Zn |
|---|---|---|---|---|
| Absorption λ/nm | 228.8 | 327.4 | 217.0 | 213.9 |
| Lamp current/mA | 4 | 4 | 5 | 5 |
| Slit width/nm | 0.5 | 0.5 | 1.0 | 1.0 |
| Background correction | Off | Off | On | On |
| Flame type | Air-acetylene, oxidizing | |||
| Signal acquisition | 5 s equilibration, mean of 3 × 3 s readings | |||
| Calibration range/mg L–1 | 0–4 | 0–25 | 0–15 | 0–10 |
| MDL/mg L–1 | 0.005 | 0.05 | 0.05 | 0.05 |
A TCLP batch typically involved extraction of 16 samples, including a blank, a duplicate, and the TV-tf standard, and standard additions were made to one of the filtrates to assess analyte recovery. To enable comparison of Casey TCLP data with that from an independent certified laboratory on return to Australia, acidified sub-samples of filtrates were stored at 4 °C for analysis by ICP-OES, and a subset of soil samples was selected for replication of the TCLP.
Results and discussion
Total metals by EDXRF
Calibration data and method detection limits (MDLs) for the EDXRF determination of total metals are shown in Table 3. Spectral interference was important for measurement of two elements in soil from the landfill. The low concentrations of As could not be measured accurately owing to interference from the more abundant Pb, and all samples were reported conservatively at <20 mg kg–1 on comparison with spectra for the TV-tf. Cadmium could not be quantified at all by the spectrometer, as a consequence of its low concentration (≤20 mg kg–1) and interference by elements, such as K, but fortunately for the assessment program, its main significance was as a leachable contaminant. Analytical accuracy was monitored directly by measurement of QC standards. XRF data deviated from certified values by an average of 28% for the six metals of principal interest: Fe, Cr, Ni, Cu, Pb, and Zn (excluding As and Mn, with a limited set of certified values). Generally, greater deviation was observed for metals at low concentrations close to the MDL, and hence better accuracy was achieved overall for Pb, Zn and Fe (many samples with high concentrations), compared to Ni, Cr, and Cu. For Cu, bias averaged 40% overall, but was only ∼10% at higher concentrations typical of contaminated soil.| Parametera | Ca | K | Fe | Mn | As | Cr | Ni | Cu | Pb | Zn |
|---|---|---|---|---|---|---|---|---|---|---|
| a All data are mg kg–1 except where indicated (wt%); LOD: limit of detection (± std dev), based on a 100 s live-time measurement; MDL: method detection limit (99% confidence level); Reporting limit: minimum concentration reported for the Thala Valley remediation. | ||||||||||
| Calibration minimum | 1.1% | 0.3% | 0.9% | 30 | 10 | 10 | 10 | 25 | 10 | 10 |
| Calibration maximum | 7.3% | 4.5% | 11% | 1.1% | 790 | 0.5% | 0.95% | 2.0% | 2.7% | 2.0% |
| R 2 | 0.971 | 0.952 | 0.936 | 0.995 | 0.973 | 0.993 | 0.999 | 0.983 | 0.997 | 0.997 |
| LOD | 12 (3) | 25 (16) | 14 (7) | 11 (4) | 7 (4) | 13 (5) | 11 (4) | 9 (3) | 11 (4) | 9 (3) |
| MDL | 0.15% | 0.05% | 0.43% | 66 | 13 | 114 | 39 | 51 | 45 | 19 |
| Reporting limit | — | — | — | — | 20 | 100 | 40 | 50 | 45 | 20 |
Precision was assessed by analysing duplicates selected at three different stages of the method (Table 4). A typical analysis batch of 15 samples included three sets of single-sample duplicate measurements, two sets of loose-powder duplicates, and a pair of duplicate samples, as well as replicate measurements of CRMs. XRF measurements of the nine metals in CRMs were reproducible, on average, to 9 ± 7% over three months, indicating long-term stability for the instrument. Similar (and for some metals, better) reproducibility was obtained for soil samples (6 ± 5%), which confirmed that they were essentially as homogeneous as standards under the measurement conditions. The reproducibility of preparing and measuring loose powders from a soil sample (9 ± 6%) was statistically equivalent to the measurement precision for single samples or standards. This demonstrated that the sample preparation scheme provided a sample of sufficient homogeneity for reproducible analysis by XRF, although a higher degree of heterogeneity was apparent for some metals, such as Cu and Pb. Limited comparison with the ICP-OES technique (duplicate analysis of six soils) indicated that XRF precision was higher for Cu, Pb and (marginally) Zn, but lower for Cr, Ni and Fe.
