Sustainability through green processing – novel process windows intensify micro and milli process technologies
Volker
Hessel
*ab,
Dana
Kralisch
c and
Ulrich
Krtschil
b
aEindhoven University of Technology, Department of Chemistry and Chemical Engineering, 5600 MB, Eindhoven, P.O. Box 513, the Netherlands. E-mail: V.Hessel@tue.nl; Fax: +31 40 247 4959; Tel: +31 40 244 6653
bInstitut für Mikrotechnik Mainz GmbH, Carl-Zeiss-Str.18-20, D-55129, Mainz, Germany. E-mail: hessel@imm-mainz.de
cFriedrich-Schiller University, Institute for Technical Chemistry and Environmental Chemistry, Lessingstr. 12, D-07749, Jena, Germany. E-mail: dana.kralisch@uni-jena.de; Fax: +49/3641/948402; Tel: +49/3641/948457
First published on 12th September 2008
Abstract
Drawing on sustainability for chemical production processes demands the prior integration of sustainability aspects during process development, when further environmental impacts and production costs become predefined. Micro and milli process technologies provide novel ways for process improvement combined with ecological and economic advantages. This is demonstrated by current developments in this area. In this context, the idea of “Novel Process Windows” is discussed referring to examples of actual research. A short overview on how to assess sustainability using established tools of evaluation is given as well. This is rounded up with a recent case study of the Kolbe–Schmitt synthesis, performed to disclose the key drivers of further green process development.
 Volker Hessel Volker Hessel | Prof. Dr Volker Hessel, born 1964, is part-time professor at Eindhoven University of Technology and Director R&D at the Institut für Mikrotechnik Mainz for Micro and Milli Process Technologies. He investigates applications in mixing, fine chemistry, fuel processing, heterogeneous catalysis, and since recently the exploration of “Novel Process Windows” (AIChE award in Process Development, 2007). Prof. Hessel is chair of the SynTOP and IMRET-10 Conferences. |
 Dana Kralisch Dana Kralisch | Dana Kralisch studied environmental chemistry at the Friedrich-Schiller-University (FSU), Jena, Germany, and obtained her PhD in 2006. In her work she concentrates on the integration of sustainability criteria into chemical process development focusing on micro reaction technology and bacterial produced nanocellulose. She leads the green process engineering and evaluation research group at the Institute of Technical and Environmental Chemistry, FSU. |
 Ulrich Krtschil Ulrich Krtschil | Ulrich Krtschil joined the Institut für Mikrotechnik Mainz GmbH (IMM) in Mainz, Germany in 2004. He is involved in several public and industrial funded R&D projects and due to his industrial background he is mainly concerned with process and plant design. Born 1954, he concluded his studies in chemical engineering at the Technische Hochschule Merseburg in 1975 as a graduate engineer. From 1975 to 2004 he was employed at the Jenaer Glaswerk and later as a project manager at Schott Engineering and QVF Engineering in Mainz. |
Green processing
Improving sustainability in all areas, e.g. science, business, society as well as policy, is the biggest challenge in our century. It was after the publication of the report “Our Common Future” by the Brundtland commission in 1987 that the idea of sustainable development started to spread throughout the world.1 Since this time, this concept has been defined more precisely in a wide range of agreements, action plans, national programmes, and scientific studies.e.g.2 Thus, sustainability has become a continuing evolutionary process with adjusting definitions and is therefore highly complex. One indicator for this is the many different interpretations of what constitute the terms sustainability and sustainable development in the current literature.Industrial production must respond to this changing need in order to satisfy the increasing product requirements demanded by the customer and the environmental, economic and social constraints of the industrial-scale process itself. The chemical industry, as one of the biggest global players, is exceptionally placed to discover innovative ways of providing novel sustainable solutions for clean technologies, thus reducing pollution levels and the consumption of exhaustible resources. But, radical improvements can only be achieved by a complete rethink of production processes, including new reactor configurations and novel process conditions (the so-called “Novel Process Windows”, discussed below) for chemical syntheses. A consequent decoupling of economic growth from the environmental impact is essential.3 In the last couple of years “green engineering” and “green chemistry” approaches have focused on achieving this goal, but in different ways. While “green engineering” deals with the design of novel equipment and new production methods, using multifunctional reactors, new operating modes, or microtechnology for process intensification, “green chemistry” primarily means new chemical synthesis strategies, chemistry based on renewable recources or waste reduction by yield and selectivity improvements.4 A combination of both could become a key function for sustainable chemical process development! This point will be discussed in detail by means of current activities in the context of green proccessing in microreactors.
Equally important for successful implementation of the idea of sustainability into chemical processes is the integration of appropriate assessment methods. Already during the stage of process development, the environmental impact and economic consequences become mostly predefined within the decision for a specific technology or the specific process conditions. Against this background, the integration of sustainability criteria in this early phase of the decision-making process can give crucial support. While a methodological and sound approach for the concerted assessment of all three main criteria of sustainability still do not exist – a broad range of environmental, cost and, in combination, eco-efficiency evaluation methods are available and can be used for sustainable process development. An overview of the application of such evaluation methods in the context of process intensification by microreaction technology is provided. This is followed by an outlook section on new research activities and trends in microreaction technology in “Novel Process Windows”. A case study is provided to demonstrate the favourable combination of process design and evaluation during the early stages of development.
Micro and milli process technologies and process intensification
Micro and milli process technologies enable a new, revolutionary type of chemical processing in continuous-flow mode. The class of microreactors comprise reactors structured by modern microfabrication techniques with characteristic internal dimensions typically in the range of 50 to 1000 µm.5–14 Milli process technology is the dimensional extension, often using commercial capillaries and tubes with mm-internals; sometimes filled with foams or fibres as structured packings.So far, micro process technology is mostly applied to chemical reactions;5,7 fewer applications concern unit operations themselves, such as mixing and heat exchange.6 Just recently, the focus has been set also on separations.15,16 Besides chemical applications, energy generation is a major application, in particular where it concerns fuel processing to make hydrogen for fuel cells.6,16 Explorative investigations to introduce the technology are being undertaken in the food, personal care, and consumer goods sectors.17
The miniaturisation of flow dimensions improves considerably mass and heat transfer, even though diffusion often is the only mechanism active in a laminar flow (see Fig. 1).5,6 The fast mixing and steep temperature changes as well as the precise setting of reaction temperature without thermal overshooting provide new options and higher performance for fast mixing or heating of sensitive chemical reactions. Selectivity is increased by suppressing side reactions and by the additional effect of precise setting of residence time the content of follow-up reactions can be reduced. The operation at the kinetic limit, not superimposed by transport phenomena, allows one to reach high space-time yields so that compact reactors and plants result. The small hold-up reduces the damage potential of explosions or other hazardous states of maloperation. Moreover, the small dimensions can prevent explosions intrinsically, when e.g. the large channel interfaces favour radical termination over radical generation and growth which is the flame-arrestor effect. The symmetry-based hierarchical reactor design (channel to plate–to stack–to multitude of stacks) and the flexibility in operation provide a high degree of modularity to adapt to different campaigns or to adjust the production rate, bridging to process system engineering. A key to system efficiency is the integration of diverse functions in one apparatus or along a process line, e.g. giving rise to integrated heat exchanger reactors or membrane reactors.18
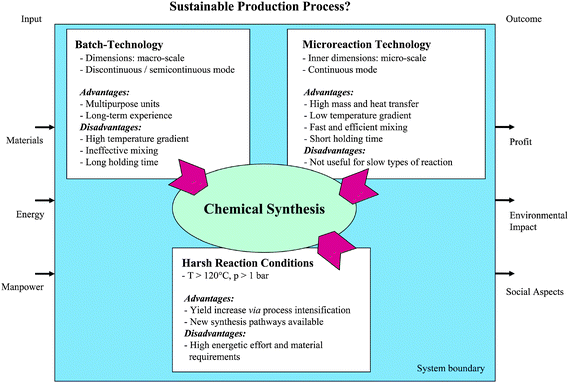 | ||
| Fig. 1 Variables for sustainable processing. | ||
The impact of microreactors is not only given for screening in the pharmaceutical and process development in the fine-chemical industries, but becomes more and more relevant for pilot and production operation, i.e. producing chemicals.19 Even the projection to bulk-chemical scale is currently being investigated.19
An excellent overview about the state of the art in process intensification is provided by the “European Roadmap for Process Intensification” (available at http://www.senternovem.nl/energytransition/downloads). “Process intensification” provides radically innovative principles (“paradigm shift”) in process and equipment design which can benefit (often with more than a factor of two) process and chain efficiency, capital and operating expenses, quality, wastes, process safety and more. Process intensification differs from process optimisation (“Performance improvement of existing concepts”) and process system engineering (“Multi-scale integration of existing and new concepts”).
