Sequestration of atmospheric CO2 in global carbon pools
R. Lal
Carbon Management and Sequestration Center, The Ohio State University, Columbus, Ohio 43210, USA
First published on 3rd July 2008
Abstract
Sequestering atmospheric CO2 is necessitated by its present concentration of 385 ppm and increasing at the rate of 2 ppm y−1. Increase in atmospheric emission of CO2 with the attendant global warming and environmental degradation are driven by global energy demand. In comparison with the emission of 300 Pg C between 1850 and 2000, total emission during the 21st century is estimated at 950 to 2195 Pg, with an annual rate of emission of 20 to 35 Pg C y−1. Reduction of CO2 atmospheric loading can be achieved by biological, chemical and technological options through either reducing or sequestering emissions. This article describes technological options of sequestering atmospheric CO2 into other global pools. Geologic sequestration involves underground storage of industrially emitted CO2 into the geosphere for long-term and secure storage. Liquefied CO2 is injected about 1000 m below the ground surface either in stable porous rocks, oil wells, coal beds, or saline aquifers. Co-injecting CO2 along with H2S and SO2 is also possible. Over time, the trapped CO2 reacts with minerals to form carbonates, enhances oil recovery, or displaces coal bed methane. Deep injection of CO2 under the ocean creates a CO2 lake which eventually penetrates into the sediments. Iron fertilization is another technique of enhancing the C pool in marine biota, notably phytoplankton. The so-called “biological pumping” is based on the “iron hypothesis” of transferring C to the ocean floor. Terrestrial C sequestration is based on the natural process of photosynthesis. Transfer of CO2 into the biotic pool and soil C pool via humification and formation of secondary carbonates has numerous ancillary benefits through enhancement of ecosystem services. Soil C sequestration is essential to improving soil quality, increasing use efficiency of agronomic input, and advancing world food security. It is also needed to improve water quality by filtration and denaturing of pollutants, and enhancing biodiversity by saving land for nature conservancy. Soil C sequestration is a low hanging fruit, and a bridge to the future until low-C or no-C fuel sources take effect.
 R. Lal R. Lal | Rattan Lal studies soil C dynamics in relation to climate change, food security, and environment. He researches soil structure, and the fate of carbon transported by erosion. Data from field experiments is synthesized to relate soil C pools to agronomic productivity, and use efficiency of input. Rates of soil C sequestration, its residence time and processes are assessed. |
I. Introduction
Three important and inter-related global issues of the 21st century are: (i) atmospheric concentration of CO2 at 385 ppm in 2008 (+37.5% compared with the pre-industrial level of 280 ppm) and increasing at the rate of 2 ppm y−1 (0.52% y−1) with the attendant impact on the current and projected global warming, (ii) world annual energy use of 500 EJ (Exajoule = 1018 Joules, 1 Quad = 1015 BTU = 1.05 EJ), increasing at the rate of 2.2% y−1 and projected to be 537 EJ by 2010, 590 EJ by 2015, 637 EJ by 2020, 687 EJ by 2025 and 737 EJ by 2030,1 and (iii) food—insecure population of about 1 billion and increasing because of an increase in price of energy and the related input (e.g., fertilizer, irrigation), decline in per capita arable land area (caused by conversion to urban/industrial uses and increasing susceptibility to soil degradation), and reduction in per capita availability of renewable fresh water resources for agricultural use. Increasing energy demand is a major cause of CO2 emission. Fossil fuel combustion for energy production emits between 0.14 to 0.28 Mg C Mwh−1 of energy.2 Thus, fossil fuel combustion and other anthropogenic activities have strongly impacted the atmospheric enrichment of several greenhouse gases (GHGs) with the attendant impact on climate change. Relative emission of GHGs comprises 79.9% energy-related CO2, 9.5% CH4, 5.8% N2O, 3.0% non-energy CO2 and 1.8% other gases (http://www.climatetechnology.gov). With regards to the energy budget of the Earth, however, radiative forcing or the global warming potential of different GHGs must also be considered. Therefore, mitigating the increase in atmospheric abundance of CO2 necessitates identification of options which: (i) reduce emissions by using low-carbon or no-carbon fuel sources, (ii) enhance energy use efficiency by minimizing losses, and (iii) sequester atmospheric CO2 into other reservoirs with secure storage and long residence time. With increasing reliance on fossil fuel as the dominant energy source for most of the 21st century, disposal of CO2 by engineering, chemical and biological techniques is important to reducing the atmospheric loading and minimizing the risks of climate change. Total emission of C, with business as usual during the 21st century, is estimated at 950 to 2195 Pg compared to only 300 Pg emitted between 1850 and 2000.3 The rate of annual emission by 2100 is estimated at 20 to 35 Pg C y−1 compared with the 1990 baseline emission rate of only 5.5 Pg y−1.3 It is thus imperative that the anthropogenic emissions of CO2 be sequestered in other global pools for long-term and secure storage. This article collates and synthesizes research information, and describes technological options of C sequestration to off-set anthropogenic emissions due to fossil fuel combustion, land use change and other activities to stabilize the atmospheric concentration of CO2 at a desired level (e.g., 550 ppm).II. The global carbon cycle
The five global C pools are interconnected (Fig. 1), and the flux among these pools is strongly influenced by anthropogenic perturbations. The gross primary production ranges from 90 to 130 Pg C y−1 (mean of 120 Pg C y−1), which is balanced by plant respiration of 40 to 60 Pg y−1 and decomposition of soil organic matter (SOM) of 40 to 68 Pg C y−1.4 The anthropogenic emissions involve two principal components: fossil fuel combustion of >7.5 Pg C y−1 during 2000 s and land use conversion (deforestation) and soil cultivation of about 1.6 Pg C y−1.5 Total anthropogenic emissions of about 9.1 Pg C y−1 are balanced as follows: (i) retention in the atmosphere by 4.1 Pg C y−1 (45%), uptake by ocean by 2.5 Pg C y−1 (27.5%), and absorption by an unidentified terrestrial sink by 2.5 Pg C y−1 (27.5%). It is argued that the capacity of the land-based sink may be decreasing.6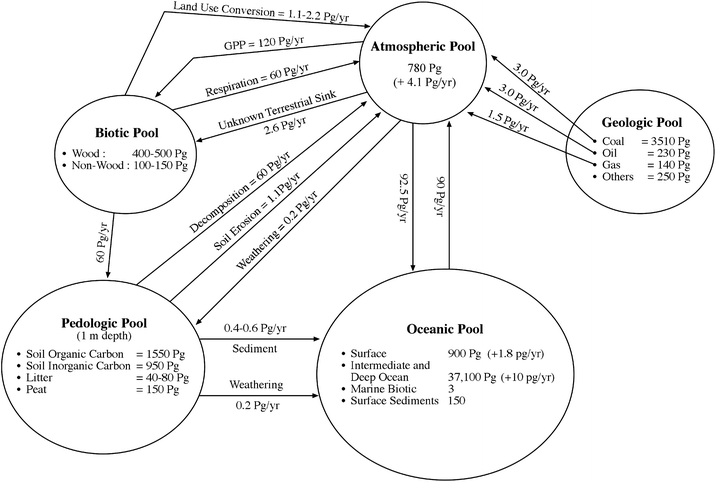 | ||
| Fig. 1 Estimates of the global pools and fluxes between them.1,4,5,7,152 | ||
For assessing whether the terrestrial biosphere is a net C sink, it is relevant to know its C budget at different spatial scales and its sink capacity. Ruddiman8,9 observed that the terrestrial C pool has been a source of the atmospheric CO2 ever since the dawn of settled agriculture about 8000 to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 years ago and of CH4 since cultivation of rice paddies and domestication of livestock about 5000 years ago. The amount of CO2 emitted from the terrestrial biosphere into the atmosphere is estimated at 320 Pg from pre-historic times up to 1850,8 and 136 ± 55 Pg between 1850 and 1998.10 Until the 1940s, more CO2 was emitted by land use change than by fossil fuel combustion, and presently about 18% of the total annual emission (1.6 Pg out of 9.1 Pg) in 2008 comes from deforestation, biomass burning and soil cultivation. In comparison, CO2 emissions by fossil fuel combustion between 1850 and 1998 is estimated at 270 ± 30 Pg C. Such estimates of the historic C loss are important because these statisitics provide a reference point with regards to the potential sink capacity of the terrestrial biosphere. Assuming that the estimates by Ruddiman are approximately correct, total C emission from the terrestrial biosphere is about 456 Pg. With 4 Pg of C being equal to 1 ppm of atmospheric CO2,11 the potential sink capacity of the terrestrial biosphere is about 114 ppm. Further assuming that 40 to 50% of the historic C loss can be resequestered over the next 40 to 50 years (by 2050 to 2060), the strategy of restoring soils and vegetation in world's ecosystems can transfer atmospheric CO2 by 45 to 55 ppm (average of 50 ppm) into the terrestrial biosphere. Therefore, the strategy of C sequestration in soil and biota is an important option that requires a critical and an objective evaluation vis-à-vis other technological options of stabilizing the atmospheric CO2 concentration.
000 years ago and of CH4 since cultivation of rice paddies and domestication of livestock about 5000 years ago. The amount of CO2 emitted from the terrestrial biosphere into the atmosphere is estimated at 320 Pg from pre-historic times up to 1850,8 and 136 ± 55 Pg between 1850 and 1998.10 Until the 1940s, more CO2 was emitted by land use change than by fossil fuel combustion, and presently about 18% of the total annual emission (1.6 Pg out of 9.1 Pg) in 2008 comes from deforestation, biomass burning and soil cultivation. In comparison, CO2 emissions by fossil fuel combustion between 1850 and 1998 is estimated at 270 ± 30 Pg C. Such estimates of the historic C loss are important because these statisitics provide a reference point with regards to the potential sink capacity of the terrestrial biosphere. Assuming that the estimates by Ruddiman are approximately correct, total C emission from the terrestrial biosphere is about 456 Pg. With 4 Pg of C being equal to 1 ppm of atmospheric CO2,11 the potential sink capacity of the terrestrial biosphere is about 114 ppm. Further assuming that 40 to 50% of the historic C loss can be resequestered over the next 40 to 50 years (by 2050 to 2060), the strategy of restoring soils and vegetation in world's ecosystems can transfer atmospheric CO2 by 45 to 55 ppm (average of 50 ppm) into the terrestrial biosphere. Therefore, the strategy of C sequestration in soil and biota is an important option that requires a critical and an objective evaluation vis-à-vis other technological options of stabilizing the atmospheric CO2 concentration.