| Componenta | n | Ca | K | Fe | Mn | Cr | Ni | Cu | Pb | Zn | Range | Meanb |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a Mean precision (%RSD) for n sets of duplicates. b Mean (± std dev) precision (%RSD) for measurement of 8 metals, excluding As (no data) and Cr (less data, relatively poor precision). | ||||||||||||
| XRF of standard | 48 | 5 | 4 | 5 | 11 | 14 | 18 | 8 | 7 | 2 | 2–18 | 7 (5) |
| XRF of soil sample | 45 | 2 | 2 | 2 | 11 | 46 | 16 | 5 | 4 | 3 | 2–46 | 6 (5) |
| Loose powder prep + XRF | 34 | 3 | 3 | 5 | 13 | 48 | 22 | 11 | 11 | 7 | 3–48 | 9 (6) |
| Sampling + sample prep + XRF | 11 | 5 | 7 | 6 | 16 | 75 | 20 | 29 | 14 | 15 | 5–75 | 14 (8) |
| Total (sample) variance | 206 | 26 | 26 | 65 | 31 | 62 | 49 | 87 | 94 | 113 | 26–113 | 62 (34) |
The overall uncertainty of the measurement of total metals in a soil sample collected from a waste container during remediation (sampling + sample preparation + XRF), averaged 21% for nine metals, and ranged from 5–7% for Ca, Fe and K, and to 75% for Cr. Excluding Cr (mostly ≤MDL), precision averaged 14% (eight metals), with Cu least precise (Table 4). This measurement uncertainty represented 36% of the total or ‘geochemical’ variance of the data set. For Cu, Pb and Zn, the proportions were 37, 17, and 15%, respectively, and are within or (for Cu) slightly outside the optimum range (≤20%) for reliable geochemical analytical methods.21–23 Despite the high heterogeneity of the soil, especially for Cu, measurement uncertainty was generally dominated and hence limited by the XRF analysis component (sample preparation + XRF), which contributed 63% on average, and was only <50% for Cu and Zn.
The independent ICP-OES data obtained for the subset of landfill soils differed from XRF data by an average of 59% for nine metals, and with only minor exception, were lower (Fig. 2). This was attributed largely to the inability of partial digestion techniques to yield true total concentrations, especially for metals that are predominantly geogenic and concentrated within the relatively inert soil matrix.20,24–26 In accordance with this, poorest correlation between the data sets was found for Ca, K, Ni and Cr. For the contaminant metals Fe, Cu, Pb, and Zn (mean difference 30–50%), correlation was better at higher concentrations, probably because a larger proportion of the metal was available for digestion in contaminated samples (more fines, more labile phases) compared to clean samples. More complete digestion and sample homogeneity could also account for the closer agreement obtained between XRF and ICP-OES data for the finer-powdered QC standards (mean difference 35%, with Fe, Cu, Pb and Zn, 7–26%). Exceptions for soils where ICP-OES > XRF were attributed to sample heterogeneity (e.g. the presence of metal particles in the digest).
 | ||
| Fig. 2 Comparison of EDXRF and digestion/ICP-OES data (Fe, Cu, Pb, Zn) for Thala Valley soils. The 1 : 1 correlation with ±25% and ±50% error is shown on the graphs. | ||
Leachable metals by the TCLP
FAAS proved satisfactory for the analysis of TCLP extracts for Cd, Cu, Pb, and Zn, with adequate detection limits and sensitivity for waste classification and site validation purposes. Routine QC procedures for FAAS included duplicate measurement of standards and samples to assess precision, and analysis of a spiked extract. Calibration drift was minimal and recovery of known additions quantitative, averaging 103 ± 5% (n = 15).Precision data for the TCLP are summarised in Table 5. Spectrometer precision averaged 2% and was lowest for Pb (10–20% for one third of duplicates), the most problematic element to measure. Extraction and analysis of the four metals in duplicate soil samples was reproducible to 11%, compared to 5% for the more homogeneous TV-tf standard. Overall reproducibility of leachable metals analysis for a sample collected from a waste container (sampling + TCLP + AAS) was considerably poorer and averaged 40%. This constituted 20–40% of the total (‘geochemical’) variance of the leachable metals data set. In contrast to the analysis of total metals, most of the variance for leachable metals was attributable to sample heterogeneity, instead of sample preparation and analysis.