The “European Roadmap for Process Intensification” also provides main drivers to use process intensification, specific to four industry sectors, grouped also by weighting factors. The industry sectors are: PETCHEM denoting petrochemicals/bulk chemicals, FINEPHARM speciality chemicals/pharmaceuticals, INFOOD food ingredients, and CONFOOD consumer food. The FINEPHARM part (and to a lesser extent the PETCHEM part) are relevant to the study in this paper (see Fig. 2).
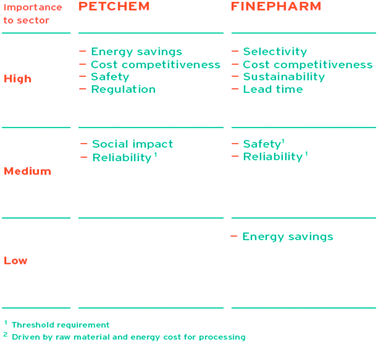 | ||
| Fig. 2 Drivers for using process intensification, allocated to four industry sectors, grouped also by weighting factors. (Courtesy of Senternovem, Creative Energy, Energy Transition). | ||
Green processing in microreactors
Two excellent overviews about this research area have been given by Haswell and Watts10 and, more recently, by Mason et al.,20 but some outstanding examples for green chemistry in microreactors are given below.Increased yields and selectivities
Increased yields and selectivities are directly tangible to “green” improvements in chemistry when using micro process technology for process intensification.21 A selection of several examples, including many of the established name reactions, are given in5,7,10,20 (see also below).Increased reaction rates
Step-change increases in reaction rates which come from increases in temperature, pressure, or concentration (solvent-free operation), are given in more detail in examples in the chapter “Sustainable Processing in Novel Process Windows”.Simplification of process flow
A simplification of the process flow8 can be achieved via fast, simultaneous carrying out of process steps (such as multiple reactant dosing), function integration (combined reaction and separation), or reducing downstream expenditure and energy efforts through better upstream performance. The latter is shown for the phenylboronic acid synthesis of Clariant Company in Frankfurt, Germany.22 Microreactor processing could be made at ambient temperature instead of cryo conditions and the purer raw product eliminated the need for the capital- and energy intensive distillation.Accessibility of exothermic and thermal runaway reactions
Nitration reactions are fast and exothermic which leads to the formation of undesired products. Lonza Company in Visp, Switzerland compared the autocatalytic nitration of phenol in a microreactor with that run in a batch reactor.23 Calorimetric analysis of the batch processing revealed even at a small scale (1 l) an increase of 55 K. The hot-spot in the microreactor, contrarily, was only about 5 K which resulted in higher selectivity and a yield of 75% as opposed to 55% for the batch. Moreover, the purity was also increased by reducing the fraction of polymeric byproducts by a factor of 5.Increased safety
Microreactors allow a reduction of the risk potential when dealing with toxic or explosive reagents, which makes formerly unscalable reactions accessible to further development. This was initially demonstrated with fluorinations, microreactors made available the direct route with elemental F2 instead of the tedious 4-step Balz–Schiemann route (Fig. 3).24–26 By virtue of exploiting the fast kinetics in the explosible regime, very high space–time yields can be achieved, at orders of magnitude above the performance of conventional batch processing.25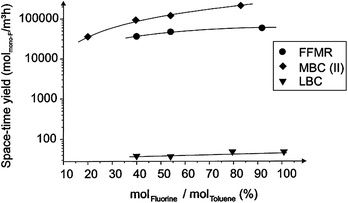 | ||
| Fig. 3 Space–time yields for the direct fluorination using elemental fluorine in a falling film microreactor (FFMR), a micro bubble column (MBC), and a laboratory bubble column (LBC).25 (Courtesy of Elsevier). | ||
Gas–liquid oxidations using oxygen or ozone in microreactors offer new ways of process control to reduce total oxidation and to avoid uncontrolled release of large reaction heats and the shift of processing out of regular operation.20,27 If that type of processing is done photochemically, the high-intensity light exposure poses another safety problem. For the addition of singlet oxygen to α-terpene, the presaturation of oxygen was eliminated and the yield of the product ascaridole was increased by about 20%.
Further demonstrations of safe microreactor processing with hazardous and explosive substances were made for diazomethane reactions,29,30 high-energy nitration reactions,31 and diazo ring expansions.32 Reactions with diazo reagents are typically highly exothermic, and in some cases, they can release significant quantities of N2 gas, creating hazardous conditions. The reaction of N-Boc-4-piperidone with ethyl diazoacetate on a laboratory scale, although done at 90% yield, was stopped due to the unacceptable temperature increase of up to 45 °C and corresponding reactor overpressurisation.31 Using microreactor operation (89% yield), however, the throughput could be raised to 91 g h−1.
Increased efficiency – less input and waste
The reduction of waste is one more green principle. A major portion of the waste in a chemical reaction is the solvent, which typically constitutes 90% or more of the overall mixture. Microreactors allow one to reduce the content of the solvent by increasing the concentration of reactants, demonstrated for the solvent-free operation (pure reagents) of the exothermic Paal–Knorr reaction.33 Further examples of increased efficiency in terms of low-volume optimisation, on-line reaction monitoring, elimination of scale-up, or multiple transformations are given.20Methodological approaches
But how can green and sustainable processing be measured and assessed? A broad range of evaluation approaches still exist to assess the environmental impact as well as economic efficiency of (chemical) products or processes already within the design/development stage. They range from easily calculable metrics to complex life cycle approaches.Well established single metrics, useful for a first examination of alternative synthesis pathways already during R&D, are, e.g. the E-factor, suggested by Sheldon34 or Mass Loss Indices (MLI).35 These characterise the mass of waste produced per unit mass of product or the masses of all substances, used in a process, related to the product mass, respectively. Further metrics have been suggested in this context, e.g. by Trost36 and Heinzle et al.37 Up-stream and down-stream processes are mostly not included in the calculation. In general, the majority of environment-related metrics are mass-based.38 They are usually easy to calculate and therefore widely used.
