Hydrogen nexus in a sustainable energy future
A.
Sartbaeva
a,
V. L.
Kuznetsov
a,
S. A.
Wells
b and
P. P.
Edwards
*a
aDepartment of Chemistry, Inorganic Chemistry Laboratory, University of Oxford, South Parks Road, Oxford, UK OX1 3QR. E-mail: peter.edwards@chem.ox.ac.uk
bDepartment of Physics and Centre for Scientific Computing, University of Warwick, Gibbet Hill Road, Coventry, UK CV4 7AL
First published on 2nd July 2008
Abstract
The vast majority of power/energy generation in our global economy is based on the chemical element carbon, specifically fossil carbon (coal) and hydrocarbon (oil and gas) fuels. Such an economy is not sustainable in the medium- to long-term for two fundamental reasons; first, there exists only a finite amount of fossil fuel, and, second, the carbon dioxide released during the combustion of fossil fuels induces anthropogenic climate change with costly and potentially disastrous consequences. Current energy technologies cannot support both a reduction in carbon dioxide emissions and an ever-expanding global economy. It is now recognized that hydrogen may be one of the leading contenders as a potential solution for a sustainable alternative to fossil fuels, especially for transport and for heat and power generation and energy storage. However, any transition from a carbon-based (fossil fuel) energy system to a hydrogen-based economy involves significant scientific, technological and socio-economic barriers. Our aim is to illustrate the “hydrogen nexus” as a possible connection or link from today's carbon economy to a sustainable energy future centred on hydrogen.
Dr Asel Sartbaeva was born in Kyrgyzstan. She received her MSc degree at the Kyrgyz-Russian Slavic University in Bishkek, and MPhil and PhD degrees at Cambridge University. She has worked in the Department of Physics at ASU on glasses, zeolites and superconductors. In 2007, she was awarded a Glasstone Research Fellowship at Oxford University. Her main research interests are hydrogen storage materials and flexible frameworks. |
Dr Vladimir Kuznetsov received MSc and PhD from Moscow State University and is currently a Research Fellow at the Inorganic Chemistry Laboratory, University of Oxford. His research interests centre on energy materials, including hydrogen storage materials, transparent conducting oxides, semiconductors and also issues related to hydrogen energy economy. He has published over 50 journal papers and 6 book chapters. |
Dr Stephen A. Wells obtained his PhD from Cambridge University. He has worked in mineral physics at the Royal Institution, London and in biophysics at Arizona State University. He is now a researcher at the University of Warwick in the Centre for Scientific Computing. His principal interest is in the development and use of simplified simulation methods for the study of mineral and biological structures. |
Prof. Peter P. Edwards received his BSc and PhD from Salford University; he is currently Head of Inorganic Chemistry at the University of Oxford and Management Director of the UK Sustainable Hydrogen Energy Consortium. His research centres on hydrogen storage materials, inorganic electronic materials, metal nanoparticles and high-temperature superconductors, with a common theme being the Metal–Insulator Transition. |
1. The carbon economy
The development of the global economy has been based on the existence of large reservoirs of carbon-based fossil fuels, which we use as primary energy sources for electricity, industry, transport and heat. Such reservoirs represent energy harvested from the sun and stored by living organisms over hundreds of millions of years, and we are depleting them at roughly a million times their original rate of deposition. Our current energy scenario is represented schematically in Fig. 1, which centres primarily, therefore, on carbon-based materials.1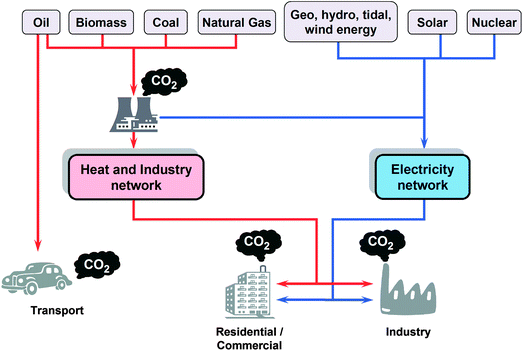 | ||
| Fig. 1 The carbon economy: the overwhelming majority of our current energy needs are met by the combustion of fossil carbon and hydrocarbon fuels. Blue arrows represent non-carbon, potentially sustainable and renewable, energy flows, while red arrows indicate energy streams based on the combustion of carbon compounds, principally from fossil fuels, with the concomitant release of carbon dioxide (modified from Marbán and Valdés-Solís1). | ||
The arguments as to why energy scenarios now merit serious and urgent attention are well documented – and accepted.2–6 The core issues centre on:
• Anthropogenically-induced climate change and the need to reduce CO2 emissions from fossil (carbon-based) fuels
• Security of energy supply in an interconnected and uncertain and politically unstable world
• Uncertainty about the long-term availability of conventional fossil fuels
• The strong connection between increased energy demand and economic growth
• The vexing issue of fuel poverty and energy inequalities – both nationally and internationally.