| Componenta | n | Cd | Cu | Pb | Zn | Range | Meanb |
|---|---|---|---|---|---|---|---|
| a Mean precision (%RSD) for n sets of duplicates. b Mean (± std dev) precision (%RSD) for measurement of 4 metals. c Estimated uncertainty because many values <MDL. | |||||||
| FAAS of TCLP extract | 7–15 | 5c | 6c | 7 | 1.0 | 1–7 | 5 (3) |
| TCLP of TV-tf | 6 | 9 | 2 | 5 | 2 | 2–9 | 5 (3) |
| TCLP of soil | 13–15 | 12 | 6 | 22 | 5 | 5–22 | 11 (8) |
| Sampling + TCLP of soil | 11 | 53 | 45 | 41 | 20 | 20–53 | 40 (14) |
| Total (sample) variance | 207 | 191 | 132 | 106 | 108 | 106–191 | 134 (40) |
To validate the leachable metals analysis performed at Casey Station during the remediation, a comparison was made between results generated on-site and data determined independently in Australia several months later. This involved ICP-OES measurement of TCLP extracts prepared and preserved at Casey, as well as repetition of the complete TCLP. The same subset of soil samples employed for validation of the EDXRF method was also used here, and the results of the comparison are in Fig. 3. The difference between the ICP-OES and FAAS data sets for 27 TCLP extracts prepared at Casey averaged 23%, with ICP-OES generally lower. This was poorer than the correlation achieved between Casey FAAS and ICP-MS data during method development in early 2003, with a mean difference 5 ± 2% for 17 extracts. TCLP extracts are typically complex mixtures that may suffer from matrix interference problems when analysed by FAAS or ICP-OES. During FAAS at Casey, difficulties encountered initially with Pb were overcome by employing matrix-matched standards and dilution. Similar problems were reported for TCLP analyses by the independent laboratory, which did not employ matrix-matched standards. The discrepancy between the data sets may therefore be systematic error arising from a failure to resolve matrix interferences in the ICP-OES technique.
 | ||
| Fig. 3 Comparison of TCLP measurements made at Casey by FAAS for Cd, Cu, Pb and Zn with independent data: ICP-OES data for Casey extracts (◆) and independent TCLP (extraction + ICP-OES) (□). The 1 : 1 correlation with ±25% and ±50% error is shown on the graphs. | ||
Less satisfactory agreement was found when the complete TCLP was repeated in Australia. With the exception of Zn, correlations between the independent and Casey TCLP data were significantly poorer than for the extract samples, and the difference between them averaged ∼50–60% for Cd, Pb and Zn (∼0.1, 9 and 4 mg L–1, respectively), and 400% (1 mg L–1) for Cu. For Zn and Cu, the independent results were nearly all greater than Casey values, whereas some of the Pb and Cd data were much lower. Furthermore, the precision of the independently performed TCLP, evaluated by six sets of duplicates including the TV-tf standard, averaged 31%, approximately three times poorer than that achieved at Casey.