Examples for cost indices used to estimate economic efficiency during early stages of process design are the Total Annualised Profit per Service Unit (TAPPS) suggested by Hoffmann et al.39 Further, simplified methods for capital budgeting, e.g. the Net Present Value (NPV), are used for this purpose.
Kheawhom and Hirao40 proposed an approach which combines optimisation of the metrics Environmental Performance and Economic Performance in order to guide environmental benign process design. This optimisation is based on a synthesis problem as well as a range of objectives and constraints defined beforehand. Further work has been done in this context, e.g. by Stefanis et al.41 and Azapagic and Clift.42
Contrary to a limited evaluation of single process units, life cycle approaches covering all life cycle stages of a process, product or service system from extracting and processing raw materials, manufacturing, transportation and distribution, use/reuse, recycling and waste management. The Life Cycle Assessment (LCA) methodology offers the possibility to quantify and assess the environmental aspects and potential impacts associated with these processes, product or service systems in order to choose the least burdensome.43 For this purpose, the calculated mass and energy flows are assigned to different environmental impact categories. Examples of this are resource depletion, global warming, acidification, human and ecotoxicity potential as well as land use. Thus, a wide range of actual environmental problems are covered. LCA studies in the context of chemical processes have been presented by, e.g. Jödicke,44 Burgess and Brennan,45 Hellweg46 and Kralisch and Kreisel.47 In recent times, the LCA methodology was supplemented by the Life Cycle Costing (LCC) approach. It refers to all costs associated with the system as applied to the defined life cycle.48 LCC has been developed for the combined evaluation of the ecological as well as the economic part of sustainability and thus, aids more sustainable business practices.49 The eco-efficiency analysis by BASF is one example of the successful implementation of these ideas into bulk chemical production and practice.50
Evaluation of micro process technology
As discussed previously, microreaction technology provides the potential for radical improvements to chemical processes regarding both environmental as well as economic sustainability.The economic drivers for introducing this technology are, first of all, substantial reductions in capital expenditure through lower investment and operational costs on the one hand and lower energy requirements on the other hand. For the first time, this topic was intensely discussed between experts during the AIChE Spring Meeting held in Orlando, FL, USA, in 2006. The following aspects were highlighted as key figures of a commercial breakthrough of microprocess engineering: process intensification (esp. in terms of selectivity), standardisation, availability of skilled employees and serious cost analyses to quantify the economic potential. The latter aspect has been examined only by some working groups so far. Their results will be summarised in the following.
The Merck Company, Darmstadt, Germany, together with the Technical University Clausthal presented in this context a four-staged potential analysis. Schmalz et al.51 suggest defining the potential of microreaction technology as its efficiency concerning the possible reduction of costs during process development and production compared to batch-wise working operations. From this, the potential is differentiated between the theoretical, technical, material and economical potential. The theoretical potential describes the maximum of attainable savings, whereas the technical potential specifies possible savings considering existing unit operations. The material potential is quantified as the savings of raw materials in relation to the batch technology. Finally, the economic potential sets the savings calculated on the basis of the material potential in relation to economic key indicators. From this, the maximum of attainable savings depends on the unit operations, which are technically feasible and meaningfully applicable. Cost savings from using microreaction technology were discussed by the authors for the example of nitration reactions.
Roberge et al.52 from Lonza Company, Visp, Switzerland have investigated benefits and drawbacks of microreaction technology in the fine chemical and pharmaceutical industry. They stress the potential of microreaction technology to reduce labour costs by highly automated and efficient processes. The outcome of their reaction analysis, detailing capital (CAPEX) and operational (OPEX) costs for several pilot microchemical processes, is that up to 50% of the studied reactions at Lonza would have a sufficiently rapid kinetic to be operated in a microreactor (see Fig. 4). For 44% of them a microreactor would be the preferred reaction device. The authors argued that the handling of solids remains one of the main hurdles that reduces the number of reaction candidates to less than 20%. Thus, they emphasise the development of multi-purpose microreactor modules that can deal with solid phases.
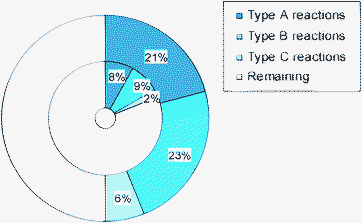 | ||
| Fig. 4 Classification of a portfolio of reactions by Lonza Company: very fast, mixing limited (Type A); fast, heat-transfer limited (Type B); with potential for micro processing due to other reasons, e.g. safety (Type C). Outer circle: all reactions; inner circle: reactions with exclusion of gas–liquid and solid-containing processes.52 (Courtesy of Wiley-VCH). | ||
An additional example is the investigation of the formation of the 4-cyanophenylboronic acid by Krtschil et al.53 An economic fine chemical process of the customised chemical producer AzurChem GmbH in Wiesbaden, Germany serves as the basis of the economic evaluation. Besides the assessment of the real existing microchemical process a number of case scenarios were described. As an example, capacity increase was considered. The authors argued that this can be reached by enlargement of the internal dimensions in a certain range without losing performance, called “smart dimensioning” as well as by “external numbering-up”. In the former case scenario two variants, assuming 5- and 10-fold increases in capacity were considered, in the latter case 10 microdevices are assumed to be working in parallel. Based on these improvements order-of-magnitude changes in productivity can be expected by microreaction technology which, respectively, may decrease the plant size per given production rate, targeting operator salaries and plant investments. The results further point out that an optimisation of operational (variable) costs can be the key driver to develop a business perspective for microprocess engineering. From this, two major trends were visible. The first relies on the synthesis of high valued products from expensive raw materials. Then, even the high operator costs, which otherwise may dominate the overall costs, are outpaced. The second strategy is based on reducing the operator costs by process intensification through microprocess engineering. In both cases, the equipment costs (microstructured reactors and balance-of-plant equipment) have a low share. Thus, the authors concluded that these costs should have a minor impact on the decision to go for the novel technology, but rather, the latter has to be proved regarding expected process optimisation and reliability.
Besides possible cost improvements a considerable reduction of the environmental impact is commonly expected from the implementation of microreaction technology into industrial processes.54 As discussed before, microstructured devices are often orders of magnitude more efficient with regard to heat and mass transfer, compared to large-scale batch reactors.7 This effect should lead to lower cooling and heating power requirements. Further, reductions of educt and solvent demand can be expected in response to the excellent control of heat transfer, which may result in improved yield, selectivity and product quality.55 Especially fast and highly exothermic reactions if performed in microreactors should be environmentally benign.