Against this backdrop, there is considerable international interest in the possibility of the so-called “hydrogen economy” to tackle many (perhaps all) of these issues.3–5 The vision is for hydrogen to be used as an energy vector allowing, notably, vehicles to be powered by hydrogen fuel cells. However, there are major scientific, technical and socio-economic challenges7–9 to be overcome in relation to the production, storage and transportation of hydrogen before its widespread use as, for example, a private-vehicle fuel would ever be feasible. Here we outline just some of the challenges, the complexity of which demand an integrated, multidisciplinary and medium- to long-term approach, but it is clear that the broad chemical sciences and engineering knowledge and expertise base could provide the major springboard to the resolution of these critically-important problems.
2. Hydrogen nexus
By “hydrogen nexus” we mean that hydrogen may fulfil part of the central role currently occupied by carbon, and provide the means of connection and link to a sustainable energy economy. Energy which was originally obtained from the sun is stored as chemical potential energy within chemical bonds in carbon-rich fossil fuel materials and is liberated by oxidation, generating CO2 as the principal by-product.7–10 There is no such reservoir of “fossil hydrogen”. In a sustainable energy future, energy obtained from renewable resources such as solar, wind and wave will be stored as chemical potential energy in hydrogen and hydrogen-rich materials, and liberated by oxidation generating only water at the point of use as the principal by-product.7–11 In the same way as our carbon economy uses not only elemental carbon but also carbon compounds (particularly hydrocarbons), so a hydrogen economy will utilise not only elemental hydrogen, but also hydrogen-rich chemical compounds, such as ammonia, urea, formates and others.At the outset, it is important to note that unlike coal, gas or oil, hydrogen is not a primary energy source. Its role more closely mirrors that of electricity as an “energy carrier” which is first produced using energy from another source, and then transported for future use where its latent energy, stored in the chemical bond, can then be utilised. Hydrogen can be stored as a fuel and utilised in transportation and distributed heat and power generation using fuel cells, internal combustion engines or turbines,1,3–5,7–12 and, importantly, a hydrogen fuel cell produces only water at its point of use.
Hydrogen can also be used as an attractive storage medium for electricity generated from intermittent, renewable resources such as solar, wind, wave and tidal power (Fig. 2); it thereby provides the solution to one of the critical issues associated with sustainable energy; namely the vexing problem of intermittency of supply. As long as the hydrogen is produced from non-fossil fuel feed stock, it is a genuinely green or sustainable fuel. Moreover, locally-produced hydrogen allows the introduction of renewable energy to the transport sector, provides potentially large economic and energy security advantages, and provides the benefits of an infrastructure based on distributed generation. It is this key element of the energy storage capacity of hydrogen which provides the potent link between sustainable energy technologies and a sustainable energy economy, generally placed under the umbrella of “hydrogen economy”; the hydrogen nexus provides therefore the link to sustainability.