Classification of soil for treatment and disposal and site validation
During remediation of the Thala Valley landfill, over 200 soil samples were analysed for total and leachable metals. The data were used to classify ∼1000 tonnes of waste soil for treatment and disposal according to Tasmania State Government guidelines (Table 1), and validate the satisfactory remediation of the site. Total and leachable concentrations of major contaminants and geogenic metals determined for soil samples from waste containers are summarised in Table 6. Leachable metal concentrations were generally the controlling variables in this classification process, and were sometimes high even when total metals were low. The dominance of leachable contaminants in the soil, consistent with significant contaminant mobility at the site,27 necessitated chemical fixation prior to disposal of the waste in Australia.28| Descriptiona | Parameterb | Ca % | K % | Fe % | Mn | Cr | Ni | Cu | Pb | Zn | Cd-TCLP | Cu-TCLP | Pb-TCLP | Zn-TCLP |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a Total concentration data are mg kg–1 on a dry matter basis except where specified (wt%); leachable metals data (M-TCLP) are mg L–1. b For calculation of statistics, data <MDL (or the reporting limit) were set equal to this value. | ||||||||||||||
| All samples from waste containers | Mean | 2.2 | 1.7 | 6.0 | 700 | 140 | 65 | 640 | 1640 | 1210 | 0.11 | 0.8 | 27 | 13 |
| n = 166 | Std dev | 0.6 | 0.4 | 3.7 | 210 | 90 | 35 | 490 | 1340 | 1230 | 0.20 | 1.0 | 25 | 12 |
| Min | 1.4 | 0.34 | 1.9 | 266 | <100 | <40 | <50 | 63 | 69 | <0.005 | <0.05 | 0.17 | 0.27 | |
| Median | 2.2 | 1.7 | 5.3 | 668 | <100 | 64 | 558 | 1510 | 1010 | 0.073 | 0.46 | 22 | 11 | |
| Max | 5.1 | 2.5 | 28 | 1560 | 833 | 450 | 2620 | 7190 | 9010 | 1.7 | 6.0 | 143 | 83 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||||||||
| L4 waste soil | Mean | 2.2 | 1.5 | 7.0 | 750 | 150 | 65 | 800 | 2110 | 1560 | 0.15 | 1.1 | 35 | 16 |
| n = 126 | Std dev | 0.6 | 0.4 | 3.6 | 190 | 100 | 39 | 460 | 1210 | 1230 | 0.22 | 1.1 | 23 | 12 |
| Min | 1.4 | 0.34 | 2.3 | 342 | <100 | <40 | 74 | 112 | 81 | 0.008 | <0.05 | 5.3 | 0.49 | |
| Median | 2.1 | 1.5 | 6.5 | 718 | <100 | 62 | 677 | 1770 | 1220 | 0.10 | 0.68 | 29 | 14 | |
| Max | 5.1 | 2.3 | 28 | 1560 | 833 | 450 | 2620 | 7190 | 9010 | 1.7 | 6.0 | 143 | 83 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||||||||
| L3 waste soil | Mean | 2.3 | 2.2 | 2.7 | 550 | 108 | 67 | 150 | 190 | 156 | 0.013 | 0.23 | 1.6 | 1.6 |
| n = 27 | Std dev | 0.5 | 0.1 | 0.4 | 160 | 33 | 9 | 110 | 72 | 62 | 0.005 | 0.23 | 1.0 | 0.9 |
| Min | 1.6 | 1.8 | 1.9 | 266 | <100 | 45 | <50 | 85 | 83 | <0.005 | <0.05 | 0.55 | 0.37 | |
| Median | 2.4 | 2.2 | 2.7 | 546 | <100 | 67 | 114 | 191 | 142 | 0.010 | 0.18 | 1.1 | 1.4 | |
| Max | 3.4 | 2.5 | 3.6 | 938 | 262 | 84 | 539 | 311 | 311 | 0.024 | 1.2 | 3.5 | 4.0 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||||||||
| L1-2 waste soil | Mean | 2.6 | 2.2 | 2.5 | 460 | <100 | 66 | 80 | 100 | 91 | 0.012 | 0.08 | 0.34 | 0.6 |
| n = 13 | Std dev | 0.4 | 0.1 | 0.5 | 120 | — | 10 | 49 | 33 | 39 | 0.005 | 0.09 | 0.11 | 0.4 |
| Min | 1.7 | 1.9 | 2.0 | 281 | — | 47 | 50 | 63 | 69 | <0.005 | <0.05 | 0.17 | 0.27 | |
| Median | 2.7 | 2.1 | 2.4 | 479 | — | 68 | 70 | 93 | 80 | 0.009 | <0.05 | 0.30 | 0.46 | |
| Max | 3.2 | 2.4 | 3.7 | 670 | — | 84 | 241 | 169 | 219 | 0.024 | 0.38 | 0.51 | 1.9 | |
Of the 166 waste containers sampled and analysed, 76% were classified as highly contaminated L4 waste, owing to high concentrations of total and/or leachable Pb (L3-4 total, L4 leachable). Total Cu and Zn were high (typically L2, some Cu L3), and leachable Cd, Cu and, in fewer cases, Zn, were also significant in L4 samples (two-thirds L3–4 for at least another metal). The 27 samples classified as contaminated L3 waste owed this to leachable Pb only, except for one also with leachable Cu. Generally, total concentrations of Cu, Pb or Zn were at L1, but some were L2. The remaining 13 containers had total metals mostly at levels of fill material, although low concentrations of leachable Pb and Zn were also measured. Because of this and also quarantine regulations, these were allocated to the low-level contaminated soil category.