III. Strategies for managing atmospheric CO2 enrichment
The CO2 loading of atmosphere can be reduced by biological, chemical, and technological options using two strategies: adaptive options and mitigative options (Fig. 2). Adaptive options are based on adjustments in land use systems within the terrestrial and aquatic biosphere (e.g., forests, deserts, agriculture, wetlands, coastal ecosystems). Mitigation options involve two approaches: reducing emissions and sequestering emissions. Reducing emission involves using techniques to enhance energy use efficiency, making life style changes, and substituting fossil fuels by low-C or no-C fuel. Sequestering emission involves transfer of atmospheric CO2 into other pools where it is securely stored and has a minimal chance of leakage back into the atmosphere. There are several options of C sequestration, including geologic, oceanic, chemical, and terrestrial (Fig. 2).12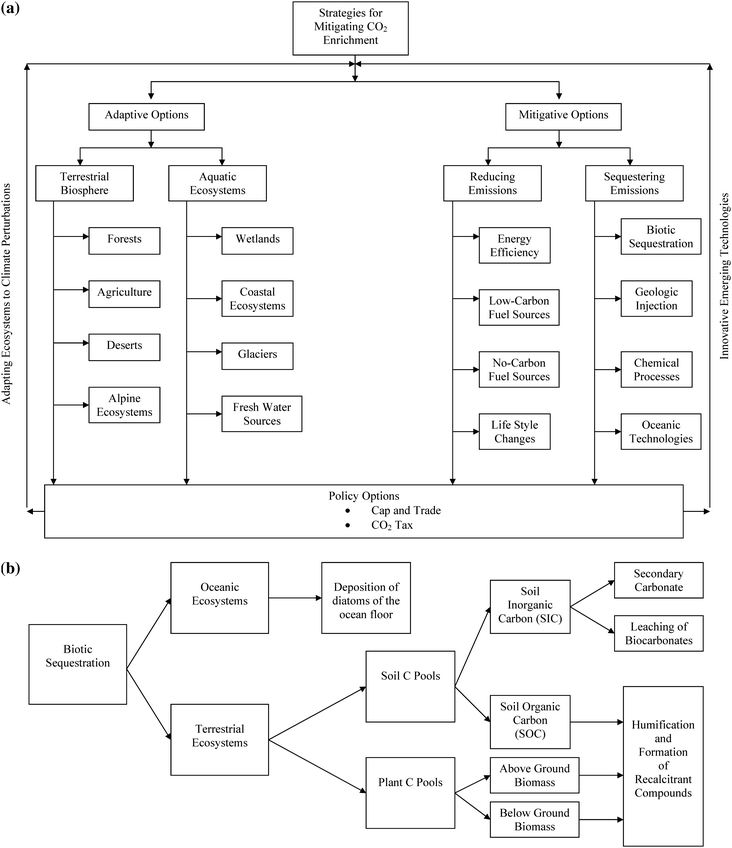 | ||
| Fig. 2 (a) Technological options for adapting to and mitigating atmospheric abundance of CO2. (b) Processes of carbon sequestration within the biosphere. | ||
a. Geologic sequestration
Underground storage of industrially emitted GHGs or geologic sequestration involves transfer of atmospheric CO2 into geologic strata for long-term and secure storage. It comprises capture, purification, liquefaction, transport and injection of CO2 from a point source into deep (∼1000 m) geologic strata or the geosphere. Industrially emitted CO2 can be injected into several geologic formations including depleted or active oil reservoirs, unmineable coal seams, and saline aquifers.13,14 Furthermore, co-injecting CO2 with H2S or SO2 (products of coal gasification and combustion) is also an option to mitigate disposal of these gases.15 Over time, the trapped CO2 reacts with minerals and organic substances to form carbonates of Ca, Mg, and Fe etc.2,16–19 The process has been used since the 1970s to primarily enhance oil recovery (EOR) from old wells.20 Similar to EOR, injection of CO2 in coal seams displaces coal bed methane (CBM). Because CO2 is strongly absorbed onto the coal,21 unmineable coal seams are a possible option for geologic storage of CO2.22–24 Liquefied CO2 can also be injected into saline aquifers25,26 where it slowly reacts with minerals to form carbonates.Despite its potential, the geologic sequestration techniques are a work in progress.12 There remains much to be learned, especially with regards to the rate at which specific geologic formations can accept CO2 and the impact of injection into the neighboring wells. The cost of drilling and constructing a safe and secure pipeline is another major consideration. Measurement, monitoring and verification (MMV) for any leakage is an important issue that must be addressed. Because of the potentially adverse effects of CO2 leakage (such as from Lake Nyos in Cameroon in 1986)27 leakage verification is an important consideration for any undertaking of geologic sequestration.28 There is also a strong need to assess degradation of well cement by CO2 under geologic sequestration conditions.29 Monitoring seismic activity is also important to secure storage of injected CO2.30 Assessment of injected CO2 with rock interaction is also important.17 An integrated decision support system is needed for management of CO2 in geologic storage.31 Therefore, managing the risks of geologic storage is a crucial issue,32 which is subject to regulations by EPA.33 Despite these limitations, geologic sequestration is an attractive option to energy industry and policy makers because of its potentially large storage capacity.12
b. Oceanic sequestration
Similar to the terrestrial C cycle, the marine C cycle also plays a major role in controlling the atmospheric abundance of CO2. Being the largest pool, ocean is the ultimate reservoir for global C. Oceanic uptake presently accounts for about one-third or 2.5 Pg out of 7.5 Pg of emission from fossil fuel combustion in 2008 (Fig. 1).34 The temperature of the ocean and partial pressure of CO2 in the atmosphere are important factors. With temperature lag between the ocean and the land and rapid increase in atmospheric abundance of CO2, the oceanic uptake is expected to increase over time.35 Therefore, oceanic fertilization is one of the strategies for sequestration of atmospheric CO2.36-38 There are two options for CO2 sequestration in ocean: (i) deep injection of liquefied CO2, and enhancement of uptake by the marine phytoplankton through micronutrient fertilization. Injection of CO2 under the ocean is one of a wide range of engineering techniques of disposing industrial point-source emission. The density of liquid CO2 is higher than that of CO2 for depths below 3000 m.39,40 The liquid CO2 injected creates a pool which eventually seeps into the sediments through gravity-driven displacement of seawater. Several chemical and hydrodynamic processes can occur at the CO2 (liquid)/saline water interphase including: (i) counter-current diffusion of the two phases, (ii) convective or mass flow driven by ocean currents, (iii) chemical reaction leading to formation of carbonates and bicarbonates, and (iv) salting out.40 Porosity and permeability of the sediments are important factors determining the deep penetration of liquid CO2 into the sediments. It is argued that deep oceanic injection could absorb a large proportion of anthropogenic emissions for many centuries.41,42The process of oceanic uptake by marine phytoplankton is termed “biological pumping” of C from the upper layers into the deep sea. Reduction in concentration in the upper layers through biotic uptake increases flux from the atmosphere into the ocean. The magnitude of the “biological pumping” is presumably limited by the lack of some micronutrients. The so called “iron hypothesis” is based on the assumption that lower atmospheric CO2 during glacial times was due to iron fertilization of the surface waters by enhanced dust deposition.43,44 In this regard, the Southern Ocean is recognized as the oceanic body most sensitive to climate change.45 Application of iron has shown a strong increase in biological production of marine phytoplankton46-56,151 Though C is not removed permanently, it is sequestered for up to a thousand years. Phytoplankton bloom, which plays a crucial role in pelagic food webs and “biological pumping”, is also influenced by species composition57 and availability of macronutrients such as phosphates (HPO4) and nitrates (NO3). Phytoplankton growth is limited by micronutrient (Fe) deficiency on ∼25% of the oceanic surface and by macronutrient (NO3, HPO4) on ∼75% of the oceanic surface.58 Fertilizing the ocean with Fe may increase primary productivity in high nitrate low chlorophyll (HNLC) regions such as the Southern Ocean.51 Besides, some phytoplanktons also produce dimethyl sulfide which acts as a cloud condensing nucleus. Increase in cloud cover increases albedo and lowers temperature.59 The seemingly vast potential of ocean fertilization on enhancing the “biological pumping” is constrained by several adverse environmental impacts12,60 and uncertainties,61 which must be objectively addressed.
c. Chemical weathering and CO2 sequestration
Chemical weathering of rocks and minerals at the continental scale is a geological process that moderates atmospheric concentration of CO2 on a geological time scale.62,63 A fraction of biomass-derived CO2 in the soil is diverted to the soil inorganic carbon (SIC) pool through weathering of Ca/Mg-bearing silicates, and of carbonates (e.g., limestone). The weathering of silicate minerals moderates atmospheric CO2 concentration on the millennial time scale, and that of carbonates on the centennial scale.64–66 Furthermore, rates of carbonate mineral weathering are orders of magnitude faster than those of silicate weathering.67| (Ca, Mg) SiO3 + CO2 ↓ → (Ca, Mg) CO3 + SiO2 | (1) |
The downward arrow indicates uptake or sequestration of atmospheric CO2. Predominant minerals involved in this reaction are plagioclase, olivine, pyroxene, and volcanic glass. These minerals are weathered into alumino-silicates or clay minerals (e.g., kaolinite, halloysite, imogolite, allophane). A specific reaction is as follows:70
| CaAl2Si2O8 + 2CO2 ↓ + 3H2O → Al2Si2O5 (OH)4 + Ca2+ + 2HCO3− → CaCO3 + CO2 + H2O | (2) |
Transport of Mg2+, Ca2+ and HCO3− from the weathering site leads to net removal of CO2 from the atmosphere.
In contrast with weathering of Ca/Mg silicates, weathering of Na–K silicates (feldspars, white mica) into clays (e.g., kaolinite) does not cause net removal of atmospheric CO2.70
| CaCO3 + CO2 ↓ + H2O → Ca++ + 2HCO3 → CaCO3 + CO2 ↑ + H2O | (3) |
The upward arrow indicates emission of CO2 back into the atmosphere. Thus, weathering of limestone is a CO2 neutral process.70
| CO2↓ + H2O → 2H+ + CO32− | (4) |
| CaSiO3 + 2H+ → Ca2+ + SiO2 + H2O | (5) |
| Ca2 + + CO32− → CaCO3 ↓ | (6) |
These reactions are accentuated either through energy-consuming pre-treatment or high Ca2+ concentration.