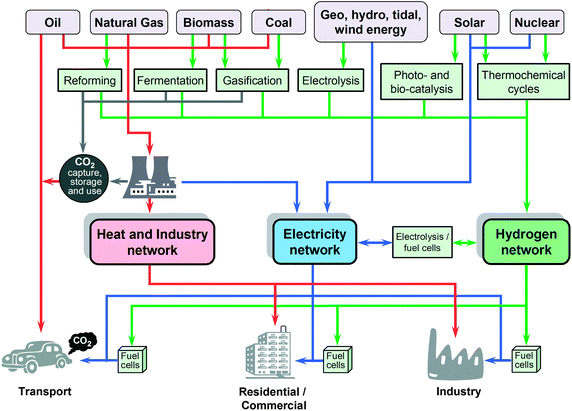 | ||
| Fig. 2 Hydrogen nexus: a possible model for a hydrogen energy economy. Note that in this view, renewable energies are considerably intensified (compared with Fig. 1) and hydrogen fuel cells are employed as critical technologies (modified from Marbán and Valdés-Solís1). | ||
3. Hydrogen production and distribution
The efficient and sustainable generation of hydrogen is necessary for the development of an ultimately sustainable hydrogen economy. Even though hydrogen is the most abundant chemical element in the universe, on Earth, it is invariably bound up in chemical compounds with other elements. It is therefore produced from other hydrogen-containing sources using energy, such as electricity or heat.12–15 The various avenues1 for producing hydrogen are highlighted in Fig. 2, derived from carbon systems, through renewable sources, and finally to nuclear.At present, hydrogen is produced in large quantities by steam reforming of hydrocarbons, generally methane.14,15 Of course, this method yields carbon dioxide as a by-product, but no more than burning the same amount of methane. Carbon dioxide emissions, the principal cause of global climate change, could be managed at large scale facilities through sequestration, which usually involves the capture and storage of CO2 underground (e.g. in depleted natural gas and oil wells or geological formations). Carbon capture and storage depends on the ability to capture CO2 – typically at such power plants, transportation by pipeline over (usually) significant distance and sequestration underground safely, reliably and durably. CO2 sequestration is currently portrayed as a potentially large-scale option for solving the CO2 challenge. But large-scale capture would also allow the possibility of CO2 conversion into synthetic fuels, where captured CO2 could potentially be converted to a liquid hydrocarbon or other fuel. This opens up the exciting possibility of recycling CO2 in association with renewable energy/hydrogen sources to selectively synthesise fuels based not only on carbon, hydrogen and oxygen (to yield hydrocarbons, alcohols, etc.), but also in combination with nitrogen to synthesise urea, formates and derivatives, etc. Critically, a full life cycle analysis of all such potential conversions must be carefully considered from scientific, technological and socio-economic stand points. This is undoubtedly a grand challenge for the chemical sciences and engineering – and to our sister disciplines beyond!
Hydrogen can be produced by splitting water through various processes including electrolysis, photo-electrolysis, high-temperature decomposition and photo-biological water splitting (Fig. 2).13,15,16 The commercial production of hydrogen by electrolysis of water achieves an efficiency of 80–85%; however, due to a high electricity cost, the cost of hydrogen is 3 to 5 times higher than that produced from fossil fuels.13,15 In addition, since a significant proportion of electricity is currently generated by burning of fossil fuels, the production of hydrogen by water electrolysis using grid electricity inevitably produces significant carbon dioxide emissions at the point of electricity generation. Electricity derived from renewable sources of energy (e.g. wind, tidal, wave) is potentially carbon-free and might provide local sources of sustainable hydrogen, but certainly it will not meet the volumes of hydrogen required globally for the new energy source.16
Production of hydrogen by the electrolysis of water has an intrinsic advantage in that the process does not depend on the source of the electricity: this aspect makes hydrogen most attractive as a potential energy store in conjunction with intermittent energy sources such as wind or wave power. However, sunlight may be more efficiently used to split water directly by photocatalysis than by electrolysis, as this could bypass the relatively low efficiencies of solar electricity generation. The holy grail of hydrogen production will be the efficient, direct conversion of sunlight through a photocatalytic process that utilises solar energy to split water directly to its constituents, hydrogen and oxygen, without the attendant use of electricity.17 This ideal production route, therefore, is to harness the power of the sun to split water from the oceans. The solar photodecomposition of water is probably the only major and long-term solution to a CO2-free route for the mass production of the huge volumes of hydrogen needed for the future hydrogen economy. Realisation of low-cost and efficient direct production of hydrogen from solar energy requires major scientific breakthroughs in developing innovative materials, emerging physical phenomena, novel synthetic techniques and new design concepts.