Kralisch and Kreisel56,47 investigated the pros and cons of microreaction technology compared to macro-scaled batch technology using the LCA methodology. The motivation for this study was the question of whether ecological improvements can be expected for the chosen model reaction performed in the microstructured device Cytos® produced by CPC Systems GmbH, Germany. Such improvements were the premise for the construction of a continuously operated pilot plant based on the Cytos® microreactor. Additionally, the influence of the life time of the microstructured devices as well as of the peripheral equipment was estimated in order to obtain insight into the ecological hot-spots of the system. A conventional macro-scaled semi-continuously operated batch process was chosen as a reference process. The comparison of both technological systems was performed by means of the two-step synthesis of m-anisaldehyde. The improved heat transfer in the micro-scale system allows for the fast and strongly exothermic reaction to be conducted isothermally at moderate temperatures. Although not all parameter specifications of the alternative reaction technologies under investigation could be determined at this stage of process development, a range of helpful information, insights into the interrelationships within these systems, as well as the influence of single life cycle stages on the overall results was gained. The calculation of case scenarios allowed for the investigation of a range of possible parameter values, and sensible assumptions and estimations based on generic data proved useful to calculate the proportion and relevance of single modules. The results obtained within this LCA clearly indicate that significant ecological advantages can be gained from the transfer of a macro-scaled batch synthesis to continuous microreactor processing. In case of the two-step synthesis of m-anisaldehyde, this outcome was found by the authors on laboratory scale as well as on production scale synthesis. It was primarily related to the avoidance of a cryogenic system by using microreaction technology resulting in a considerable decrease of the energy demand during synthesis.
Sustainable processing in novel process windows
Idea
Microreaction technology enables, as mentioned before, improving processes to reach operation conditions which are not accessible, or which are at least unscaleable, with conventional equipment. So far this has, here and there, been used with regard to safety directives, e.g. to allow process development with processes which otherwise could not be investigated.The idea to deliberately explore harsh or otherwise very unusual process conditions for process intensification of chemical reactions, is a recent concept termed “Novel Process Windows”. This has given rise to a German research cluster with five projects being funded (http://www.dbu.de).
The means for Novel Process Windows are summarised.9 The general idea is to operate at conditions which considerably speed up conversion rates, while maintaining selectivity. This is achieved, e.g., by step-change increases in temperature,57–60 pressure,61,62 or concentration (solvent-free operation),9 or by simplifying the process flow and protocol, via fast, simultaneous carrying out of process steps (reactant dosing),9 as well as function integration (membrane reactor).9 These measures, albeit each it stands to reason, could hardly be exploited so far, since operation typically would be beyond the capability of standard equipment. In addition, all these actions are accompanied by strong heat release and may induce notably side reactions. This, however, can be both controlled at the micro scale by virtue of the much improved mixing and heat transfer and, most importantly, by the use of very short residence times. Finally, it is not surprising that our chemical experience, which relates to processing in the range from some ten minutes to – more often – some hours is not adequate to judge the suitability of process windows, when undertaken in a few seconds.
Current implementation of novel process windows into chemical process design – high temperature, high pressure
Recently, a directed and deliberate search for novel process windows and their use for microreactor processing has been initiated.Initial research was done on increasing temperature which was found to increase reaction rates by orders of magnitude.57–59 The impact of increasing pressure is more manifold; it may be applied simply to avoid boiling under high-temperature conditions,57–59 also, for increasing mass transfer for gas–liquid reactions,60 changing the reaction mechanism through solubility effects (supercritical state),61 and using steric effects (volume of transition state) to guide product and isomer selectivity.61
Among the first examples to demonstrate the effect of drastically increasing temperature was the aqueous Kolbe–Schmitt reaction of resorcinol to 2,4-dihydroxy benzoic acid (Scheme 1) using a pressurised capillary heated by an oil bath and a micro heat exchanger for temperature quenching downstream.57 Experiments were conducted at a pressure of 40–70 bar, a temperature of 100–220 °C, and reaction times of 4–390 s. It was cross-checked and confirmed that the textbook recommended reaction time of 2 h at least roughly constitutes the actual kinetically needed reaction time at the standard batch conditions of 100 °C and 1 bar.
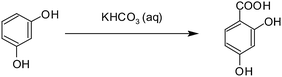 | ||
| Scheme 1 Synthesis of 2,4-dihydroxy benzoic acid | ||
The industrial production method is similar to this microreactor processing.632,4-Dihydroxy benzoic acid is produced via conversion of resorcinol with aqueous potassium carbonate solution at 100 °C and applying a carbon dioxide pressure of 4.5 bar. A byproduct 2,6-dihydroxy benzoic acid is formed,63 which is favoured at high temperatures and long residence times.64 Due to continuous processing and to its small dimensions the experimental set up offers the capability to improve selectivity. The process variant of the Kolbe–Schmitt synthesis used here is different from that used for the synthesis of monohydroxycarboxylic acids like acetylsalicylic acid which is a gas–solid reaction performed in pressurised vessels (autoclaves) at high temperatures.63 Even when these conditions are similar to the novel process windows' approach and when they further are combined with new reaction routes based on reactive intermediates, e.g. using alkali metal aryloxides, reaction times up to 2 h are still needed.65
On the contrary, using the high-temperature microreactor processing method as described above the Kolbe–Schmitt synthesis was completed within less than one minute at comparable yields to batch operation, i.e. a reduction of reaction time by a factor of ∼450 at comparable yields was achieved (see Fig. 5). Accounting for the reactor volume as well, this corresponds to an increase in space–time yield by factor 440.
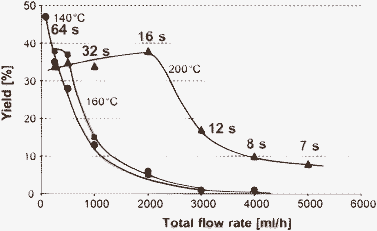 | ||
| Fig. 5 Yield for various flow rates for the aqueous Kolbe–Schmitt reaction of resorcinol to 2,4-dihydroxy benzoic acid using a pressurised capillary, demonstrating feasibility of reaction times of some 10 s.57 (Courtesy of American Chemical Society). | ||
As is to be expected, the high temperature processing principally enhances side and consecutive reactions, most remarkably at long residence times. Decarboxylation of the main product is a function of temperature (see Fig. 6), evident from the synthesis of 2,4,6-trihydroxy benzoic acid from phloroglucinol being even more labile to thermal destruction due to the enhanced electron richness of the aromatic core.58 Here, good performance is achieved only in a small process window which demands accurate process control.
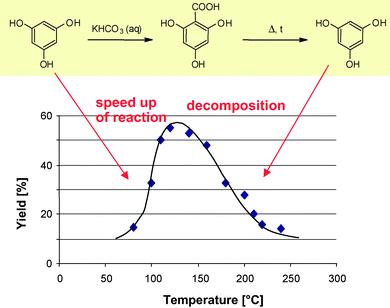 | ||
| Fig. 6 Yield for various flow rates for the aqueous Kolbe–Schmitt reaction of resorcinol to 2,4-dihydroxy benzoic acid using a pressurised capillary, demonstrating feasibility of reaction times of some 10 s.58 (Courtesy of Wiley-VCH). | ||
Similar process intensification by pressurised high-temperature operation was obtained for the Michael addition with several α,β unsaturated amines and esters.62 The faster kinetics led to an increase of the heat released which demanded efficient heat removal and avoidance of thermal overshooting. Yields ranging from 95–100% were obtained, at reaction times of a few seconds to some 10 min (dependent on the substrate), instead of the generally used 24 h batch operation; operation at high pressure and temperature was established. High productivity was achieved, giving a space–time yield increase by a factor of about 650, in the best case.