IV. Utilization and recycling of CO2
Rather than treating it as a waste (garbage) to be disposed of in underground or undersea reservoirs, industrially emitted CO2 is an important resource with numerous applications. Indeed, with increasing world population and dwindling supplies, industrially emitted CO2 must be recycled and used as a raw material for a range of chemical and biological products including enhancing agronomic/food production. For production of chemicals, CO2 can be used as a source of C, co-reagent or a solvent in a wide range of industrial processes, ref. 2,75,76 outline several chemical reaction for using CO2 as a source of carbon in a variety of synthetic processes. Using CO2 to produce biomass and create bioeconomy, based on enhanced production in both terrestrial and marine ecosystems, has a vast economic potential awaiting to be realized (Fig. 3). In addition to the CO2 fertilization effect and improving agronomic and forestry production, biomass produced through growth of macro- and micro-algae and cyanobacteria using bioreactors has major applications in biofuels, biochemicals, and biochar (Fig. 4). One kg of biofuel is obtained from 5–10 kg of biomass.77 An important variant of this process is C sequestration in terrestrial ecosystems both as forest biomass and SOM in agroecosystems. The latter, with significant implications to soil quality, water quality and global food security, is described in the following sections.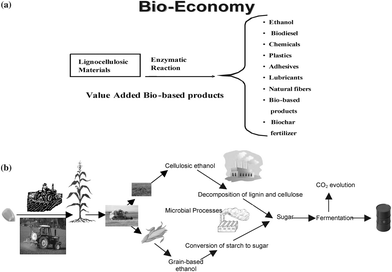 | ||
| Fig. 3 The bioeconomy based on biomass. | ||
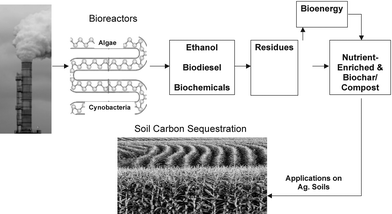 | ||
| Fig. 4 Using industrial carbon dioxide in bioreactors to produce biofuel, biochar for use as soil amendment, and other bioproducts. | ||
Chemical and engineering barriers to lignocellulosic biofuels are being broken through scientific advances.78 To be economic, production of biofuels must be linked with that of biomaterials. The strategy is to fully integrate the agro–biofuel–biomaterial–biopower cycle.79 Such an integration requires a well planned road map for the biorefineries, chemical processing industries, and farming systems of the 21st century.
V. Soil organic matter in agroecosystems
The importance of organic matter (OM) or more appropriately the SOM to soil quality, its capacity to produce economic goods and perform ecosystem services, has been recognized for millennia. The humus, highly reactive and decomposed component of SOM, impacts soil's physical, chemical and biological properties, and imparts the characteristic dark brown color which is synonymous with the soil of a good quality. Allison80 stated that “soil organic matter has, since the dawn of history, been the key to soil fertility. For 8000 years or more, man has appreciated the fact that dark soils are usually productive soils”. Homer (Greek Poet 900–700 BC) emphasized regular manuring of vineyards, probably because its application enhanced SOM concentration and improved productivity. Xenophon (434–335 BC) stated that “the estate has gone to ruins because someone didn't know it was well to manure the land”.81 Similar to the European farming, the significance of manuring to enhancing soil quality has long been a cultural tradition in Asia. King82,83 stated that “China, Japan, Korea and other countries of eastern Asia have a long tradition of recycling OM to enhance soil fertility”. It was partly because of manuring that the settled agriculture in these densely populated countries has been practiced for 3 to 5 millennia. Similar to East Asia, manuring also has been used since antiquity in the Mediterranean region,84 in the Middle East since prior to the biblical era,85,86 and since ∼1500 BC in South Asia (SA) region by the Harappan civilization in the Indus Valley. Two books written about 300 BC “Arthashastra” by Kautilya and “Krishi” vividly describe the benefits of using animal manure to improving soil fertility.87The use of manuring continued at the onset of the Christian era. Feller88 described the historical developments with regard to the importance of SOM. He observed that the Roman philosopher Virgil89 (1 AD) implied “humus” as soil, and used the words “humus”, “solum” and “terra” interchangeably. Ibn-Al-Awan, a 12th century Moorish philosopher, wrote in the book “Kitab-Al-Filaha” (Book of Agriculture) that “the first step in the science of agriculture is the recognition of soils and of how to distinguish that which is of good quality and that which is of inferior quality. He who does not possess this knowledge lacks the first principles and deserves to be regarded as ignorant”. He further stated that “one must also take into consideration the depth of the soil, for it often happens that its surface layer may be black”.90 The emphasis on the black color of the surface layer signifies the importance that he gave to the beneficial effect of humus on soil quality, because in general soils of darker color have high SOC content and are of better physical, chemical and biological quality.
The recognition of the importance of humus to soil quality increased with the development of modern farming in the early 19th century. In the book “Principles of Rational Agriculture” (published in German in 1809 and translated in French and English in 1811), Thaër91 defined that humus “is the residue of animal and vegetable putrefaction. It is a black substance”.88 Muller92 described different types of horizons containing humus as mull (mould) and torf (peat).
The knowledge of soil science, as a distinct discipline, increased progressively during the first half and drastically during the second half of the 20th century. Waksman93 stated that “The importance of humus in human economy seldom receives sufficient emphasis. Suffice to say that it probably represents the most important source of human wealth on this planet. Nature has stored in and upon the earth, in the form of humus, the source of a vast amount of readily available energy, a large part of carbon needed for life processes, and most of the combined nitrogen, so much needed for plant growth”. In the USDA's Year Book of Agriculture “Soil and Men”, Albrecht94 stated the importance of SOM to soil's quality. He wrote that “the high productivity of most virgin soils has always been associated with the high content of SOM, and the decrease in supply with cultivation has generally been paralleled by a corresponding decrease in productivity.” Albrecht also narrated that “Soil organic matter is one of our most important national resources; its unwise exploitation has been devastating, and it must be given its proper rank in any conservation policy as one of the major factors affecting the levels of crop production in the future.” Sir Albert Howard95 stated that “In a fertile soil, the soil and plant come into gear in two ways simultaneously. In establishing and maintaining these contacts, humus is essential. It is, therefore, a key material in the life cycle. Without this substance, the wheel of life cannot function effectively.” Sir Albert Howard96 further stated that “humus is the most significant of all Nature's reserves.”
The importance of SOM as a moderator of the global climate began in the later part of the 20th century. Jenny97 stated that contributions of SOM to atmospheric CO2 appear underestimated. Since then, the literature is replete with the data on the importance of enhancing SOM and sequestering C in soil and terrestrial ecosystems to mitigate the climate change by off-setting fossil fuel emissions.98–102 It is also hypothesized that emission of CO2 from soil into the atmosphere began with the onset of settled agriculture, and that of CH4 with cultivation of rice paddies and domestication of animals.8,9 With 1 Pg of soil C being equivalent to 0.47 ppm of atmospheric CO2, emissions from world soils could have contributed 37 ppm of the total increase of 105 ppm or 35% of the cumulative gain in the atmospheric abundance from 280 ppm in ∼1750, to 385 ppm in 2008. For each 4 Pg of fossil C burned, the atmospheric abundance of CO2 increases by 1 ppm.11
The biotic sequestration of CO2 through photosynthesis is a natural process. It involves natural and managed interventions for retaining a small fraction of approximately 120 Pg of CO2-C annually photosynthesized by terrestrial and aquatic biospheres. Being a natural process, it is cost effective, has numerous ancillary benefits, and little or none environmental/health risks (Table 1). Despite its positive impact on numerous ecosystem services, there are several uncertainties in the SOM pool with regards to the projected climate change (Table 2). Understanding basic processes governing biogeochemical cycling of C is essential to minimize these uncertainties.
| Direct benefits | Ancillary benefits and ecosystem services |
|---|---|
| 1. Improves soil structure and tilth | 1. Sequesters atmospheric CO2 |
| 2. Reduces soil erosion | 2. Enhances soil's ability to oxidize CH4 |
| 3. Decreases non-point source pollution | 3. Restores degraded ecosystems |
| 4. Purifies water | 4. Increases soil/terrestrial biodiversity |
| 5. Denatures pollutants | 5. Enhances use efficiency of input (water use efficiency, nutrient use efficiency) |
| 6. Increases plant available water | 6. Improve wildlife habitat |
| 7. Stores plant nutrients | 7. Decreases nutrient and water loss from the ecosystem |
| 8. Improves crop/biomass yield | 8. Enhances ecosystem resilience |
| 9. Provides food/energy for soil biota | 9. Strengthens recycling mechanisms |
| 10. Buffers impact of perturbation on soil properties | 10. Improves the environment |
| Will climate change: | Will soil processes: |
|---|---|
| (1) Amplify SOM depletion | (1) Have mitigative impact |
| (2) Exacerbate soil erosion | (2) Adversely impact agronomic yield |
| (3) Alter global C cycle more drastically | (3) Increase the land-based C sink |
| (4) Affect NPP through CO2 fertilization effect | (4) Decrease SOC pool through C-input in soil at high temperatures |
VI. Carbon sequestration in terrestrial ecosystems
The strategy of C sequestration in terrestrial ecosystems is based on the natural photosynthetic process of transferring atmospheric CO2 into the biomass (eqn (7)),70| 106CO2 + 16NO3 + HPO42− + 122H2O + 18H = C106H263O11N16P + 138O2 | (7) |
Most of the 120 Pg of C photosynthesized annually is balanced either by direct plant respiration or by microbial decomposition. However, even if 6 to 7% of 120 Pg photosynthesized annually can be retained in the biosphere, it can effectively off-set anthropogenic emissions due to fossil fuel combustion and cement manufacturing estimated at 7.5 Pg y−1,5 and 1.6 Pg y−1 by deforestation and land use conversion6 (Fig. 1). Choosing a judicious land use and adopting recommended soil/plant management can enhance retention of some photosynthetic C in the terrestrial/marine biosphere (Fig. 5). The processes can be enhanced through managing coupled cycles of C, N and H2O which would positively influence soil quality, environment quality and the biomass productivity. Managing terrestrial C sequestration would also advance food security because of increase in use efficiency of agricultural input. There is a wide range of agricultural production systems for enhancing energy use efficiency. Important among these are (Fig. 6): (i) no-till or conservation tillage systems with mulching and cover cropping for seedbed preparation and weed control, (ii) integrated nutrient management (INM) along with biochar and slow release formations for soil fertility management, (iii) water harvesting and recycling with drip/furrow irrigation, and (iv) use of improved germplasms tolerant to biotic and abiotic stresses (Fig. 6). Some studies have shown that Bt (Bacillus thuringiensis) plant residues have a high lignin content103–105 and thus more resistant to decomposition than non-Bt residues.106,107
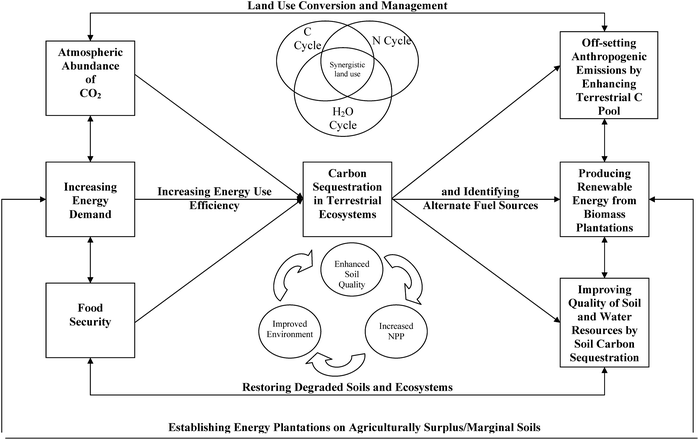 | ||
| Fig. 5 Addressing global issues of atmospheric enrichment of CO2, increasing energy demand and exacerbating food insecurity through carbon sequestration in terrestrial ecosystems. | ||
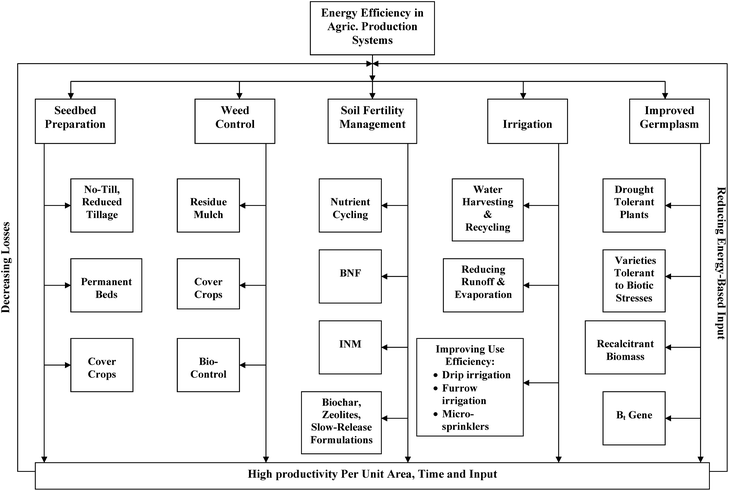 | ||
| Fig. 6 Increasing energy use efficiency in the agricultural production system. | ||
Strategies for C sequestration in terrestrial ecosystems are outlined in Fig. 7. The goal is to: (i) enhance production per unit area, time and input on existing farmland through agricultural intensification, (ii) control soil erosion and restore degraded soils, (iii) undertake afforestation and reforestation of degraded and marginal soils, (iv) establish biofuel plantations, and (iv) use biochars as a soil amendment (Fig. 7). The objective is to enhance ecosystem services in terms of water quality, nutrient cycling, biodiversity, increase in NPP, and off-setting emissions.