The challenge is to develop a photocatalyst which is durable, both against the attack of the highly reactive oxygen species formed during the splitting of water and against damage from the light itself, is cheap to produce in large quantities, and makes use of abundant, visible-spectrum photons rather than ultraviolet light. A promising approach is to make use of nanostructured catalysts. The energy gap of many materials, in particular inorganic materials, may be altered by size effects in nanoparticles, possibly allowing us to tune photocatalytic materials to absorb wholly in the visible spectrum. Materials currently under investigation include nanostructures of titania,18,19cadmium (selenide/sulfide) supported in mesoporous silica,20 and metal nanoparticles.
An intriguing possibility is also to recruit biological catalytic molecules, that is, enzymes, and modify them by genetic engineering. A recent development in this field has been the modification of a bacterial hydrogenase (which produces hydrogen from formate) to increase by thirty-fold its rate of hydrogen production.21,22 The energetic efficiency of photosynthesis in plants, which is relevant to the economics of biofuel production, depends upon an inefficient initial catalysis step by the enzyme Rubisco,23 and efforts are under way to develop more efficient forms of the enzyme.24
Nuclear energy can be used to produce hydrogen by the thermochemical electrolysis of water and this method has the potential to meet the future high demand for hydrogen. Next generation advanced nuclear reactors are also being developed that will enable high-temperature water electrolysis (with less electrical energy needed) or thermochemical cycles that will use heat and a chemical process to dissociate water. One additional benefit of high-temperature water electrolysis is the possibility to shift the main production between electricity and hydrogen during the day and night demand cycles thus using the extra energy output at night for storing energy in the form of hydrogen. Fusion power, if successfully developed, would also be a clean, abundant, and carbon-free resource for hydrogen production.
The economic viability of any hydrogen production method will be strongly affected by regional factors (availability of renewable energy sources, delivery approaches, taxation etc.). It is anticipated that in any fully developed hydrogen economy, hydrogen will be produced both centrally in large energy complexes and also locally – in refuelling stations, communities, and on-site at customers' premises.15,16 The development and implementation of such a diverse range of hydrogen production techniques15,25 requires substantial technological advances, and with them the social acceptability of any such development.26 In an extensive survey, Dutton has examined the principal technologies for producing hydrogen, for example from wind energy.27 From a particular UK perspective, he finds that a fully developed hydrogen economy from renewable, carbon-free sources would require at least a doubling of current electrical energy demand!
4. Hydrogen storage for fuel cell vehicles
On-board hydrogen storage for fuel cell vehicles is another particularly challenging area, which is widely recognised as a critical enabling technology for the successful commercialization and market acceptance of hydrogen-powered vehicles.3–5,7,9 The accepted target is a reliable, safe and economical method to store between 4 to 5 kg of hydrogen (sufficient for a drive range of some 400–500 km) whilst minimising volume, weight, storage energy, cost and refuelling time and, just as important, in providing prompt hydrogen release on demand.At present, the critical technological barrier centres on the lack of a safe, low-weight, low-cost and high performance hydrogen storage method with a high energy density.9,12Hydrogen contains more energy on a weight-for-weight basis than any other element or substance. Unfortunately, since it is the lightest chemical element, it also has a very low energy density per unit volume.