Another investigation was concerned with changing the product type for the Heck aminocarbonylation reaction using high pressure and temperature, operating above the boiling point of toluene.60 A special silicon microreactor for operation at pressures >100 bar was fabricated by a self-developed solder-based sealing technique.
One example investigated was the aminocarbonylation of 3-iodoanisole and morpholine at 7.9 bar (Scheme 2).60
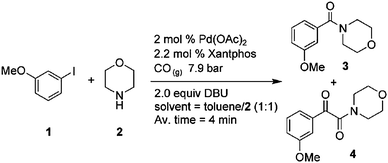 | ||
| Scheme 2 Heck aminocarbonylation reaction. | ||
It was found that the aminocarbonylation in the microreactor at elevated CO pressures yielded notable amounts of α-ketoamide in addition to the amide in contrast to conventional benchtop reactions at near atmospheric pressure. This was explained by the improved mass transfer via the larger gas–liquid interface, resulting in a higher CO concentration in solution. A significant shift towards amide formation is observed especially for temperatures above the boiling point of the solvent at atmospheric pressure (toluene, 110 °C) (see Fig. 7).
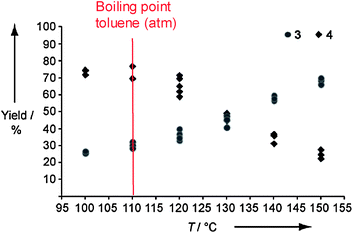 | ||
| Fig. 7 Steering product selectivity between α-ketoamide and amide for the Heck aminocarbonylation reaction by changing temperature; for definition of the products 3 and 4 see above.60 (Courtesy of Wiley-VCH). | ||
Beyond the issue of changing product distribution, the pressurised microreactor could show its potential by expanding the process windows (from 4.5 to 14.8 bar and 98 to 160 °C). This leads to greater flexibility for loading and sampling than can be achieved with conventional high-pressure chemical equipment such as a Parr bomb or autoclave.
The influence of pressure on speeding up chemical reactions was demonstrated for the esterification reaction of phthalic anhydride with methanol in a glass microreactor at pressures up to 110 bar and using supercritical CO2, which is generated inside the microreactor, as a co-solvent61 (and see62 for more examples). Substantial rate enhancements were obtained, e.g. a 53-fold increase at 110 bar and 60 °C. Supercritical CO2 as a co-solvent led to a 5400-fold increase as compared to batch experiments at 1 bar and 60 °C.
This increase in rate constants was explained by changes in activation energy and pre-exponential factor by the supercritical CO2 from data gathered in a kinetic study.61 The origin was not totally clear. One speculation was concerned with a catalytic process occurring at the sidewalls of the microreactor due to the large surface area offered. Another explanation assumes a change in reaction mechanism. Close to its critical conditions CO2 has a positive effect on the cluster formation between phthalic anhydride and methanol molecules. Localised regions with high reactant concentrations result, which may serve as microscopic pockets for reactions.
The use of high pressure to exploit steric effects via the volume of transition state is given in examples of the Diels–Alder reaction and nucleophilic substitution of halogenated nitro aromates by voluminous cyclic amines.62 Rate enhancements up to a factor of 3 when operating at a pressure of 600 bar were determined for the nucleophilic substitution. The pressure impact on the Diels–Alder reaction is due to their negative activation volumes, which are usually rather high in the range of −25 to −45 cm3 mol−1. This was monitored for the stereoselectivity of the reaction of 2-furylmethanol and 3-furylmethanol with various maleimides. Endo/exo product ratios of the first reactant were impacted by pressure, but not for the second. This is in line with the corresponding negative reaction volumes as calculated from minimised structures of the endo/exo products.
Economic and ecological implications of novel process windows for one case study
Cost improvements may be achieved not only by exploiting the engineering potential of microstructured reactors, but by using the latter to utilise essentially “Novel Process Windows” e.g. at high temperatures combined with high pressures to result in substantially shortened residence times.9This is exemplified in one running case study of the Kolbe–Schmitt synthesis for which the potential of applying novel process windows is discussed above. This approach allows for a targeted development of sustainable processes starting already on the R&D level. The idea is to formulate case scenarios based on experimental data and predictions, providing an insight into the influence of single parameter improvements, potentially gained in further research work, to the overall ecological and economic consequences.
Economic implications
The investigated process here is the aqueous Kolbe–Schmitt synthesis of 2,4-dihydroxy benzoic acid from resorcinol in an aqueous solution of potassium hydrogen carbonate under high temperature, 200 °C, and high pressure, 40 bar, in a continuous working microreaction plant.66 The reaction times could be reduced by three orders of magnitude, from some hours for the batch process to some tens of seconds. The achieved selectivity was 45%.57The base case was calculated with a five-tube reactor allowing a theoretical production rate of 4.4 tons/year (assuming 8000 h/year).66 The product related fixed cost of about 1 €/kg product, derived from the investment cost of the plant by reckoned up with a amortisation time of 7 years, are very small compared with the operational costs of 91 €/kg product. The main and approximately equal portions of the operational costs are the raw material (prices for 25 kg units) and the operator's salary when a quarter of the hourly wage rate of manpower is assumed (Fig. 8).
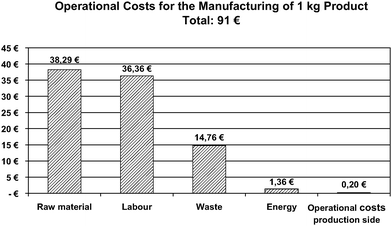 | ||
| Fig. 8 Shares of variable costs of the realised process.66 | ||
 | ||
| Fig. 9 Shares of variable costs using tenfold process intensification.66 | ||
With this rough cost analysis, the optimisation of the operational (respectively variable) costs could be identified as the decisive point for a commercially profitable microreaction process.66 This is in line with the quest to go from microreactor engineering to microreactor process engineering. The study showed up two ways for cost-efficient microreactor operation. In the case of the synthesis of high-value products from expensive raw materials the high operator costs, which otherwise dominate, are of less relevance.53 On the other hand, process intensification through micro process engineering can reduce the operator costs compared to the batch.66 The equipment costs, consisting of the microstructured reactors and the balance-of-plant equipment, have a low share in both cases and thus should have a minor influence on the decision for the implementation of this novel technology.
Ecological impact
Besides cost analyses some first estimations of environmental impacts have been done to improve the ongoing process development. To compare the different process alternatives of the Kolbe–Schmitt synthesis, which have been discussed already, the global warming potential (GWP), resulting from the production of one kilogram product (unpurified) in a microreactor on lab-scale (T = 180 °C, p = 40 bar, yield 43%), was calculated and compared to the batch scenario (T = 100 °C, p = 1 bar, yield 40%), discussed previously (assumption: production target: 0.5 kg h−1).The GWP is an environmental impact category, typically used in life cycle analyses.67 It is calculated from the sum of the global warming potential of different green house gases, which will be emitted to the environment during the whole life-cycle of a product or process. Such green house gases enhance radiative forces, causing the temperature at the earth's surface to rise. The equivalence factor of this impact category is related to the impact of the green house gas carbon dioxide.