Site validation was carried out on four occasions during remediation, with a total of 38 samples collected and analysed. There were improvements in both total and leachable metal levels in soil as the excavation proceeded (Fig. 4 and 5). The first set of validation samples was collected from an area ∼500 m2 excavated at the front of the landfill, adjacent to Brown Bay. Analysis of soil revealed a reduction in total metal concentrations compared to data collected before clean-up in early 2003, although half of the samples were still highly contaminated by Pb (L3–4). Leachable concentrations of Pb, Cd and Zn remained high, and the soil was classified as highly contaminated L3–4. After further excavation of ∼100 m2 of the northern section of the landfill by ∼0.5 m, analysis demonstrated that remediation to background levels had been achieved. The soil was clean in appearance—generally grey in colour, sandy, and marine shells were present. Unfortunately, further excavation of the larger, southern section of the landfill-front was not possible, owing to flooding by meltwater and then the sea. A third set of samples was collected from ∼300 m2 excavated at the western end of the site. Remediation to low contaminant levels was verified, bentonite fabric was overlain, and the area used to stockpile soil from the landfill in excess of what could be placed into the available waste containers, for future removal or treatment.
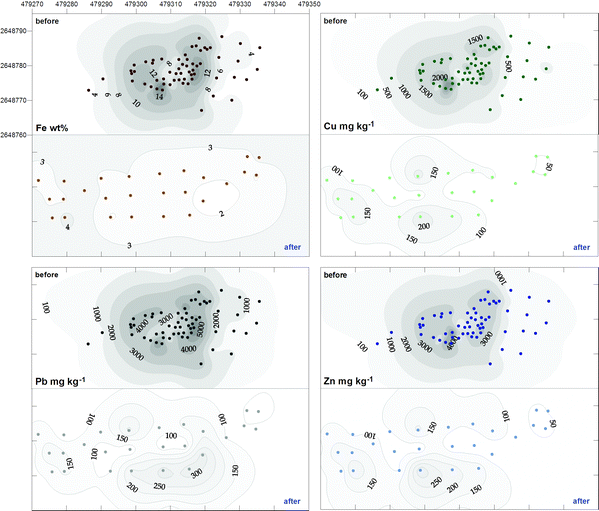 | ||
| Fig. 4 Changes in the total concentrations of Fe, Cu, Pb and Zn in soil from the Thala Valley landfill, before and after remediation. Pairs of ‘before’ and ‘after’ plots are orientated north; eastings and northings in 10 m intervals. Data representing the easterly section of the site before remediation were collected after partial excavation, and true contaminant levels were likely to have been greater. | ||
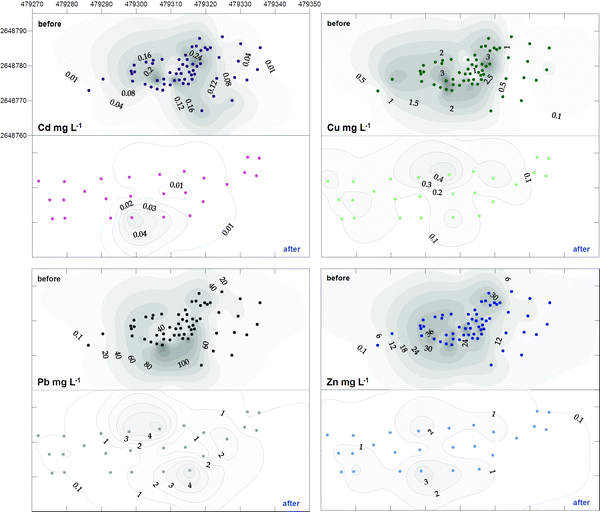 | ||
| Fig. 5 Changes in the leachable concentrations of Cd, Cu, Pb and Zn in soil from the Thala Valley landfill, before and after remediation. Pairs of ‘before’ and ‘after’ plots are orientated north; eastings and northings in 10 m intervals. See also notes to Fig. 4. | ||
Final site validation for the remediation was carried out over ∼600 m2 in the middle of the landfill, located between the waste stockpile and the new marine boundary. This included some of the most highly contaminated parts of the site. It was found that total contaminant concentrations in soil had been reduced to that recommended as suitable for fill material L1 or low level contaminated soil L2 (marginally for Pb in a few samples only), but some leachable residues remained (mainly Pb at L3–4, but less than pre-clean-up levels). This indicated that the bulk of the contaminants had been removed from the site and only residual amounts remained—that remediation close to the minimum acceptable level had been achieved. Further excavation of the area was not carried out, especially since the area was inundated by seawater during a high tide only hours after sampling.