Present hydrogen storage options for automotive applications have centred upon high-pressure (up to 700 bar) gas containers or cryogenically cooled (liquefied) fluid hydrogen (Fig. 3). One downside of these methods is a significant energy penalty – up to 20% of the energy content of hydrogen is required to compress the gas and up to 40% to liquefy it. Another crucial issue that confronts the use of high-pressure and cryogenic storage centres on public perception and acceptability is associated with the use of pressurised gas and liquid hydrogen containment. Hydrogen storage requires a major technological breakthrough and this is likely to occur in the most viable alternative to compressed and liquid hydrogen, namely the storage of hydrogen in solids or liquids. Interestingly, several classes of solid state hydrogen storage materials demonstrate higher energy density than those of liquid hydrogen (Fig. 3) and they are centred on metal hydrides formed from alloys (e.g.LaNi5) and chemical hydrides of the light chemical elements (e.g. MgH2 or LiBH4etc.).28 Solid state hydrogen storage promises a real and significant breakthrough, but much research and development is still needed to understand the physical and chemical processes governing hydrogen storage and release, and to improve hydrogen absorption/desorption characteristics. Again, this is a complex challenge, necessitating close interactions between chemists, material scientists, physicists and engineers; a most attractive feature of this challenge is the “natural” multidisciplinarity of the problem at hand. From such a multidisciplinary approach, one hopes to see the evolution of new concepts and ideas, for as the recent US National Academies report notes5 “…success in overcoming the major stumbling block of on-board storage is critical for the future of transportation use of fuel cells.”
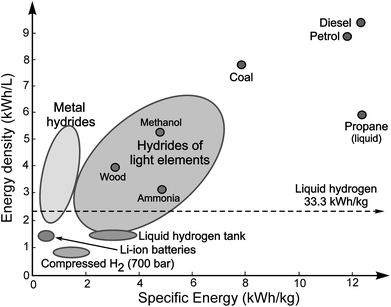 | ||
| Fig. 3 Energy densities of various energy storage materials and technologies, illustrating the respective volumetric and gravimetric densities. | ||
5. Fuel cells
Once hydrogen has been produced and stored, it is most efficiently used for power generation in a fuel cell, an electrochemical device in which hydrogen is combined with oxygen – without combustion – to produce dc electricity. Because fuel cells are not subject to the limitations of the Carnot cycle, they convert fuel into electricity at more than double the efficiency of internal combustion engines. In transportation, hydrogen fuel cell engines operate at an efficiency of up to 65%, compared to 25% for present-day petrol driven car engines. When heat generated in fuel cells is also utilised in Combined Heat and Power (CHP) systems, an overall efficiency in excess of 85% can be achieved.12 The chemical energy density of hydrogen is significantly higher than that found in electric battery materials29–31 (Fig. 3) and hydrogen fuel cells could also deliver much longer operational lifetime than that of electric batteries. Unlike internal combustion engines or turbines, fuel cells demonstrate high efficiency across most of their output power range. This attractive scalability makes fuel cells ideal for a variety of applications from mobile phones to large-scale power generation. The hydrogen economy will therefore involve the widespread use of fuel cells for efficient, clean, quiet and local generation of electricity for both static and transport uses (Fig. 2).Several types of fuel cells suitable for different energy applications at varying scales have been developed but all share the basic design of two electrodes (anode and cathode) separated by a solid or liquid electrolyte. Hydrogen (or a hydrogen-containing fuel) and oxygen are fed into the anode and cathode of the fuel cell and the electrochemical reactions assisted by catalysts take place at the electrodes. The electrolyte enables the transport of ions between the electrodes while the excess electrons flow through an external circuit to provide electrical current. Fuel cells are classified according to the nature of their electrolyte, which also determines their operating temperature, the type of fuel and a range of applications. The electrolyte can be acid, base, salt or a solid ceramic or polymer that conducts ions.