The results obtained for the analysis of the alternative processes concern real process parameters obtained on laboratory-scale and the resulting yields of 2,4-dihydroxy benzoic acid. For this purpose, the demand of electrical current was estimated using given power consumption of the electrical devices multiplied by an efficiency factor for heat transfer (oil bath: 40%). The estimated global warming potential includes all life-cycle-steps starting from the extraction of fossil energy materials up to the energy supply for the reaction process. Furthermore, the overall effort to supply the educts and solvents and for the treatment of accrued waste water has been included into the balance. The values were determined using the ecoinvent database by Frischknecht et al.68 The results pointed out a similar global warming potential for the microreaction process (29 kg CO2 kg−1 product) compared to the batch system (33 kg CO2 kg−1 product). Thus, further development has to been done to reach significant ecological improvements.
Conclusion
Milli and micro process technologies have considerable potential for green processing which relies on speeding up reactions, increasing selectivity, safe operation in explosive regimes, and other process intensification due to increased mass and heat transfer, controllable short residence times, and other advantages for chemical processing. Following the implementation of the new, innovative technology into chemical pilot and even production operation, first ecological and economical analyses were made which make visible the environmental impact and economic efficiency. In the public domain, this is so far only done with a few examples, which makes it difficult, even for the experts, to have a generic view. Certainly, much more information is available on the company side with internal calculations.Beyond the little information on the proven industrial impact, this paper aims to emphasise that much stronger process intensification improvements may be expected when deliberately addressing novel process conditions (“Novel Process Windows”) far from the current practice of organic chemistry. This may be done using state-of-the-art microstructured apparatus which contrarily means that their process intensification potential is not fully exploited using the present chemical protocols.
Nonetheless, there are examples in literature which use novel process windows and some are cited here. A broad and deliberate approach, however, has been missing for a long time and only recently is this seemingly being addressed, as e.g. given by the new German research cluster “Novel Process Windows” of the Deutsche Bundesstiftung Umwelt (DBU). We aim in this paper, to highlight some initial ecological and economic considerations for one of the corresponding projects which is concerned with the intensification of the aqueous Kolbe–Schmitt synthesis (see above and also the outlook for more details).
While such up-front analysis of the technology implementation is undoubtedly abridged and limited in details, it still has a big advantage in that the major directions to achieve cost competitiveness and environmental acceptance are identified.
We think this is a small contribution to avoid only technologically driven developments for new innovations, and to consider rather the market view as the major driver. This is especially valid for the chemical industry with its huge, long-time operating plants, which create a conservative attitude to- and big hurdles for- implementation of innovations. An early-bird view on relevant decision drivers and business arguments is needed to counterbalance the soft human factor and convince decision makers.
Outlook: The potential of combining PI technologies
The combination of PI technologies offers the potential to use their complementary features at best to further maximise processing. This is only on the horizon for current processing, with initial approaches on combined microwave–microreactor operation being demonstrated. In the following, the main conclusions of running cost- and LCA analyses on the combination of PI technologies, to be published elsewhere as technical paper, are given since these provide a good outlook in the context of this review.The aqueous Kolbe–Schmitt reaction from resorcinol to 2,4-dihydroxy benzoic acid57 is taken once more as an example, see technical information given above. Recent improvements to the Kolbe–Schmitt synthesis refer to using ionic liquids to allow higher product concentrations (solvent-less processing) using a microwave as an alternative energy source for fast heating up, and thus making more efficient use of the reaction capillary (higher average reaction temperature).69 It was found that the new approaches can increase the yields when compared to the conventionally heated Kolbe–Schmitt process, from about 40 to 55–60% under comparable conditions, and allow operation at a much lower reaction temperature.57
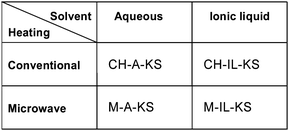
This leads to the definition of three new processes, one being the combined use of microwave and ionic liquids (see Fig. 10).69 The drawbacks of such upgraded process flows are that ionic liquids are costly materials typically available in small amounts and microwaves require expensive equipment with high energy effort. The question is – can this be counterbalanced by higher yields and achievable throughputs. The cost analysis depicted below exceeds previous investigations confined to conventionally heated micro process engineering (CHAKS type).69
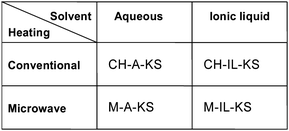 | ||
| Fig. 10 Novel Process Windows for process intensification: CHAKS: Conventionally Heated Aqueous Kolbe–Schmitt process; MAKS: Microwave Aqueous Kolbe–Schmitt process; CHILKS: Conventionally Heated Ionic Liquid Kolbe–Schmitt process; MILKS: Microwave Ionic Liquid Kolbe–Schmitt process (not considered in this study, but elsewhere69). | ||
In Fig. 11, the share of variable costs for the microwave-type MAKS process is given. The capital costs for all processes given in Fig. 8 were calculated as well, but have only a very minor share of the total product costs and are not further considered. Thus, the variable costs largely impact and approach the total product costing.
 | ||
| Fig. 11 Summary of the impact of the different Novel Process Windows approaches on variable costs. Listed are variable costs of the unpurified product, considering only reaction costs. CHILKS* denotes the fictive, highly process intensified CHILKS process given below. | ||
Not surprisingly, the preliminary analysis shows that both approaches need further improvement to become superior to the simple conventionally heated micro process; and much more improvement is needed for the ionic liquids than for the microwaves.
However, the cost analysis indicates that the microwave aqueous Kolbe–Schmitt process (MAKS) can already be cost-efficient under specific conditions for lab-scale synthesis (assumptions: production target: 0.5 kg h−1; real best yield of 52%; 7.5 l h−1 in 7 parallel capillaries; 160 °C; 8 bar).69 This is due to raw material costs, as a major cost portion, being decreased by the higher degree of conversion of the reactants due to the high yields of the microwave process when compared to conventional heating (CHAKS). Labour costs, related to the reduction of reaction time, could not be reduced in the first run due to current technological limitations of the setup, although this is to be expected from the faster heating to reaction set temperature by virtue of using microwaves.
The conventionally heated, ionic-liquid based Kolbe–Schmitt process (CHILKS) is less cost-efficient (assumptions: production target 0.5 kg h−1; real best yield of 59%; 6 l h−1 in 35 parallel capillaries; 180 °C; 35 bar; ionic liquid 50% ethyl based methyl imidazolium hydrogen carbonate (Scheme 3) diluted with water; loss of only 0.5% ionic liquid for each step, rest being recycled; real current cost ionic liquid: 3275 €/kg).