Concentrations of total and leachable contaminant metals in soil samples from the landfill were highly correlated. This can be succinctly demonstrated through principal component analysis (PCA) of the data.26,29 Total concentration variables for Cr and Mn were omitted from this analysis (many Cr < MDL, poor accuracy for Mn) but Ca and Ni were retained, giving an 11-variable data set with 238 soil samples collected from waste containers and the site over the entire course of the remediation. The resultant PCA demonstrated that one factor explained most of the sample variance (71%), with all concentration variables, other than Ca and Ni, highly correlated—factor loadings ranged in magnitude from 0.81–0.99 (Table 7). Potassium was negatively correlated with Fe and other contaminants, an effect attributed to dilution of the native, clean soil (e.g.feldspars) with contaminated fines. Because correlation between these nine variables is lower in clean soil, high loading of factor 1 can be associated with soil contamination. An additional two factors were required independently to explain most of the variance for Ca and Ni (mainly geogenic and not correlated with the other metals or each other), and 89% of the total sample variance. The remaining 11% of the variance can be attributed to the random uncertainty or ‘noise’ in the XRF and TCLP methods.
| Factor | EV a | ∑fvarb | Fe | Cu | Pb | Zn | K | Ca | Ni | Cd-TCLP | Cu-TCLP | Pb-TCLP | Zn-TCLP |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a Eigenvalue. b Cumulative proportion of total variance explained by factor. | |||||||||||||
| 1 | 7.8 | 71% | 0.93 | 0.96 | 0.97 | 0.99 | –0.87 | 0.15 | –0.27 | 0.88 | 0.81 | 0.92 | 0.96 |
| 2 | 1.0 | 80% | –0.03 | –0.07 | –0.04 | –0.04 | –0.22 | 0.97 | –0.09 | –0.09 | 0.02 | –0.08 | –0.06 |
| 3 | 1.0 | 89% | 0.12 | –0.06 | –0.10 | –0.02 | –0.11 | –0.14 | –0.94 | –0.14 | –0.003 | –0.10 | –0.06 |
Overview of the chemical assessment program
The XRF and TCLP methods employed to perform the on-site analysis of total and leachable metals in soil at the landfill were capable of achieving the data quality and practical objectives of the chemical assessment program. The methods were of sufficient sensitivity, accuracy and precision to classify excavated soil for treatment and disposal, and to ascertain if the site had been remediated acceptably. The accuracy and precision of the EDXRF determination of total metals was analyte dependent and poor in some cases (e.g. mainly geogenic Cr, Mn, and Ni), but it was satisfactory for the major contaminants—typically ±10–30%. For total metals, sample preparation plus XRF made a greater contribution to total measurement uncertainty than sampling did. In contrast, sampling was the main source of variance in the analysis of leachable metals by TCLP.The analysis of total metals in soils by EDXRF at Casey Station was consistent with an independent digestion/ICP-OES determination, but there was greater discrepancy between TCLP data generated on-site and that obtained later in Australia. Although this was not sufficient to alter the waste classification of soil made at Casey, it illustrated the difficulties in achieving reproducible results with the TCLP, owing to inter-laboratory analytical differences in technique, or the effects of chemical change, such as oxidation, on sample leachability.10,13 Regardless, on-site analysis of leachable metals was clearly advantageous for logistical purposes (e.g. planning of treatment procedures prior to the arrival of waste soil in Australia), and to expedite site remediation.
The chemical assessment program was designed to minimise analytical turnaround time, but this was more difficult to achieve. A team of three people provided sufficient labour to manage the work load: three for sampling, two for sample preparation and chemical analysis, and one for waste tracking and data management. Sampling and sample preparation were the most time-consuming and labour-intensive components. The TCLP required more time and labour than total metals analysis, owing to the extraction and filtration steps, and use of non-automated FAAS. Data for total and leachable metals in soil were typically available within 3–4 days of sampling; for total metals only, this could be reduced to 1–2 days. While not problematic for the classification of soil for disposal, with a complete data set only required prior to arrival of waste containers in Australia, it was less satisfactory for site validation work, because the data had an immediate and critical influence on the remediation design.