However, various major technological hurdles must still be overcome before fuel cells can compete effectively with conventional energy conversion technologies. The key scientific and technical challenges facing fuel cells are cost reduction and increased durability and performance of materials and components. This requires intensive research in the development of safe operation of improved or new materials and could provide the commercial viability of fuel cells in both stationary and mobile applications. Hydrogen fuel cell cars, currently the focus of intense development activity worldwide, are not expected to reach mass market until 2015, or possibly beyond. A recent status report31 on hydrogen fuel cell vehicles from General Motors points out that cost, range and refuelling figures are presently inferior as compared to internal combustion engines. However, the authors also note that the current figures are much better than those of advanced battery electric vehicles and projections show that hydrogen fuel cells can ultimately become cost competitive with internal combustion engines.
6. Challenges: scientific, technological and socio-economic
Any possible transition to a widespread hydrogen economy will probably require many decades. However, one notes that Iceland plans to create the world's first hydrogen economy by 2030. The timescale and evolution of such a transition is the focus of many “roadmaps” emanating from Japan, Canada and the EU (amongst many others).2–4,32,33 For example, the European Commission has very recently endorsed the concept of a Hydrogen and Fuel Cell Technology Platform with the expenditure of € 2.8 billion over a period of ten years, and the European Parliament has voted overwhelmingly in favour of such a major advance in energy policy.34 The introduction of hydrogen as an energy carrier has also been identified33 as a possible strategy for moving the UK towards its voluntary adopted targets for carbon dioxide reduction of 60% of current levels by 2050. Table 1 summarises the forecasts of several roadmaps for deployment status and targets for hydrogen technologies and fuel cell applications today and up to 2050.| Technology | Today | 2020–2025 | 2050 |
|---|---|---|---|
| EU, Portable fuel cells sold per year | n.a. | 250 million | n.a. |
| EU, Fuel cell vehicles sold per year | n.a. | 0.4–1.8 million | n.a. |
| Fuel cells (€ kW−1) | 6000–8000 | 400 | 40 |
| High temperature fuel cells (€ kW−1) | 8000–10000 | 800 | 200 |
| Carbon capture and sequestration (CCS) (€ per ton CO2) | 20–30 | 4–8 | 3–6 |
| Hydrogen produced from coal with CCS (€ GJ−1) | 8–10 | 7–9 | 3–5 |
| Hydrogen transportation/storage cost (pipeline, 5000 kg h−1, 800 km) (€ GJ−1) | 10–15 | 3 | 2 |
| EU, Stationary fuel cells (CHP) sold per year | n.a. | 2–4 GW | n.a. |
One can therefore summarise the key scientific and technological challenges for any transition to a hydrogen economy; these are:
(a) The cost of hydrogen production must be lowered to a level comparable with the energy cost of petrol, a target which becomes easier to achieve as the cost of hydrocarbon fuels continuously increases. Such a “cost equivalence” is necessary in order that the penetration of hydrogen into the fuel sector becomes economically possible. This initial step may not necessarily involve truly sustainable methods of hydrogen production; transitional methods such as hydrogen production from hydrocarbons may still be useful in enabling (or demonstrating) the transition from an economy dominated by carbon-fuel-consuming combustion systems to hydrogen fuel cell systems.
(b) A sustainable and CO2-free route to the mass production of hydrogen at a competitive cost is vital in the long term. As we have seen, it is likely that solar methods, either using electrolysis by photoelectricity or using photocatalytic methods, will be the dominant technology; however, wind, wave, geothermal and nuclear electricity generation will all contribute.