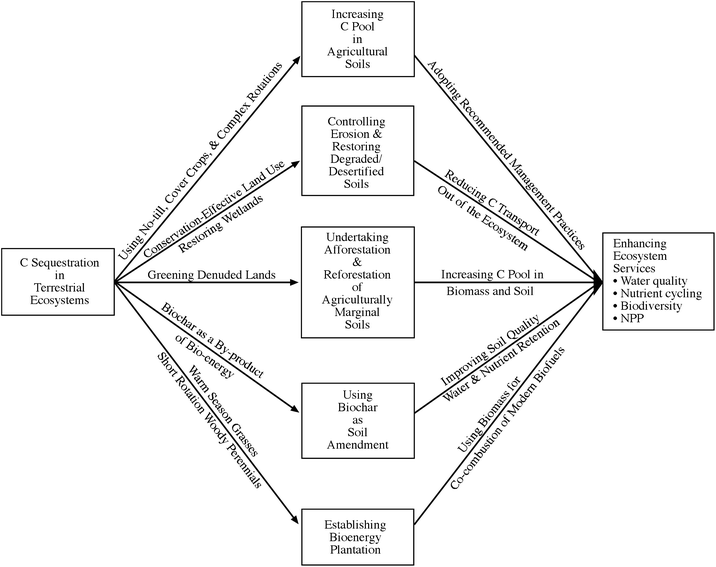 | ||
| Fig. 7 Strategies for carbon sequestration in terrestrial ecosystems. | ||
Soil and crop management practices which enhance the input of biomass-C into the soil (e.g., no-till farming, and using fertilizer, manure, irrigation, mulch) normally increase the ecosystems C pool:10,101 although net gains in C pool depend also on the hidden C costs of the techniques used.108 Some of the management practices which require special mention are briefly describe below:
(a) Agricultural liming
Use of lime on acid soils may affect weathering of silicates and export of bicarbonates. Oh and Raymond109 observed that net atmospheric C sequestration by liming through transport of bicarbonates in streams in the Ohio River Basin was 9.5 kg C ha−1 y−1. However, the effect of liming on biomass production, SOC dynamics or the emission of CO2 reaction in acidic soil was not assessed. Further, the net effect of liming on soil C pool depends on soil-specific factors. In Scotland, Rangel-Castro et al.110,111 observed that more soil C was lost than gained through photosynthesis because of pasture liming from 1990 to 1998. These researchers observed that turnover of the recently assimilated 13C compounds was faster in microbial biomass from limed than that from unlimed soil, suggesting that liming increases incorporation by microbial communities of root exudates.(b) Nitrogen management
The nitrogen cycle strongly interacts with the C cycle, and N is needed in creation of biomass C into stable soil humus. Further, N availability may also increase the photosynthetic reaction (eqn (7)) in N-limited soils both in agricultural and forest ecosystems. Indeed, the CO2 fertilization effect may also be limited by the lack of adequate N.112,113 The impact of N fertilizer on biomass production is likely to increase in developing countries such as Africa, where the rate of fertilizer use is at present only 5 kg ha−1 y−1 compared with ∼200 kg ha−1 y−1 in developed countries. In addition to N, soils of the tropics are also deficient in P and cations (Ca2+, Mg+). Unless, other nutrients are available, increase in application of N may accentuate losses by volatization (N2O) and leaching (NO3), and depletion of the SOC pool.114,115 The Haber-Bosch process of producing reactive N as a mineral fertilizer has saved billions from starvation,116 and lack of its application in Africa and elsewhere is the cause of perpetual food insecurity, hunger and malnutrition.While judicious use of reactive N as a fertilizer in agroecosystems is essential, its impact on SOC dynamics remains to be a controversial topic,117 and needs to be critically and objectively assessed. This debate must be resolved on the basis of systematic, well designed and long-term field experiments, because the effect of N may depend on other management parameters. For example, Russell et al.118 assessed the impact of N fertilization rate and cropping systems on C pool in soils of Iowa. Grain and biomass yields increased with increase in N input. The SOC concentrations were significantly higher only in systems containing alfalfa in the rotation cycle. In China, Zhu et al.119 reported that it was the application of farm yard manure for 25 years rather than N fertilizer that increased SOC concentration. In Canada, Malhi et al.120 observed that total SOC was generally more with than without straw retention for all levels of N application. A long-term study in Illinois, USA, showed that the SOC pool increased with an increase in the rate of N application.121 Sainju et al.122 reported that the SOC concentration in 0–30 cm depth increased when cover crops (hairy vetch, Vicia villosa Roth; rye; Secale cereale L) were included in the rotation and N fertilizer was applied at the rate of 120 to 130 kg N ha−1 y−1. In Brazil, Diekow et al.123 observed that the average SOC sequestration rate of legume-based cropping systems was 1.42 Mg C ha−1 y−1 with N application. These studies show that effectiveness of N application for SOC sequestration depends on residue retention as mulch, inclusion of cover crops in the rotation cycle and adoption of complex crop rotations, and use of INM practices such as application of compost, farm yard manure and biochar.
(c) Biochar
Another mechanism of enhancing the SOC pool is through application of black C or biochar extracted from controlled combustion of biomass. The biochar C applied to soil has a long residence time. Using biochar is mimicry of the natural process in which some biomass from forest and grassland fires is not fully burnt but is “carbonized” and added to the soil as a relatively recalcitrant charcoal or biochar.124 Using this principle, there are some man-made soils with high concentration of C. These soils in the Amazon are called “Terra Preta do Indio” (Indian Black Earth).125,126 Ever since the discovery of “Terra Preta do Indio”, large pockets of permanently fertile soils in otherwise highly leached and acid soils of the Amazon region, there is a growing interest in using biochar as a soil amendment.127–129 The application of biochar to soil as an amendment is considered an option to off-set emissions while improving soil quality—a new green130 or a charcoal vision.131 Consequently, pyrolysis plants are being established132,133 to commercialize the production of nutrient fortified biochar as a soil amendment. While some consider black C sequestration as an alternative to bioenergy,134 others have reported an increase in losses of forest-derived humus and loss of native soil C due to application of biochar.135 Similar to biofuel, there is also a strong need for complete life cycle analysis of the biochar production system, application, and fate of charcoal vs. native C in soil humus.VII. Biofuels and the carbon cycle
Increase in the energy cost and threat of global warming because of the atmospheric enrichment of CO2 have increased emphasis on biofuel production. Production of biofuels affect the global C cycle directly and indirectly. Directly, biofuels avoid emissions of CO2 by merely recycling it. Indirectly, production of biofuels can accentuate CO2 emissions by using reactive nitrogenous fertilizers and other energy-based input in production of corn grains and soybeans (USA), and sugarcane (Brazil). These 3 crops require the use of N-fertilizers (soybeans require less N than corn and sugarcane) which normally has low use efficiency of ∼30%. Thus, N-intensive biofuels could negate any savings in C emission.114 Removal of crop residues for ethanol production can degrade soils by accelerated erosion and exacerbate emission of CO2. Conversion of natural to agricultural ecosystems can enhance gaseous emissions from land use change.136 Deforestation of tropical rain forest for additional land can exacerbate CO2 emission.137The biomass production potential of energy crops ranges from 10 to 22 Mg ha−1 y−1 for short rotation woody crops in the U.S. and 20 Mg ha−1 y−1 in Brazil with a global average of about 10 Mg ha−1 y−1.79 The biomass productivity of energy plantations can be increased by identifying biotic and abiotic constraints of species to be grown in an ecoregion and addressing those constraints through site-specific research including: (i) tolerance to drought, cold and heat, (ii) resistance to pests and diseases, (iii) addressing dormancy, floral sterility and delayed leaf senescence, (iv) more carbon allocation to stem diameter vs. height growth, (v) more shoot : root ratio to optimize the aboveground (stem) biomss, (vi) high H2O, N and nutrient use efficiency, (vii) high biomass production per unit area and time, (viii) readily processable cellulose, hemicollulose and lignin in the biomass, (ix) desired biomass composition, and (x) potential to produce value added chemicals.79
Despite the high biomass production strategy, the competition for natural resources (land area, water, nutrients needed for food production) is a concern that is likely to exacerbate with an increasing world population. Two issues that need an objective and critical appraisal with regards to biofuels are:138 (i) the magnitude of emissions avoided from use of biofuels, and (ii) the impact of alternative land use strategies on C sequestration in the biosphere. Production of biofuels is a viable option if the net amount of emission avoided exceeds the quantity of C sequestered in the terrestrial biosphere. The net amount of emission avoided includes the C equivalent of energy required to produce biofuel (e.g., fertilizer, pesticdes, processing). The data in Table 3 by Righelato and Spracklen138 show that afforestation of an equivalent area would sequester 2 to 9 times more C over a 30 year period than the emissions avoided by the use of biofuels. It is estimated that 10% substitution of petrol by biofuels would require additional 43% of the current cropland area in the U.S. and 38% in Europe.139 Deforestation and biomass burning of the tropical rainforest lead to a large up-front emission. Of all the options, conversion of woody biomass to ethanol may be compatible with afforestation because many biofuels have greater aggregate environmental costs than petroleum.