While the labour costs are comparable between the MAKS, CHAKS and CHILKS processes, the major burden for the latter is its high (raw material) costs of the ionic liquid material. The high costs of the ionic liquid even counterbalance and mask the positive effect on the raw material costs stemming from the higher yield obtained by CHILKS, even assuming a high recycle ratio and a very small loss of 0.5% of the ionic liquid, much below current technical limits. Thus, this impact is intrinsic, regardless of any process improvements, as long as the price of the ionic liquid stays high. Thus, the only option to make the CHILKS process cost-efficient is to considerably reduce the costs of the ionic liquid, to work undiluted, and to have much higher throughput. Therefore, a more than 20-fold cost reduction of the price of the ionic liquid is needed, which approaches the costs of a very few, mass produced ionic liquids these days. A first and still approximate analysis of the environmental burden resulting from single aspects of the alternative ways of processing the Kolbe–Schmitt-synthesis by means of the global warming potential has two solutions:
(1) The results (Fig. 12) actually point out a similar global warming potential for MAKS compared to CHAKS and the batch system. But, the increased energy demand in case of the MAKS synthesis can be counterbalanced by higher yields, so that further yield increase should allow for ecological improvements.
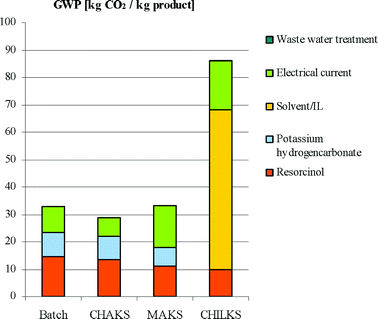 | ||
| Fig. 12 Estimated global warming potential. | ||
(2) In case of the CHILKS process, a very efficient work-up and long-term recycling of the ionic liquid is necessary, before the benefits in performance will outweigh the disadvantages, resulting from the additional effort for the ionic liquids supply.
Prospectively, these first estimations will be extended by the analysis of the work-up procedure and different recycling as well as scale-up scenarios cross-linked to further research work.
Thus, not surprisingly, the preliminary analysis shows that both approaches will need further improvements to be superior to the simple conventionally heated micro process; and much more improvement will be necessary for the ionic liquids than for the microwaves.
Acknowledgements
The authors like to thank Dr Annegret Stark from Jena University for her expert information concerning the ionic liquids used in the Kolbe–Schmitt synthesis and Sabine Hübschmann from Jena University for the estimation of the global warming potential for the different process alternatives of the Kolbe–Schmitt-synthesis considered here.References
-
G. H. Brundtland, For World Commission on Environment and Development, Our Common Future, Oxford University Press, Oxford, UK, 1987 Search PubMed
.
- United Nations Conference on Environment and Development (UNCED), Rio de Janeiro, June 3–14, 1992 Search PubMed.
- OECD Environmental Outlook, Paris, 2001.
-
P. T. Anastas and J. Warner, Green Chemistry: Theory and Practice, Oxford University Press, London, 1998 Search PubMed
.
-
V. Hessel, S. Hardt and H. Löwe, Chemical Micro Process Engineering – Fundamentals, Modelling and Reactions, Wiley-VCH, Weinheim, 2004 Search PubMed
.
-
V. Hessel, H. Löwe, A. Müller and G. Kolb, Chemical Micro Process Engineering – Processing and Plants, Wiley-VCH, Weinheim, 2005 Search PubMed
.
- K. Jähnisch, V. Hessel, H. Löwe and M. Baerns, Angew. Chem., Int. Ed., 2004, 43, 406 CrossRef
.
- V. Hessel, P. Löb and H. Löwe, Curr. Org. Chem., 2005, 9, 765 CrossRef CAS
.
- K. F. Jensen, Chem. Eng. Sci., 2001, 56, 293 CrossRef CAS
.
- S. J. Haswell and P. Watts, Green Chem., 2003, 5, 240 RSC
.
- P. D. Fletcher, S. J. Haswell, E. Pombo-Villar, B. H. Warrington, P. Watts, S. Y. F. Wong and X. Zhang, Tetrahedron, 2002, 58, 4735 CrossRef CAS
.
- A. Gavriilidis, P. Angeli, E. Cao, K. K. Yeong and Y. S. S. Wan, Trans. Inst. Chem. Eng., 2002, 80/A, 3 Search PubMed
.
- K. Geyer, J. D. C. Codee and P. H. Seeberger, Chem.–Eur. J., 2006, 12, 8434 CrossRef CAS
.
- J. G. Kralj, R. Hemantkumar, R. Sahoo and K. F. Jensen, Lab Chip, 2007, 7, 256 RSC
.
- A. Aota, M. Nonaka, A. Hibara and T. Kitamori, Angew. Chem., Int. Ed., 2007, 46, 878 CrossRef CAS
.
-
G. Kolb, Fuel Processing for Fuel Cells, Wiley-VCH, Weinheim, 2008, p. 382 Search PubMed
.
- P. Löb, V. Hessel, A. Hensel and A. Simoncelli, Chim. Oggi - Chem. Today, 2007, 25(3), 26 Search PubMed
.
- P. Löb, V. Hessel, U. Krtschil and H. Löwe, Chim. Oggi - Chem. Today, 2006, 24(2), 46 Search PubMed
.
-
V. Hessel, P. Löb and H. Löwe, ‘Industrial Microreactor Process Development up to Production’, in Microreactors in Organic Chemistry and Catalysis, ed. T. Wirth, Wiley-VCH, Weinheim, 2008, pp. 211–275 Search PubMed
.
- B. P. Mason, K. E. Price, J. L. Steinbacher, A. R. Bogdan and D. T. McQuade, Chem. Rev., 2007, 107, 2300 CrossRef
.
- R. J. J. Jachuck, J. G. M. Lee, D. Kolosta, C. Ramshaw, P. Valachis and S. Yanniotis, Green Chem., 1999, 2, G15 Search PubMed
.
- V. Hessel, C. Hofmann, H. Löwe, A. Meudt, S. Scherer, F. Schönfeld and B. Werner, Org. Process Res. Dev., 2004, 8, 511 Search PubMed
.
- L. Ducry and D. M. Roberge, Angew. Chem., Int. Ed., 2005, 44, 7972 CrossRef CAS
.
- R. D. Chambers and R. C. H. Spink, Chem. Commun., 1999, 10, 883 RSC
.
- K. Jähnisch, M. Baerns, V. Hessel, W. Ehrfeld, V. Haverkamp, H. Löwe, C. Wille and A. Guber, J. Fluorine Chem., 2000, 105(1), 117 CrossRef CAS
.
- N. de Mas, A. Günther, M. A. Schmidt and K. F. Jensen, Ind. Eng. Chem. Res., 2003, 42(4), 698 CrossRef CAS
.
- B. K. Vankayala, P. Loeb, V. Hessel, G. Menges, C. Hofmann, D. Metzke, U. Krtschil and H.-J. Kost, Int. J. Chem. React. Eng., 2007, 5, A91 Search PubMed
.
- R. C. R. Wootton, R. Fortt and A. J. de Mello, Org. Process Res. Dev., 2002, 60 Search PubMed
.
- M. Struempel, B. Ondruschka, R. Dauteb and A. Stark, Green Chem., 2008, 10, 41 RSC
.
-
S. Löbbecke, J. Antes, W. Ferstl, D. Bošković, T. Türcke, M. Schwarzer and H. Krause, ‘Microreactors for Processing of Hazardous and Explosible Reactions’, in IChemE Symposium Series No. 153: Proceedings of 12th International Symposium Loss Prevention and Safety Promotion in the Process Industries, 22–24 May, 2007, Edinburgh, Scotland Search PubMed
.