(c) A safe and efficient national infrastructure for hydrogen delivery and distribution must be developed. Industrialised nations already have systems in place which achieve safe and efficient distribution of gaseous, liquid and solid fossil fuels; however, the fundamentally different characteristics of hydrogen as compared to hydrocarbons mean that a hydrogen system faces particular challenges. Predictions of the necessary global investment to supply hydrogen to the world's transport sector are in the range of several hundred billion dollars over several decades. This level of investment is not insurmountable (in the long term) but a major infrastructure investment might be seen as premature until several of the key hydrogen technologies outlined here are developed. A related consideration is the competition with the other competing technologies, e.g. biofuels and battery-electric vehicles.
(d) Hydrogen storage materials and systems must be developed which far outperform current high-pressure and cryogenic systems, particularly if hydrogen is to see widespread use in private fuel-cell vehicles. More compact, low-weight, low-cost, safe and efficient storage systems operating at or near room temperatures and low pressures will need to be developed for automotive as well as for stationary applications. It is becoming increasingly accepted that the solid-state hydrogen storage using compounds of light elements may represent perhaps the only viable method of achieving a high weight percent and high volume density of stored hydrogen. The grand challenge reflects the fact that, at present, no known material meets these critical requirements.
(e) The preparation of regulations and safety standards at national, EU and international levels.
(f) Fuel cell technology is relatively well developed. The key scientific and technical challenges facing fuel cells are cost reduction and improving the performance, durability and safety of materials and components for both stationary and transport applications to enable them to compete with conventional combustion technologies.
Conclusions
Our current economy, based on energy from fossil carbon, is not sustainable in the medium to long term, because of both the finite quantity of fossil fuels available to us, and the environmental issues attendant on significantly increasing atmospheric CO2 levels. An attractive answer to this rapidly advancing problem is to shift towards a “hydrogen nexus”– to develop a hydrogen economy in which hydrogen occupies the central position currently occupied by carbon as the principal chemical currency for our energy needs. It is important also to stress that hydrogen could also increasingly play a key role in the generation, distribution and consumption of a future carbon-neutral/negative energy system. For example, a process of hydrogen generation using water as a feedstock, with nitrogen and carbon (in the form of CO2) as intermediates and carriers and solar, wind and nuclear energy as conversion energy sources to yield not only sustainable hydrogen, but sustainable ammonia and, with that, their conversion to suitable hydrogen (energy) carriers such as ammonium carbonate, formate and urea. In this paper we have briefly reviewed just some of the intrinsically “chemical” challenges of hydrogen production, storage and utilisation. Creating the hydrogen nexus will require the best thinking from the brightest minds to solve not only the clear challenges in the chemical science and engineering, but also the political and economic issues attendant on fundamentally restructuring a global economy. There are exciting scientific opportunities here to solve what has been termed as the greatest challenge of this century.6Acknowledgements
The authors would like to acknowledge funding from EPSRC. AS thanks the Samuel and Violet Glasstone Research Fellowship and SAW thanks the Leverhulme Trust for funding.References
- G. Marbán and T. Valdés-Solís, Int. J. Hydrogen Energy, 2007, 32, 1625–1637 CrossRef CAS.
- B. D. Solomon and A. Banerjee, Energy Policy, 2006, 34, 1318–1318 CrossRef.
- European Hydrogen & Fuel Cell Technology Platform, http://https%3A//www.hfpeurope.org/hfp/keydocs.
- US Department of Energy: Hydrogen Posture Plan, http://www.hydrogen.energy.gov/.
- The Hydrogen Economy: Opportunities, Costs, Barriers, and R&D Needs, National Research Council and National Academy of Engineering Report, http://www.nap.edu/catalog/10922.html.
- D. A. King, Science, 2004, 303, 176–177 CrossRef CAS.
- M. S. Dresselhaus, G. W. Crabtree and M. V. Buchanan, MRS Bull., 2005, 30, 518–524 CAS.
- S. Dunn, Int. J. Hydrogen Energy, 2002, 27, 235–264 CrossRef CAS.
- G. W. Crabtree, M. S. Dresselhaus and M. V. Buchanan, Phys. Today, 2004, 57, 39–44 CrossRef CAS.