| Strategy | Process | Emissions avoided/Mg C ha−1 |
|---|---|---|
| I. Biofuel | (i) Sugarcane to ethanol | 50 |
| (ii) Wheat to ethanol | 15 | |
| (iii) Sugar beet to ethanol | 30 | |
| (iv) Maize to ethanol | 15 | |
| (v) Rapeseed to diesel | 12 | |
| (vi) Woody biomass to diesel | 55 | |
| II. C sequestration in terrestrial ecosystems | (i) Tropical forest to cropland | −182.5 (emissions) |
| (ii) Tropical cropland to forest | 160 | |
| (iii) Temperate cropland | −90 (emissions) | |
| (iv) Temperate cropland to grassland | 25 |
Detailed life cycle analysis is needed to assess the net C loss or gain upon conversion of native ecosystems to biofuel plantations. Fargione et al.140 computed ecosystem C debt and the number of years required to pay it for conversion of different native ecosystems to biofuel plantations in Brazil, Indonesia, Malaysia and the USA. Because converting native ecosystems to agricultural/biofuel plantations leads to substantial emissions of CO2 through deforestation/biomass burning and microbial decomposition, the initial loss is termed “C debt” of land use conversion. This debt is to be repaid over time through production of biofuels. The longer the duration to pay the debt, the more long lasting is the adverse impact. The data in Table 4 show that the only viable option is the biofuels from perennials grown on degraded or marginal croplands. In contrast, biofuels produced from converting rainforests, peatlands, savannas or grasslands emit 17 to 420 times more CO2 than the GHG emission over 30 years and increase over 167 years. Therefore, a critical appraisal through detailed life cycle analysis and, of food for hungry stomachs vs. tanks, are essential prior to undertaking large scale biofuel production.
| Former ecosystem | Biofuel | C Debt/Mg C ha−1 | Debt allocated to ethanol (%) | Annual payment/Mg C ha−1 y−1 | Time to repay debt/y | ||
|---|---|---|---|---|---|---|---|
| Soil | Biomass | Total | |||||
| Tropical rainforest | Palm biodiesel | 55 | 135 | 190 | 87 | 1.9 | 86 |
| Peatland rainforest | Palm biodiesel | 218 | 135 | 353 | 87 | 1.9 | 423 |
| Tropical rainforest | Soybean biodiesel | 65 | 135 | 200 | 39 | 0.25 | 319 |
| Cerrado wooded | Sugarcane ethanol | 31 | 14 | 45 | 100 | 2.7 | 17 |
| Cerrado woodland | Soybean biodiesel | 22 | 1 | 23 | 39 | 0.25 | 37 |
| Central grassland | Corn ethanol | 34 | 3 | 37 | 83 | 0.33 | 93 |
| Abandoned cropland | Corn ethanol | 18 | 1 | 19 | 83 | 0.33 | 48 |
| Abandoned cropland | Corn ethanol | 2 | — | 2 | 100 | 1.2 | 1 |
| Abandoned cropland | Prairie Biomass ethanol | 0 | 0 | 0 | 100 | 2.1 | No debt |
VIII. Secondary carbonates
The total soil C pool comprises two distinct components: SOC and SIC. The SIC pool consists of primary or lithogenic carbonates and secondary or pedogenic carbonates. Most soils of arid and semi-arid regions contain primary carbonates such as calcite (CaCO3), dolomite (CaMg(CO3)2), aragonite (polymorph of natural CaCO3) and siderite (FeCO3). Primary carbonates are derived from the weathering of soil parent material. In contrast, secondary carbonates are derived from the decomposition of Ca-bearing materials and precipitation of weathering products. Secondary carbonates occur in soils in different forms including precipitates in the interstitial spaces of parent material, prelaminated layers of carbonates, coating on the underside of stones, and as needles or crystals.141 Ryskov et al.142 described several mechanisms of formation of secondary carbonates since the second half of the Holocene in steppe soils of Russia. They divided the process into 3 stages: (i) accumulation of carbonates in the upper 2 m sequence resulting from a high groundwater level 5000–3800 years ago, (ii) redistribution of carbonates in the profile, with removal from the upper horizon and formation of an accumulation horizon 3800–2000 years ago, and (iii) stabilization of the carbonate profile. The slow process of chemical transformation shows that during dissolution, every CaCO3 molecule binds one CO2 molecule, which transforms it into biocarbonate. During crystallization, CO2 is re-emitted back into the atmosphere as carbonates are deposited. Secondary carbonates are formed as a result of precipitation that occurs when super-saturated solution is subject to evaporation, freezing, decline in partial pressure of CO2, and soil dehydration through transformation. Living organisms (microorganisms, termites, earthworms, some mushroom species, and mollusks) can also facilitate formation of secondary carbonates with dissolution of CO2 from soil air and uptake of Ca2+ and H2O from soil solution (eqn (8)–(12)).143| CO2 + H2O ↔ H2CO3 | (8) |
| H2CO3 ↔ H+ + HCO3− | (9) |
| HCO3− ↔ H+ + CO32− | (10) |
| H2CO3 ↔ 2H+ + CO3− | (11) |
| Ca2+ + 2HCO3− ↔ CaCO3 + H2O + CO2 | (12) |
Secondary carbonate accumulation commonly occurs in pH range of 7.3 to 8.5, and sufficient amount of Ca2+ must be present.
There are four general models of pedogenic carbonate formation144
(i) The per descendum model: precipitation of carbonates in the sub-soil following dissolution of pre-existing carbonates in the upper layers and their vertical translocation to sub-soil,
(ii) The per ascensum model: secondary carbonates are deposited from capillary rise of Ca2+ from shallow water tables,
(iii) The in situ model: reprecipitation of carbonates occurs close to bedrock following short-range dissolution, and
(iv) The biogenic model: biological factors (e.g., termites, microbes, and plants) accentuate the precipitation of secondary carbonates.
Measured rates of soil C sequestration through formation of secondary carbonates are low and range from 1.2 to 6 Kg C ha−1 y−1 in southwest USA145 to 12 to 17 kg ha−1 y−1 in Canada.143 With a large area of about 4 billion ha in arid and semi-arid regions, total amount of C sequestration as secondary carbonates at 5 to 10 kg C ha−1 y−1 can be 20 to 40 Tg C y−1. There have also been some concerns whether exposure of caliche to the surface may lead to its weathering and emission of CO2. Experiments conducted in Southwestern U.S. by Serna-Pérez et al.146 showed that exposed petrocalcic horizons are not actively emitting CO2.
IX. Carbon sequestration and global food security
In addition to the threat of global warming, C sequestration also impacts global food security. There are presently about 1 billion food-insecure people in the world. The population prone to food insecurity is increasing with the increase in the price of food grains, notably corn, wheat, rice and soybeans. The increase in food price, adversely affecting the poor population (<$2 day−1), are due to several complex and interacting factors. Over and above the effects of increase in price of energy ($135 per barrel of oil in May 2008), perpetuation of drought, in Australia and Ukraine among others, presumably caused by global warming, has adversely affected production of rice, wheat and other food staples. Soil degradation, coupled with high temperatures in early spring, also supposedly caused by global warming, is one of the causes of stagnating or declining productivity of the rice–wheat system in the Indo-Gangetic Basin. Soil degradation, especially by depletion of SOM and the negative nutrient budget on a continental scale, are causes of agrarian stagnation in Sub-Saharan Africa. Climate change may exacerbate the problem of low agricultural production in the West African Sahel.147–149 The benefits of improved germplasm cannot be realized unless grown under optimal soil and agronomic conditions because even the improved varieties cannot extract water and nutrients from impoverished soils. The problem of food insecurity for the world's poor is to some extent also affected by the emphasis on biofuels (see section VII). Production of corn grain based ethanol and soybean derived biodiesel affects food security directly by increasing the price of food grains, and indirectly by competition for land, water and fertilizers for establishing energy plantations.Carbon sequestration, by natural or pedologic/engineering/industrial processes, is directly or indirectly linked to all causes of world's food insecurity. For example, climate change and drought are caused by atmospheric enrichment of CO2. Soil degradation and nutrient mining/imbalance are caused by depletion of SOC pool by perpetual use of extractive farming practices. Use efficiency of input (e.g., fertilizers, irrigation) can only be increased if SOC pool can be raised above the critical level of 1.1 to 1.2% in the root zone, which in most soils of sub-Saharan Africa and South Asia is presently about 0.1 to 0.2%. Use of nutrient-fortified biochar, a by-product of the pyrolysis of biomass conversion into liquid biofuels, can enhance the SOC pool and improve soil quality.
Enhancing and maintaining soil quality (physical, chemical and biophysical) are essential pre-requisites to sustaining agronomic production to meet the increasing food demands of the world. Restoring and maintaining the SOC pool are essential to improving soil quality. Lal150 estimated that increasing the C pool in the root zone of world cropland soils can increase food grain production by 32 ± 11 million tons y−1 and root/tuber production by 11 ± 4 million tons y−1. This important inter-connectivity is one of the several reasons why C sequestration in the terrestrial biosphere (especially in world's soils) is called a “win-win-win strategy”. It mitigates climate change by off-setting fossil fuel emissions, improves environment especially the water quality by filtering/denaturing pollutants and by reducing erosion and non-point source pollution, and increases agronomic production by enhancing soil quality and use efficiency of all inputs. Indeed, some have termed atmospheric CO2 as a misplaced resource. While the CO2 fertilization effect may contradict somewhat the logic of calling CO2 “the misplaced resource”, its transfer into soil is definitely beneficial to restoring numerous ecosystem services, the most important of which are mitigating climate change while advancing food security and eliminating of worlds hunger and malnutrition.
X. Conclusions
Transfer of atmospheric CO2, presently at 780 Pg and increasing at the annual rate of 4 Pg, to other pools (oceanic, geologic, biotic and pedologic) is essential to minimizing the risks of global warming and environmental degradation. Engineering techniques of oceanic and geologic sequestration have a high sink capacity, but are constrained by high costs and risks of leakage necessitating regulatory measures and the establishing of protocols for measurement, monitoring and verification. In addition to injection of CO2 deep into the ocean, Fe fertilization in the Southern Ocean can enhance “biological pumping” through increased productivity of phytoplankton and other marine biota. Geologic sequestration into oil wells is done to enhance its recovery and in unmineable coal seams to enhance displacement of coal bed methane. In strong contrast to the engineering techniques, there are three natural processes of C sequestration: weathering of Ca/Mg silicate minerals, formation of secondary carbonates, and photosynthesis followed by humification of biomass. Photosynthesis, humification and formation of secondary carbonates increase the terrestrial (soil and biota) C pools. Weathering of silicates and formation of secondary carbonates are slow processes and operate on centennial to millennial (geologic) time scales. Yet, industrial processes of conversion of point-source CO2 into carbonates is being tried to mimic the natural process.The pedologic or soil C sequestration has numerous ancillary benefits through restoration of ecosystem services. Important among these are improvement in soil quality, increase in biodiversity, restoration of degraded soils and ecosystem, and reduction in the rate of enrichment of atmospheric CO2. Improvement in soil quality is essential to enhancing agronomic production but also minimizing soil erosion risks, decreasing non-point source pollution and sedimentation, and reducing risks of hypoxia of coastal ecosystems.