-
J. Antes, T. Türcke, E. Marioth, F. Lechner, M. Scholz, F. Schnürer, H. H. Krause and S. Loebbecke, ‘Investigation, analysis and optimization of exothermic nitrations in microreactor processes’, in Microreaction Technology – IMRET 5: Proceedings of the 5th International Conference on Microreaction Technologyed. M. Matlosz, W. Ehrfeld, J. P. Baselt, Springer-Verlag, Berlin, 2001, pp. 446–454 Search PubMed
.
- X. Zhang, S. Stefanick and F. J. Villani, Org. Process Res. Dev., 2004, 8(3), 455 Search PubMed
.
- S. Taghavi-Moghadam, A. Kleemann and K. G. Golbig, Org. Process Res. Dev., 2001, 5, 652 Search PubMed
.
- R. A. Sheldon, Chem.-Tech. (Heidelberg), 1994, 24, 38 CAS
.
- G. Koller, D. Weirich, F. Brogli, E. Heinzle, V. H. Hoffmann, M. A. Verduyn and K. Hungerbuhler, Ind. Eng. Chem. Res., 1998, 37, 3408 CrossRef CAS
.
- B. M. Trost, Science, 1991, 254(5037), 1471 CrossRef CAS
.
- E. Heinzle, D. Weirich, F. Brogli, V. H. Hoffmann, G. Koller, M. A. Verduyn and K. Hungerbuehler, Ind. Eng. Chem. Res., 1998, 37, 3395 CrossRef CAS
.
-
Industrial environmental performance metrics: challenges and opportunities. Commitee on Industrial Environmental Performance Metrics, National Academy of Engineering, National Research Council, National Academy Press, Washington, DC, 1999 Search PubMed
.
- V. H. Hoffmann, K. Hungerbühler and G. J. McRae, Ind. Eng. Chem. Res., 2001, 40(21), 4513 CrossRef CAS
.
- S. Kheawhom and M. Hirao, Comput. Chem. Eng., 2004, 28, 1715 CrossRef CAS
.
- S. K. Stefanis, A. Buxton, A. G. Livingston and E. N. Pistikopoulos, Comp. Chem. Eng., 1996, 20, 1419
.
- A. Azapagic and R. Clift, Comput. Chem. Eng., 1999, 23, 1509 CrossRef CAS
.
- ISO 14040:2006 ‘Environmental management - Life cycle assessment - Principles and framework.’ and ISO 14044:2006, ‘Environmental management - Life cycle assessment - Requirements and guidelines.’, European Commitee for Standardisation, Brussels, Belgium, 2006.
- G. Jödicke, O. Zenklusen, A. Weidenhaupt and K. Hungerbühler, J. Clean Prod., 1999, 7, 159 CrossRef
.
- A. A. Burgess and D. J. Brennan, Chem. Eng. Sci., 2001, 56, 2589 CrossRef CAS
.
- S. Hellweg, U. Fischer and M. H. K. Scheringer, Green Chem., 2004, 6, 418 RSC
.
- D. " and G. Kreisel, Chem. Eng. Sci., 2007, 62(4), 1094 CrossRef
.
-
B. Blanchard and W. J. Fabrycky, Systems Engineering and Analysis. Prentice Hall, Upper Saddle River, New Jersey, USA, 1998, pp. 506 Search PubMed
.
- G. Rebitzer and D. Hunkeler, Int. J. Life Cycle Assess., 2003, 8, 253–256
.
- P. Saling, A. Kicherer, B. Dittrich-Kramer, R. Wittlinger, W. Zombik, I. Schmidt, W. Schrott and S. Schmidt, Int. J. Life Cycle Assess., 2002, 7(4), 203
.
- D. Schmalz, M. Häberl, N. Oldenburg, M. Grund, H. Muntermann and U. Kunz, Chem. Ing. Tech., 2005, 77(7), 859 CrossRef CAS
.
- D. M. Roberge, L. Ducry, N. Bieler, P. Cretton and B. Zimmermann, Chem. Eng. Tech., 2005, 28, 318 CrossRef CAS
.
- U. Krtschil, V. Hessel, D. Kralisch, G. Kreisel, M. Küpper and R. Schenk, CHIMIA 2006, vol. 60(9), p. 611.
- J. F. Jenck, F. Agterberg and M. J. Droescher, Green Chem., 2004, 6(11), 544 RSC
.
- V. Hessel, P. Löb and H. Löwe, Curr. Org. Chem., 2005, 9(8), 765 CrossRef CAS
.
- D. Kralisch and G. Kreisel, Chem. Ing. Tech., 2005, 77(6), 62
.
- V. Hessel, C. Hofmann, P. Löb, J. Löhndorf and H. Löwe, A. Ziogas, Org. Process Res. Dev., 9(4), p. 479 Search PubMed.
- V. Hessel, C. Hofmann, P. Löb, H. Löwe and M. Parals, Chem. Eng. Tech., 2007, 30(3), 355 CrossRef CAS
.
- H. Löwe, V. Hessel, S. Hubbard and P. Löb, Org. Process Res. Dev., 2006, 10(6), 1144 Search PubMed
.
- E. R. Murphy, J. R. Martinelli, N. Zaborenko, S. L. Buchwald and K. F. Jensen, Angew. Chem., 2007, 119, 1764 CrossRef
.
- F. Benito-Lopez, R. M. Tiggelaar, K. Salbut, J. Huskens, R. J. M. Egberink, D. N. Reinhoudt, H. J. G. E. Gardeniers and W. Verboom, Lab Chip, 2007, 10(7), 1345 Search PubMed
.
-
F. Benito-Lopez, PhD thesis, High Pressure: a Challenge for Lab-on-a-Chip Technology, 2007, University Twente, Enschede, The Netherlands Search PubMed
.
-
Ullmann's Encyclopedia of Industrial Chemistry, 7th edn, Wiley-VCH, Weinheim, 2008 Search PubMed
.
- D. K. Hale, A. R. Hawdon, J. I. Jones and D. I. Packham, J. Chem. Soc., 1952, 3503 RSC
.
- M. R. Samuels and R. M. Yabroff, EP1035100B1.
-
V. Hessel, U. Krtschil, P. Löb, H. Löwe, D. Kralisch, G. Kreisel, M. L. Küpper and R. Schenk. Proceedings of the 2006 AIChE Annual Meeting San Francisco, CA, November 12–17, 2006 Search PubMed
.
-
J. B. Guinée, ‘Life cycle assessment - an operational guideline to the ISO standards - part 2b’, in Minstry of Housing, Spatial Planning and the Environment and Centre of Environmental Science, ed. M. Gorree and R. Heijungs, Leiden University, The Netherlands, 2001 Search PubMed
.
- Frischknecht, et al., Ecoinvent database, Swiss Centre for Life Cycle Inventories, Switzerland, 2006, vol. 1.3.
- F. Benaskar, V. Hessel, U. Krtschil and P. Löb, unpublished results, paper in preparation, 2008 Search PubMed
.
| This journal is © The Royal Society of Chemistry 2008 |