- F. Armstrong and K. Blundell, Energy – Beyond Oil, Oxford University Press, Oxford, 2007 Search PubMed.
- R. Masel, Nature, 2006, 442, 521–522 CrossRef CAS.
- P. P. Edwards, V. L. Kuznetsov and W. I. F. David, Philos. Trans. R. Soc. London, Ser. A, 2007, 365, 1043–1056 CrossRef CAS.
- S. S. Penner, Energy, 2006, 31, 33–43 CrossRef CAS.
- P. P. Edwards, V. L. Kuznetsov and W. I. F. David, in Energy – Beyond Oil, ed. F. Armstrong and K. Blundell, Oxford University Press, Oxford, 2007 Search PubMed.
- T. I. Sigfusson, Philos. Trans. R. Soc. London, Ser. A, 2007, 365, 1025–1042 CrossRef CAS.
- J. A. Turner, Science, 2004, 305, 972–974 CrossRef CAS.
- J. R. Bolton, Solar Power and Fuels, Academic Press, New York, USA, 1977 Search PubMed.
- M. Kitano, M. Takeuchi, M. Matsuoka, J. M. Thomas and M. Anpo, Catal. Today, 2007, 120.
- M. Ni, M. K. H. Leung, D. Y. C. Leung and K. Sumathy, Renewable Sustainable Energy Rev., 2007, 11, 401–425 CrossRef CAS.
- D. S. Shephard, T. Maschmeyer, B. F. G. Johnson, J. M. Thomas, G. Sankar, D. Ozkaya, W. Zhou, R. D. Oldroyd and R. G. Bell, Angew. Chem., Int. Ed. Engl., 1997, 36, 2242–2245 CrossRef CAS.
- T. Maeda, V. Sanchez-Torres and T. Wood, Appl. Microbiol. Biotechnol., 2008, 79, 77–86 CrossRef CAS.
- J. Barber, in Energy – Beyond Oil, ed. F. Armstrong and K. Blundell, Oxford University Press, Oxford, 2007 Search PubMed.
- R. Spreitzer and M. Salvucci, Annu. Rev. Plant Biol., 2002, 53, 449–475 Search PubMed.
- M. Parry, P. Andralojc, R. Mitchell, P. Madgwick and A. Keys, J. Exp. Bot., 2003, 54, 1321–1333 CrossRef CAS.
- P. P. Edwards, V. L. Kuznetsov, M. Gersch, S. Ellis and Z. X. Guo, Hydrogen, Materials UK Energy Review 2007, Alternative Energy Technologies, mailto:http://www.matuk.co.uk/docs/4_Alternative%20Energy%20FINAL.pdf. Search PubMed.
- I. Schulte, D. Hart and R. van der Vorst, Int. J. Hydrogen Energy, 2004, 29, 677–685 CrossRef CAS.
- A. G. Dutton, Wind Eng., 2003, 27, 239–256 Search PubMed.
- W. Grochala and P. P. Edwards, Chem. Rev., 2004, 104, 1283–1315 CrossRef CAS.
- S. Young, Nature, 2001, 414, 487–488 CrossRef.
- M. Winter and R. J. Brodd, Chem. Rev., 2004, 104, 4245–4269 CrossRef CAS.
- R. von Helmolt and U. Eberle, J. Power Sources, 2007, 165, 833–843 CrossRef CAS.
- R. Pagnamenta, in The Times, London, 24 May, 2008 Search PubMed.
- E4tech, Element Energy, Eoin Lees Energy, A strategic framework for hydrogen energy in the UK, mailto:http://www.berr.gov.uk/files/file26737.pdf.
- European Parliament gives go-ahead to Fuel Cell and Hydrogen JTI, mailto:https://www.hfpeurope.org/hfp/ec_activities.
| This journal is © The Royal Society of Chemistry 2008 |