There are specific nitches where different sequestration strategies have comparative advantages. While there are challenges and opportunities for each strategy (e.g., geologic, oceanic, terrestrial), the prudent approach lies in identifying which option is environmentally and economically viable under what ecological conditions?
References
- EIA, International Energy Outlook. 2007, Energy Information Administration, Washington, DC, 2007,http://eia.doe.gov/oiaf/ieo/index.html Search PubMed.
- M. I. Hoffert, K. Caldeira, G. Benford and D. R. Criswell, et al., Advanced technology paths for global climate stability: Energy for a greenhouse planet, Science, 2001, 298, 981–987.
- IPCC, The Third Assessment Report, Ingergovernment Panel on Climate Change, Cambridge University Press, UK, 2001 Search PubMed.
- E. A. Paul and F. E. Clark, Soil Microbiology and Biochemistry, Academic Press, San Diego, 1996, 264 pp Search PubMed.
- IPCC, Climate Change 2007, The Physical Science Basis, Cambridge University Press, UK, 2007 Search PubMed.
- J. G. Canadell, C. LeQuere, M. R. Raupach, C. B. Field, E. T. Buitenhuis, and P. Ciais, et al., Contributions to acceleration of atmospheric CO2 growth from economic activity, carbon intensity and efficiency of natural sinks, 2007, http://www.pnas.org/cgi/doi/10.1973/pnas.0702737104 Search PubMed.
- R. Lal, Soil carbon sequestration impacts on global climate change and food security, Science, 2004, 304, 1623–1627 CrossRef CAS.
- W. F. Ruddiman, Plows, Plagues and Petroleum: How humans took control of climate, Princeton University Press, Princeton, 2003 Search PubMed.
- W. F. Ruddiman, The anthropogenic greenhouse era began thousands of years ago, Clim. Change, 2005, 61, 262–292.
- IPCC, Land Use, Land Use Change and Forestry, Cambridge University Press, Cambridge, UK, 2000 Search PubMed.
- W. S. Boecker, CO2 arthimatic, Science, 2007, 315, 1371 CrossRef.
- R. Lal, Carbon sequestration, Phil. Trans. R. Soc. London, Ser. B, 2007, 363, 815–830.
- S. Holloway, An overview of the underground disposal of carbon dioxide, Energy Convers. Manage., 1997, 38, 193–198.
- B. Hitchon, W. D. Gunter, T. Gentzis and R. T. Bailey, Sedimentary basins and greenhouse gases: A serendipitous association, Energy Convers. Manage., 1999, 40, 825–843 CrossRef CAS.
- T. F. Xu, J. A. Apps, K. Pruess and H. Yamamoto, Numerical modeling of injection and mineral trapping of CO2 with H2S and SO2 in a sandstone formation, Chem. Geol., 2007, 242, 319–346 CrossRef CAS.
- H. Lin, T. Fujii, R. Takisawa, T. Takahashi and T. Hashida, Experimental evaluation of interactions in supercritical CO2/water/rock minerals system under geologic CO2 sequestration conditions, J. Mater. Sci., 2008, 43, 2307–2315 CrossRef CAS.
- K. G. Knauss, J. W. Johnson and C. I. Steefel, Evaluation of the impact of CO2, co-contaminant gas, aqueous fluid and reservoir rock interactions on the geologic sequestration of CO2, Chem. Geol., 2005, 217, 339–350 CrossRef CAS.
- T. F. Xu, E. Sonnenthal, N. Spycher and K. Pruess, TOUGHREACT - A simulation program for non-isothermal multiphase reactive geochemical transport in variably saturated geologic media: Applications to geothermal injectivity and CO2 geological sequestration, Comput. Geosci., 2006, 32, 145–165 CrossRef CAS.
- K. G. Knauss, J. W. Johnson and C. I. Steefel, Evaluation of the impact of CO2, co-contaminant gas, aqueous fluid and reservoir rock interactions on the geologic sequestration of CO2, Chem. Geol., 2005, 217, 339–350 CrossRef CAS.
- R. J. Pawar, N. R. Warpinski, B. Stubbs, R. B. Grigg, J. C. Lorenz, S. P. Cooper, J. L. Krumhansl, R. D. Benson and J. T. Rutledge, Geologic sequestration of CO2 in a depleted oil reservoir, Abstr. Pap. Am. Chem. Soc., 2003, 226, U605–U605.
- H. G. Yu, G. Z. Zhou, W. T. Fan and H. P. Ye, Predicted CO2 enhanced coalbed methane recovery and CO2 sequestration in China, Int. J. Coal Geol., 2007, 71, 345–357 CrossRef CAS.
- C. N. Hamelinck, A. P. C. Faaji, W. C. Turkenburg, F. Van Bergen, H. J. M. Pagnier, O. H. M. Barzandyi, K.-H. A. A. Wolf and G. J. Ruijg, CO2 enhanced coal bed methane production in the Netherlands, Energy, 2002, 27, 647–674 CrossRef CAS.
- A. L. Goodman, R. N. Favors, M. M. Hill and J. W. Larsen, Structure changes in Pittsburg #8 coal caused by sorption of CO2 gas, Energy Fuels, 2005, 19, 1759–1760 CrossRef CAS.
- J. W. Larsen, The effects of dissolved CO2 on coal structure and properties, Int. J. Coal Geol., 2004, 60, 43–55 CrossRef.
- R. G. Bruant, A. J. Guswa, M. A. Celia and C. A. Peters, Safe storage of CO2 in deep saline aquifers, Environ. Sci. Technol., 2002, 36, 240–245.
- C. M. White, B. R. Strazisar, E. J. Granite, J. S. Hoffman and H. W. Pennline, Separation and capture of CO2 from large stationary sources and sequestration in geological formations-Coalbeds and deep saline aquifers, J. Air Waste Manage. Assoc., 2003, 53, 645–715 CAS.
- G. W. Kling, M. A. Clark, A. R. Compton, J. D. Devine and W. C. Evans, et al., The 1986 Lake Nyos gas disaster in Cameroon, West Africa, Science, 1987, 236, 169–175 CrossRef CAS.
- J. L. Lewicki, G. E. Hilley and C. M. Oldenburg, An improved strategy to detect CO2 leakage for verification of geologic carbon sequestration, Geophys. Res. Lett., 2005, 32 Search PubMed.
- B. G. Kutchko, B. R. Strazisar, D. A. Dzombak, G. V. Lowry and N. Thaulow, Degradation of well cement by CO2 under geologic sequestration conditions, Environ. Sci. Technol., 2007, 41, 4787–4792 CrossRef CAS.
- A. Kumar, A. Datta-Gupta, R. Shekhar and R. L. Gibson, Modeling time lapse seismic monitoring of CO2 sequestration in hydrocarbon reservoirs including compositional and geochemical effects, Pet. Sci. Technol., 2008, 26, 887–911 CrossRef CAS.
- X. S. Qin, G. H. Huang, H. Zhang and A. Chakma, An integrated decision support system for management of CO2 geologic storage in the Weyburn Field, Pet. Sci. Technol., 2008, 26, 813–843 CrossRef CAS.
- E. J. Wilson, T. L. Johnson and D. W. Keith, Regulating the ultimate sink: Managing the risks of geologic CO2 storage, Environ. Sci. Technol., 2003, 37, 3476–3483 CrossRef CAS.
- D. W. Keith, J. A. Giardina, M. G. Morgan and E. J. Wilson, Regulating the underground injection of CO2, Environ. Sci. Technol., 2005, 39, 499–505.
- M. Battle, M. L. Bender, P. P. Tans, J. W. C. White, J. T. Ellis, T. Conway and R. J. Francey, Global carbon sinks and their variability inferred from atmospheric O–2 and S 13C, Science, 2000, 287, 2467–2470 CrossRef CAS.
- J. W. Morse, A. J. Andersson and F. T. Mackenzie, Initial response of carbonate-rich shelf sediments to rising atmospheric pCO2 and oceanic acidification: Role of high Mg-calcites, Geochim. Cosmochim. Acta, 2006, 70, 5814–5830 CrossRef CAS.
- S. Jinning, Oceanic iron fertilization: one of the strategies for sequestration of atmospheric CO2, Acta Oceanol. Sin., 2003, 22, 57–68 Search PubMed.
- F. J. Millero, The effect of iron on carbon dioxide in the oceans, Sci. Prog., 1997, 80, 147–168 CAS.
- J. M. Song, Oceanic iron fertilization: one of strategies for sequestration atmospheric CO2, Acta Oceanol. Sin., 2003, 22, 57–68 Search PubMed.
- Q. J. Kang, I. N. Tsimpanogiannis, D. Zhang and P. C. Lichtner, Pore-scale studies of oceanic CO2 sequestration, Abstr. Pap. Am. Chem. Soc., 2004, 227, U1099–U1099.
- Q. J. Kang, I. N. Tsimpanogiannis, D. X. Zhang and P. C. Lichtner, Numerical modeling of pore-scale phenomena during CO2 sequestration in oceanic sediments, Fuel Process. Technol., 2005, 86, 1647–1665 CrossRef CAS.
- R. B. Bacastow and R. K. Dewey, Effectiveness of CO2 sequestration in the post-industrial ocean, Energy Convers. Manage., 1996, 37, 1079–1086 CrossRef CAS.
- C. S. Wong and R. J. Matear, Ocean disposal of CO2 in the North Pacific Ocean: Assessment of CO2 chemistry and circulation storage and return to the atmosphere, Waste Manage., 1997, 17, 329–336 CAS.
- J. M. Martin, Glacial to interglacial CO2 change: the iron hypothesis, Paleoceanography, 1990, 5, 1–13 CrossRef.
- A. J. Watson, D. C. E. Bakker, A. J. Ridgewell, P. W. Boyd and C. Law, Effect of iron supply on Southern Ocean CO2 uptake and implications for glacial atmospheric CO2, Nature, 2000, 730–733 CrossRef CAS.
- I. Marinov, A. Gnanadesikan, J. R. Toggweiler and J. L. Sarmiento, The Southern Ocean biogeochemical divide, Nature, 2006, 441, 964–967 CrossRef CAS.
- P. Assmy, J. Henjes, C. Klaas and V. Smetacek, Mechanisms determining species dominance in a phytoplankton bloom induced by the iron fertilization experiment EisenEx in the Southern Ocean, Deep-Sea Res., Part I, 2007, 54, 340–362 CrossRef.
- D. C. E. Bakker, A. J. Watson and C. S. Law, Southern Ocean iron enrichment promotes inorganic carbon draw down, Deep-Sea Res., Part II., 2001, 48, 2483–2507 CrossRef CAS.
- S. Blain, B. Queguiner, L. Armand, S. Belviso, B. Bombled, L. Bopp and A. Bowie, et al., Effect of natural iron fertilization on carbon sequestration in the Southern Ocean, Nature, 2007, 446, 1070–U1 CrossRef CAS.
- S. W. Chisholm, Oceanography: Stirring times in the Southern Ocean, Nature, 2000, 407, 685–687 CrossRef CAS.