Although measurement of leachable metals by TCLP requires considerable time and effort, it is the more valuable aspect of the analytical program in relation to understanding the potential mobility of contaminants in waste soil. As a consequence, there is a clear need for improvement in this component if it is to be included routinely in remote site remediation programs. For instance, with waste soils of high heterogeneity, collection of several smaller samples (e.g. 1–10 g) and measurement using quicker extraction or different extractants, may provide a better characterisation of leachable metals at the site (or inside a container of waste soil) than the TCLP with a single 100 g composite sample. Furthermore, enabling the analysis of TCLP or other extracts by EDXRF, either directly with more powerful yet transportable instruments, or following preconcentration,30–32 would allow assessment of metal-contaminated sites in remote locations to be performed entirely using EDXRF, reducing significantly the level of laboratory support required to undertake this work.30
Conclusion
Total and leachable metals analyses were used to achieve the data quality objectives for the chemical assessment of contaminated soil during remediation of the landfill at Thala Valley. The analytical program had turnaround times longer than optimal, owing mainly to the TCLP for leachable metals, but the utility of EDXRF for performing rapid, accurate and precise assessment of total metals in soil at a remote contaminated site was demonstrated. These methods can be used during completion of the Thala Valley remediation for assessment of stockpiled soil and additional excavated material, and for work at other contaminated sites associated with the Australian Antarctic Program. Improvements in analytical quality at low concentrations and reduction of analysis time would be expected from refinement of EDXRF measurement parameters and calibration. Analysis of leachable metals by TCLP is much more time-consuming and labour-intensive, and requires additional laboratory resources. However, the value of data on the potential mobility of contaminants in waste soil is high from an environmental protection and remediation viewpoint, providing strong motivation to improve the practicability of leachable metals tests for remote site assessment and validation.Acknowledgements
We thank the expeditioners at Casey Station during the 2002–2003 and 2003–2004 summer seasons for field and laboratory support, Gary Pritchard and Simon McCall (PANalytical) for technical advice and support, Veolia Environmental Services (formerly Collex) for logistic and personnel support, and Tania Raymond for editorial assistance. Laboratories involved with the independent analysis of samples were Analytical Services Tasmania, and the Central Science Laboratory and CODES, School of Earth Sciences at the University of Tasmania. We also thank three anonymous referees for their helpful comments on the manuscript.References
- J. S. Poland, M. J. Riddle and B. A. Zeeb, Polar Rec., 2003, 39, 369–384 Search PubMed.
- I. Snape, M. J. Riddle, J. S. Stark, C. M. Cole, C. K. King, S. Duquesne and D. B. Gore, Polar Rec., 2001, 37, 199–214 Search PubMed.
- J. S. Stark, I. Snape and M. J. Riddle, Ecol. Manage. Restor., 2006, 7, 21–31 Search PubMed.
- P. P. Deprez, M. Arens and H. Locher, Polar Rec., 1999, 35, 299–316 Search PubMed.
- J. S. Stark, M. J. Riddle, I. Snape and R. C. Scouller, Estuarine, Coastal Shelf Sci., 2003, 56, 717–734 CrossRef CAS.
- J. S. Stark, I. Snape and M. J. Riddle, J. Exp. Mar. Biol. Ecol., 2003, 283, 21–50 CrossRef CAS.
- J. S. Stark, I. Snape, M. J. Riddle and S. C. Stark, Mar. Pollut. Bull., 2005, 50, 276–290 CrossRef CAS.
- S. Duquesne and M. J. Riddle, Polar Biol., 2002, 25, 206–215.
- NEPC, National Environmental Protection (Assessment of Site Contamination) Measure 1999, National Environmental Protection Council Service Corporation, Adelaide, 1999 Search PubMed.
- J. Scott, D. Beydoun, R. Amal, G. Low and J. Cattle, Crit. Rev. Environ. Sci. Technol., 2005, 35, 239–332 Search PubMed.
- A. Rutter, G. Cairns, N. Plato and J. S. Poland, Polar Rec., 2003, 39, 339–346 Search PubMed.