- S. W. Chisholm, P. G. Folkowski and J. J. Cullen, Discreting ocean fertilization, Science, 2001, 294, 309–310 CrossRef CAS.
- K. H. Coale, et al, A massive phytoplankton bloom induced by an ecosystem-scale iron fertilization experiment in the equatorial Pacific Ocean, Nature, 1996, 383, 495–501 CrossRef CAS.
- P. L. Croot, U. Passow, P. Assmy, S. Jansen and V. H. Strass, Surface active substances in the upper water column during a Southern Ocean Iron Fertilization Experiment (EIFEX), Geophys. Res. Lett., 2007, 34, L03612 CrossRef.
- S. H. M. Jacquet, J. Henjes, F. Dehairs, A. Worobiec, N. Savoye and D. Cardinal, Particulate ba-barite and acantharians in the southern ocean during the European iron fertilization experiment (EIFEX), J. Geophys. Res., 2007, 112(G4), GO4006.
- A. Krishnamurthy, J. K. Moore and S. C. Doney, The effects of dilution and mixed layer depth on deliberate ocean iron fertilization: 1-D simulations of the southern ocean iron experiment (SOFeX), J. Mar. Syst., 2008, 71, 112–130 CrossRef.
- N. Meskhidze, A. Nenes, W. L. Chameides, C. Luo and N. Mahowald, Atlantic Southern Ocean productivity: Fertilization from above or below?, Global Biogeochem. Cycles, 2007, 21(2), 2006.
- M. L. Wells, Pumping iron in the Pacific, Nature, 1994, 368, 295–296 CrossRef.
- V. Smetacek, P. Assmy and J. Henjes, The role of grazing in structuring Southern Ocean pelagic ecosystems and biogeochemical cycles, Antarct. Science, 2004, 16, 541–558 Search PubMed.
- R. J. Matear and B. Elliott, Enhancement of oceanic uptake of anthropogenic CO2 by macronutrient fertilization, J. Geophys. Res., [Oceans], 2004, 109 Search PubMed.
- A. J. Watson and P. S. Liss, Marine biological controls on climate via the carbon and sulfur geochemistry cycles, Phil. Trans. Royal. Soc. London, Ser. B, 1998, 353, 41–51 Search PubMed.
- K. S. Johnson and D. M. Karl, Is ocean fertilization credible and creditable?, Science, 2002, 296, 467 CAS.
- K. O. Buesseler, S. C. Doney, D. M. Karl, P. W. Boyd, K. Caldeira and F. Chai, et al., Environment - Ocean iron fertilization - Moving forward in a sea of uncertainty, Science, 2008, 319, 162–162 CrossRef CAS.
- G. L. Foster and D. Vance, Negligible glacial-interglacial variation in continental chemical weathering rates, Nature, 2006, 444, 918–921 CrossRef CAS.
- C. S. Riebe, J. W. Kirchner and R. C. Finkel, Erosional and climatic effects on long-term chemical weathering rates in granitic landscapes spanning diverse climatic regimes, Earth Planet Sci. Lett., 2004, 224, 547–562 CrossRef CAS.
- R. A. Berner, A. C. Lasaga and R. M. Garrels, The carbonate-silicate geochemical cycle and its effect on atmospheric CO2 over the past 100 million years, Am. J. Sci., 1983, 283, 641–683 Search PubMed.
- W. H. Schlesinger, Carbon storage in the Caliche of arid soils: A case study from Arizona, Soil Sci., 1982, 133, 247–255 CAS.
- E. L. Williams, L. M. Walter, T. C. W. Ku, G. W. Kling and D. R. Zak, Effects of CO2 and nutrient availability on mineral weathering in controlled tree growth experiments, Global Biogeochem. Cycles, 2003, 17(2), 10-1–10-12.
- E. K. Berner and R. A. Berner, The Global Water Cycle: Geochemistry and Environment, Prentice Hall, Old Tappan, NJ, USA, 1987, pp. 387 Search PubMed.
- W. Seifritz, CO2 disposal by means of silicates, Nature, 1990, 1990.
- K. S. Lackner, C. H. Wendt, D. P. Butt, E. L. Joyce and D. H. Sharp, Carbon dioxide disposal in carbonate minerals, Energy, 1995, 20, 1153–1170 CrossRef CAS.
- S. R. Gislason, Chemical weathering, chemical denudation and the CO2 budget for Iceland, in Iceland: Modern Processes and Post Environments, ed. C. Casedine, A. Russell, J. Hardardottir and O. Knudsen, Elsevier, Amsterdam, 2005,pp. 289–307 Search PubMed.
- L. P. WildingRole of inorganic carbon in CO2 sequestration, in Carbon Sequestration in Soils: Science, Monitoring and Beyond, ed. N. J. Rosenberg, R. Cesar Izaurralde, and E. L. Malone, Battelle Press, Columbus, OH, 1999, pp. 147–148 Search PubMed.
- K. S. Lackner, Carbonate chemistry for sequestering fossil carbon, Annu. Rev. Energy Environ., 2002, 27, 193–232 CrossRef.
- W. J. J. Huijgen, G. J. Witkamp and P. N. J. Comans, Mineral CO2 sequestration by steel slag carbonation, Environ. Sci. Technol., 2005, 39, 9676–9682 CrossRef CAS.
- W. J. J. Huijgen and R. N. J. Comans, CO2 sequestration by mineral carbonation: literature review, Energy Research Center, Petten, The Netherlands, 2003, ECN-C-03–016.
- J. Melendez, M. North and R. Pasquale, Synthesis of cyclic carbonates from atmospheric pressure of CO2 using active aluminum (salen) complexes as catalysts, Eur. J. Inorg. Chem., 2007, 2007, 3223–3226.
- M. Aresta and A. Dibenedetto, The contribution of the utilization option to reducing the CO2 atmospheric loading: research needed to overcome existing barrier for a full exploitation of the potential of CO2 use, Catal. Today, 2004, 98, 455–462 CrossRef CAS.
- R. Lal, Carbon sequestration in soil, CAB Review #030, Wallingford, UK, 20 pp.
- T. P. Vispute and G. W. Huber, Breaking the chemical and engineering barriers to lignocellulosic biofuels, The Int. Sugar J., 2008, 110, 138–143 Search PubMed.
- A. J. Ragauskas, C. K. Williams, B. H. Davison, G. Britousek and et al., The path forward for biofuels and biomaterials, Science, 2006, 311, 484–489 CrossRef.
- F. E. Allison, Organic matter and its role in crop production, Elsevier, N.Y., 1973, 637 pp Search PubMed.
- S. L. Tisdale and W. L. Nelson, Soil fertility and fertilizers, Macmillan, N.Y., 1966, 637 pp Search PubMed.
- F. H. King , Farmers of forty centuries, Democrat, Madison, WI, USA, 1911, 438 pp Search PubMed.
- F. H. KingFarmers of forty centuries, or permanent agriculture in China, Korea, and Japan, Oxford University Press, 1926 Search PubMed.
- E. C. Semple, Ancient Mediterranean Agriculture, Agric. Hist., 1928, 2, 61 Search PubMed.
- W. C. Lowdermilk, Conquest of the land through 7000 years, Agric. Inf. Bull. (U.S. Dep. Agric.), 1953, 99, 30 Search PubMed.
- A. I. MacKay, Farming and gardening in the bible, Rodale, Emmaus, Pa., 1950, p. 280.
- R. J. Wasson, Exploitation and conservation of soil in the 3000 year agricultural and forestry history of South Asia, inSoils and Societies: Perspectives from Environmental History, ed. J. R. McNeill and V. Winiwarter, The White Horse Press, Center Conway, NH, 2006, pp. 13–50 Search PubMed.
- C. Feller, The concept of soil humus in the past three centuries, in History of Soil Science, Advances in Geoecology, ed. D. H. Yaalon, S. Berkowicz, Catena Verlag, Reiskirchen, vol. 29, 1997, pp. 15–46 Search PubMed.
- Virgil (Publius Vergiilius Marco), Georgicus. Translated by Theodore Williams, Harvard Univ. Press Search PubMed.
- A. Cherbonneau and H. Peres, “Kitab-Al-Filaha” or Le Livre de la Culture, Editions Carbonel, Alger, 1946, 29 pp. Search PubMed.
- A. Thaër 1809, Grundsätze der Rationnellen Landwirtschaft (1809–1812), Realschulbuch Ed, Berlin.
- P. E. Muller, Recherches sur les formes naturelles de l'hummus et leur influence sur la vegetation et le sol (Research on the natural forms of humus and their influence on vegetation and soil), Berger-Levrault et Cie, Paris-Nancy, 1884 Search PubMed.
- S. A. Waksman, Humus. Origin, Chemical Composition and Importance in Nature, The Williams and Wilkins Company, Baltimore, London, 2nd edn, 1938 revised Search PubMed.
- W. Albrecht, Loss of soil organic matter and its restoration. Soils and Men, in Yearbook of Agriculture, USDA, Washington, DC, 1938, pp. 347–360 Search PubMed.
- A. Howard, An agricultural testament, Oxford University Press, Oxford, 1940 Search PubMed.
- A. Howard, The Soil and Health, A study of organic agriculture, The Devin-Adam Company, New York, 2nd edn, 1952 Search PubMed.
- H. Jenny, Soil resource: origin and behavior, Springer Verlaag, New York, 1980, 377 pp Search PubMed.
- R. LalJ. M. Kimble, E. Levine and B. A. Stewart, Soil management and the greenhouse effect, Lewis Publishers, Chelsea, MI, 1995,385 pp Search PubMed.
- R. Lal, J. M. Kimble, R. Follett and B. A. Stewart, Soil processes and the carbon cycle, CRC, Boca Raton, FL, 1998, b609 pp Search PubMed.
- R. Lal, J. M. Kimble, R. Follett and C. V. Cole, The potential of U.S. cropland to sequester carbon and mitigate the greenhouse effect, Sleeping Bear Press, Chelsea, MI, 1998a, 128 pp Search PubMed.
- R. Lal, R. F. Follett and J. M. Kimble, Achieving soil carbon sequestration in the U.S.: A challenge to policy makers, Soil Sci., 2003, 168, 1–19 CrossRef.
- A. Paul, K. Paustian, E. T. Elliott and C. V. Cole, Soil organic matter in temperate agroecosystems: long term experiment in North America, CRC Publishers, Boca Raton, FL, USA, 1997, 415 pp Search PubMed.
- S. Flores, D. Saxena and G. Stotzky, Transgenic Bt plants decompose less in soil than non- Bt plants, Soil Biol. Biochem., 2005, 37, 1073–1082 CrossRef CAS.
- J. Poerschmann, A. Bathmann, J. Augustin, U. Langer and T. Gorecki, Molecular composition of leaves and stems of genetically modified Bt and near isogenic non- Bt maize: characterization of lignin patterns, J. Environ. Qual., 2005, 34, 1508–1518 CrossRef CAS.
- D. Saxena and G. Stotzky, Bt corn has a higher lignin content than non- Bt corn, Am. J. Bot., 2001, 88, 1704–1706 Search PubMed.
- M. Castaldini, A. Turrini, C. Sabrana, A. Benedetti and M. Marchionni, et al., Impact of Bt corn on rhizospheric and soil eubacterial communities and on benefitical mycorrhizal symbiosis on experimental microcosm, Appl. Environ. Microbiol., 2005, 71, 6719–6729 CrossRef CAS.
- N. W. Mungai, P. O. Motavalli, K. A. Nelson and R. J. Kremer, Differences in yields, residue composition and N mineralization dynamics of Bt and non- Bt maize, Nutr. Cycling Agroecosyst., 2005, 73, 101–109 Search PubMed.
- W. H. Schlesinger, Carbon sequestration in soils, Science, 1999, 284, 2095 CrossRef CAS.
- N. H. Oh and P. A. Raymond, Contributions of agricultural liming to riverine bicarbonate export and CO2 sequestration in the Ohio River Basin, Global Biogeochem. Cycles, 2006, 20(3), GB3012 CrossRef.
- J. I. Rangel-Castro, J. I. Prosser, C. M. Scrimgeour, P. Smith, N. Ostle, P. Ineson, A. Mehang and K. Killham, Carbon flow in upland grassland: effect of liming on the flux of recently photosynthesized carbon to rhizosphere soil, Global Change Biol., 2004, 10, 2100–2108 CrossRef.
- J. I. Rangel-Castro, J. I. Prosser, N. Ostle, C. M. Scrimgeour, K. Killham and A. A. Meharg, Flux and turnover of fixed carbon in soil microbial biomass of limed and unlimed plots of an upland grassland ecosystem, Environ. Microbia, 2005, 7, 544–552 Search PubMed.
- K. J. Van Groeningen, J. Six, D. Harris, H. Blum and C. Vankessel, Soil 13C and 15N dynamics in an N2 fixing clover system under long-term exposure to elevated atmospheric CO2, Global Change Biol., 2003, 9, 1751–1762 CrossRef.
- R. Hyvonen, G. I. Argen, T. Persson, M. F. Cotrufo and et al., The likely impact of elevated CO2, nitrogen deposition, increased temperature and management on carbon sequestration in temperate and boreal forest ecosystems: a literature review, New Phytologist, 2007, 173, 463–480 Search PubMed.
- J. N. Galloway, A. R. Townsend, J. W. Erisman, M. Bekunda, Z. Cai, J. R. Freney, L. A. Martinelli, S. P. Seitzinger and M. A. Sutton, Transformation of the nitrogen cycle: Recent trends, questions and potential solutions, Science, 2008, 320, 889–897 CrossRef CAS.
- P. A. Matson, W. H. McDowell, R. A. Townsend and P. M. Vitousek, Biogeochemistry, 1999, 46, 67 CAS.
- V. Smil , Enriching the Earth: Fritz Haber, Carl Bosch and the Transformation of World Food Production, MIT Press, Cambridge, MA, 2001 Search PubMed.
- S. A. Khan, R. L. Mulvaney, T. R. Ellsworth and C. W. Boast, The myth of nitrogen fertilization for soil carbon sequestration, J. Environ. Qual, 2007, 36, 1821–1832 CrossRef CAS.
- A. E. Russell, D. A. Laird and A. P. Mallarino, Nitrogen fertilization and cropping system impacts on soil quality in Midwestern Mollisols, Soil Sci. Soc. Am. J., 2006, 70, 249–255 CrossRef CAS.
- P. Zhu, J. Ren, L. C. Wang, X. P. Zhang, X. M. Yang and D. MacTavish, Long-term fertilization impacts on corn yields and soil organic matter on a clay loam soil in Northeast China, J. Plant Nutr. Soil Sci., 2007, 170, 219–223 CrossRef CAS.
- S. S. Malhi, R. Lemke, Z. H. Wang and S. S. Chhabra, Tillage, nitrogen and crop residue effects on crop yield, nutrient uptake, soil quality and greenhouse gas emissions, Soil Tillage Res., 2006, 90, 171–183 CrossRef.
- S. Jagadamma, R. Lal, R. G. Hoeft, E. D. Nafziger and E. A. Adee, Nitrogen fertilization and cropping systems effects on soil organic carbon and total nitrogen pools under chisel plow tillage in Illinois, Soil Tillage Res., 2007, 95, 348–356 CrossRef.
- U. M. Sainju, B. P. Singh, W. F. Whitehead and S. Wang, Carbon supply and storage in tilled and no-tilled soils as influenced by cover crops and nitrogen fertilization, J. Environ. Qual., 2006, 35, 1507–1517 CrossRef CAS.
- J. Diekow, J. Mielnicquk, H. Knicker, C. Bayer, D. P. Dick and I. Kogel-Knaber, Soil carbon and nitrogen stocks as affected by cropping systems and nitrogen fertilization in a southern Brazil Aerisol managed under no-till for 17 years, Soil Tillage Res., 2005, 81, 87–95 CrossRef.
- M. W. I. Schmidt, Biogeochemistry – carbon budget in the black, Nature, 2004, 427, 305–306 CrossRef CAS.
- W. I. Woods and J. M. McCann, The anthropogenic origin and persistence of Amazonian dark earths, Yearbook Conference of Latin Americanist Geographers, 1999, 25, 7–14 Search PubMed.
- W. Sombroek, M. De. R. Ruivo, P. M. Fearnside, B. Glaser and J. Lehmann, Amazon dark earths as carbon stores and sinks, in Amazon Dark Earths: Origin, Properties and Management, ed. J. Lehmann, D. C. Kern, B. Glaser and W. I. Woods, Dordrecht, Klumer, Academic Publishers, 2003, pp. 125–139 Search PubMed.
- W. Seifritz, Should we store carbon in charcoal?, Int. J. Hydrogen Energy, 1993, 18, 405–407 CrossRef CAS.
- J. Lehmann, A handful of carbon, Nature, 2007, 446, 143–144 CrossRef.
- H. N. Lima, C. E. R. Schaefer, J. W. V. Mello, R. J. Gilkes and J. C. Kerr, Pedogenesis and pre-Colombian land use of “Terra Preta Anthrosols” (“Indian Black Earth”) of Western Amazonia, Geoderma, 2002, 110, 1–17 CrossRef CAS.
- E. Marris, Putting the carbon back: black in the new green, Nature, 2006, 442, 624–626 CrossRef CAS.
- D. A. Laird, The charcoal vision: A win-win-win scenario for simultaneously producing bioenergy, permanently sequestering carbon, while improving soil and water quality, Agron. J., 2008, 100, 178–181 CrossRef.
- P. C. Badger and P. Fransham, Use of mobile fast pyrolysis plants to densify biomass and reduce biomass handling costs – A preliminary assessment, Biomass Bioenergy, 2006, 30, 321–325 CrossRef CAS.
- A. V. Bridgewater, D. Meier and D. Radlein, An overview of fast pyrolysis of biomass, Org. Geochem., 1999, 30, 1479–1493 CrossRef.
- M. Fowles, Black carbon sequestration as an alternative to bioenergy, Biomass Bioenergy, 2007, 31, 426–432 CrossRef CAS.
- D. A. Wardle, M. C. Milsson and O. Zackrisson, Fire-derived charcoal causes loss of soil humus, Science, 2008, 320, 629 CrossRef CAS.
- T. Searchinger, R. Heimlich, R. A. Houghton, F. Dong, A. Elobeid, J. Fabiosa, S. Tokgoz, D. Hayes and T.-H. Yu, Use of U.S. cropland for biofuels increases greenhouse gases through emissions from land use change, Science, 2008, 319, 1238–1240 CrossRef CAS.
- W. F. Laurence, Switch to corn promotes Amazon deforestation, Science, 2007, 318, 1721.
- R. Righelato and D. V. Spracklen, Carbon mitigation by biofuels or by saving and restoring forests, Science, 2007, 317, 902 CrossRef CAS.
- IEA. 2004. Biofuels for transport: An International perspective, IEA, Paris, France,http://www.iea.org/textbase/nppdf/free/2004/biofuels2004.pdf Search PubMed.
- J. Fargione, J. Hill, D. Tilman, S. Polasky and P. Hawthorne, Land clearing and the biofuel carbon debt, Science, 2008, 319, 1235–1237 CrossRef CAS.
- A. Landi, A. R. Mermut and D. W. Anderson, Origin and rate of pedogenic carbonate accumulation in Saskatchewan soils, Canada, Geoderma, 2003, 117, 143–156 CrossRef CAS.
- Ya.G. Ryskov, Ts. Kh. Tsybzhitov, Ts. Ts. Tsybikdorzhiev, S. A. Oleinik and E. A. Ryskova, Russia's soil: Is it a CO2 source or sink?, Geochem. Int., 2001, 39, 577–584 Search PubMed.
- A. R. Mermut and A. Landi, Secondary/Pedogenic carbonates, in Encyclopedia of Soil Science, ed. R. Lal, Taylor and Francis, New York, 2nd edn, 2004 Search PubMed.
- H. C. Monger, Pedogenic carbonates: links between biotic and abiotic CaCO3, 17th World Congress of Soil Sci. Synp. #20, Oral Paper #891, Bangkok, Thailand, 14–21 August 2002 Search PubMed.
- W. H. Schlesinger, The formation of caliche in soils of the Mohave desert, California, Geochim. Cosmochim. Acta, 1985, 49, 57–66 CrossRef CAS.
- A. Serna-Perez, H. C. Monger, J. E. Herrick and L. Murray, Carbon dioxide emissions from exhumed petrocalcic horizons, Soil Sci. Soc. Am. J, 2006, 70, 795–805 CrossRef CAS.
- A. Ben Mohamed, N. Van Duivenbooden and S. Abdoussallam, Impact of climate change on agricultural production in the Sahel - Part 1. Methodological approach and case study for millet in Niger, Climat. Change, 2002a, 54, 327–348 Search PubMed.
- A. Ben Mohamed, N. Van Duivenbooden and S. Abdoussallam, Impact of climate change on agricultural production in the Sahel - Part 1. Methodological approach and case study for millet in Niger, Climat. Change, 2002b, 54, 327–348 Search PubMed.
- N. Van Duivenbooden, S. Abdoussalam and A. Ben Mohamed, Impact of climate change on agricultural production in the Sahel - Part 2. Case study for groundnut and cowpea in Niger, Climat. Change, 2002, 54, 349–368 Search PubMed.
- R. Lal, Managing soils for feeding a global population of 10 billion, J. Sci. Food Agric., 2006, 2273–2284 CrossRef.
- M. J. Behrenfeld and R. T. O'Malley, et al., Climate-driven trends in contemporary ocean productivity, Nature, 2006, 444, 752–755 CrossRef CAS.
- R. A. Houghton, R. D. Boone, J. M. Melillo, C. A. Palm, G. M. Woodwell, N. Myers, B. Moore III and D. L. Skole, Net flux of carbon dioxide from tropical forests in 1980, Nature, 1985, 316(6029), 617–620 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2008 |