- A. Kinney, J. Mack and G. McKenna, in Innovative Approaches to the On-Site Assessment and Remediation of Contaminated Sites, ed. D. Reible and K. Demnerova, Kluwer Academic Publishers, Dordrecht/Boston/London, 2002, vol. 15, pp. 1–30 Search PubMed.
- USEPA, Toxicity characteristic leaching procedure, USEPA Method 1311, United States Environmental Protection Agency, 1992 Search PubMed.
- DPIWE, Classification and management of contaminated soil for disposal, Information Bulletin 105, Department of Primary Industries, Water, and the Environment, Hobart, Tasmania, 2004, (updated August 2006) Search PubMed.
- Standards Australia, Guide to the sampling and investigation of potentially contaminated soil. Part 1: Non-volatile and semi-volatile compounds, AS 4482.1-1997, Homebush, 1997 Search PubMed.
- ANZECC/ARMCANZ, Australian and New Zealand Guidelines for Fresh and Marine Water Quality, Australia and New Zealand Environmental and Conservation Council/Agricultural and Resource Management Council of Australia and New Zealand, 2000 Search PubMed.
- D. H. Loring, ICES J. Mar. Sci., 1991, 48, 101–115.
- S. C. Stark, I. Snape, N. J. Graham, J. C. Brennan and D. B. Gore, Assessment of metal contamination using XRF spectrometry and the TCLP during remediation of a waste disposal site in Antarctica. Report prepared for the 10th meeting of the Antarctic Treaty Committee for Environmental Protection, Delhi, India, April 30–May 11 2007, Australian Antarctic Division, 2007, available with Metadata record via AAD Data Centre website, http://aadc-maps.aad.gov.au/aadc/portal/ Search PubMed.
- K. L. Williams, An Introduction to X-Ray Spectrometry, Allen & Unwin, London, 1st edn, 1987, pp. 115–118 Search PubMed.
- M. J. Duane, S. Facchetti and G. Pigozzi, Sci. Total Environ., 1996, 177, 195–214 CrossRef CAS.
- C. Vanhoof, V. Corthouts and K. Tirez, J. Environ. Monit., 2004, 6, 344–350 RSC.
- M. H. Ramsey, J. Anal. At. Spectrom., 1998, 13, 97–104 RSC.
- A. Argyraki, M. H. Ramsey and P. J. Potts, Analyst, 1997, 122, 743–749 RSC.
- P. Anderson, C. M. Davidson, D. Littlejohn, A. M. Ure, L. M. Garden and J. Marshall, Int. J. Environ. Anal. Chem., 1998, 71, 19–40 CrossRef CAS.
- S. J. Goldstein, A. K. Slemmons and H. E. Canavan, Environ. Sci. Technol., 1996, 30, 2318–2321 CrossRef CAS.
- S. M. Pyle, J. M. Nocerino, S. N. Deming, J. A. Palasota, J. M. Palasota, E. L. Miller, D. C. Hillman, C. A. Kuharic, W. H. Cole, P. M. Fitzpatrick, M. A. Watson and K. D. Nichols, Environ. Sci. Technol., 1996, 30, 204–213 CrossRef.
- I. Snape, D. B. Gore, C. M. Cole and M. J. Riddle, Bull. –R. Soc. N. Z., 2002, 35, 641–648 Search PubMed.
- J. C. Brennan, I. Snape, M. J. Riddle, C. Paterson, S. C. Stark, K. Northcott and P. R. Woodberry, Contaminants in freezing ground: proceedings of the 4th International Conference Fairbanks, Alaska, 2004.
- N. Jorgensen, J. Laursen, A. Viksna, N. Pind and P. E. Holm, Environ. Int., 2005, 31, 43–52 CrossRef CAS.
- E. S. Heiden, D. B. Gore and S. C. Stark, J. Hazard. Mater., 2007 Search PubMed , submitted.
- A. Takahashi, S. Igarashi, Y. Ueki and H. Yamaguchi, Fresenius’ J. Anal. Chem., 2000, 368, 607–610 CrossRef CAS.
- R. Van Grieken, Anal. Chim. Acta, 1982, 143, 3–34 CrossRef CAS.
Footnote |
| † The HTML version of this article has been enhanced with colour images. |
| This journal is © The Royal Society of Chemistry 2008 |
