Anion receptors based on organic frameworks: highlights from 2005 and 2006
Philip A.
Gale
*,
Sergio E.
García-Garrido
and
Joachim
Garric
School of Chemistry, University of Southampton, Southampton, UK, SO17 1BJ. E-mail: philip.gale@soton.ac.uk; Fax: +44 23 8059 6805; Tel: +44 23 8059 3332
First published on 14th November 2007
Abstract
This critical review covers advances in anion complexation chemistry related to receptors based on organic frameworks in the years 2005–2006. The review covers anion receptors that employ amides and thioamides, pyrroles and indoles, ureas and thioureas, ammonium, guanidinium, imidazolium, and receptors containing hydroxyl groups. There is a discussion of anion templated assembly, followed by a short section outlining modelling studies of these systems. (226 references.)
 Sergio E. García-Garrido, Philip A. Gale and Joachim Garric | Phil Gale received his DPhil (Oxon) (1995) under the supervision of Paul Beer before moving to Jonathan Sessler's group in Austin as a Fulbright Postdoctoral Fellow. He returned to Oxford in 1997 as a Royal Society University Research Fellow, moving to the University of Southampton in 1999 where he is now Professor of Supramolecular Chemistry. A major focus of his research is the supramolecular chemistry of anionic species. He has won a number of awards including a 2005 Corday-Morgan medal and prize from the Royal Society of Chemistry. Sergio E. García-Garrido studied chemistry at the University of Oviedo and received his PhD in 2005 under the supervision of Prof. J. Gimeno and Dr V. Cadierno working in the synthesis and catalytic applications of Ru(II) and Ru(IV) complexes. He then joined the group of Prof. Gale with a two-year postdoctoral fellowship. Joachim Garric studied chemistry at the University Bordeaux I where he received his PhD in 2005 under the guidance of Dr Ivan Huc, working on the synthesis of helicoidal capsules similar to the shape of the skin of an apple peeled in a helical fashion. He has currently a postdoctoral position with Prof. Gale. |
Introduction
Anion receptor chemistry continues to be a very vigorous area of research.1–4Anions play many positive roles in biological systems but can also have deleterious effects, for example as pollutants in the environment. Much effort is currently being directed towards the use of anion receptors as membrane transport agents for chloride in biological systems, as ion-pair receptors, as sensors for the detection of biologically important anionic species and in a variety of other applications. This critical review covers developments in the years 2005 and 2006 and focuses on the design of receptors based on organic frameworks. Receptors containing metals are not included.Amide based anion receptors
Secondary amides have been widely employed as hydrogen bond donor groups in anion receptors.5 Receptors containing isophthalamide or pyridine-2,6-dicarboxamide have been synthesised by a number of groups following the independent pioneering work of Crabtree et al.6,7 and B. D. Smith and Hughes8 on these systems in 1997.Kondo and co-workers have reported the synthesis of receptors 1 and 2a–c composed of three parts: an isophthalamide core, an α-amino acid spacer, and terminal amide groups appended with either a phenyl or pyridine group.9 The four amide NH groups form a complex with Y-shaped anions such as acetate and phosphate. The complexation studies demonstrate the selectivity of compound 2c toward H2PO4− (K11(H2PO4−)/K11(AcO−) > 59.9 in 0.5% DMSO–MeCN (v/v)) illustrating the importance of the terminal pyridyl groups which are presumably accepting hydrogen bonds from the dihydrogen phosphate anion (Scheme 1).
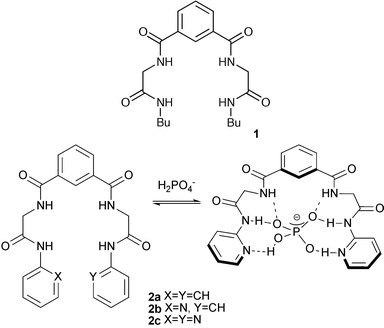 | ||
| Scheme 1 Dihydrogen phosphate complexation by receptor 2c. | ||
Gale and co-workers have previously shown that functionalised isophthalamides can form a ‘2 + 2’ double helical complex with fluoride.10 In more recent work, this group has deliberately designed receptors to form higher order complexes with anions . Specifically they have employed steric interactions in 1,3-dicarboxamidoanthraquinones 3 to ‘twist’ the isophthalamide-like anion binding site.11 Steric interactions between the amide in the 1-position and the adjacent oxygen atom force this group to twist out of plane, giving a divergent hydrogen bonding array. It was shown that receptor 3c forms a ‘2 + 2’ complex with fluoride in the solid state. This family of compounds also gives a colorimetric response to the presence of anions in solution. It has also been shown that the electrochemistry of the anthraquinone redox system dramatically changes when an appropriate ion (for example fluoride) is added to the receptor in DMSO solution. In the presence of a competitive anion , intramolecular hydrogen bonds between the amides and the semiquinone and dianion species are broken, dramatically changing the electrochemistry of this system.12
Chmielewski and Jurczak have continued their work on neutral macrocyclic tetraamides containing two 2,6-dicarboxamidopyridine or isophthalamide groups linked via short aliphatic chains.13,14 2,6-Dicarboxamidopyridines 4a–e were prepared15,16 and anion binding studies in DMSO-d6 solution revealed that the size of the receptor has a significant effect on anion affinity. Size complementarity between the putative anionic guest and the receptor cavity was found to play only a secondary role in determining selectivity, as receptor 4b was found to have the highest affinity for all the anions studied from this series of receptors (binding chloride with a stability constant of 1930 M−1vs. 65 M−1 for compound 4a). The authors postulate that this receptor has the optimal balance of preorganisation and adaptability from this series of compounds, resulting in high affinity for a variety of anionic guests .
Recently, the same group has described the synthesis and anion binding properties of macrocyclic isophthalamide receptors 5a–d.17 The stability constants of these receptors with various anions were determined by 1H NMR titrations in DMSO-d6 and, contrary to the authors' initial assumptions, the isophthalamides turned out to be weaker anionophores than their 2,6-dicarboxamidopyridine counterparts. Combined theoretical and experimental structural studies demonstrate that this unexpectedly low anion binding ability of the isophthalic acid-based receptors is due to the self-complementary nature of the isophthalic bis-amide fragments: when two such moieties are present within a sufficiently flexible macrocycle , they adopt syn–anti conformations and bind to each other by two strong intramolecular hydrogen bonds that close the macrocyclic cavity. Nevertheless, anion binding is able to break these hydrogen bonds and switch a macrocycle into a convergent all-syn conformation (Scheme 2). In a further study, a hybrid macrocycle containing both 2,6-dicarboxamidopyridine and isophthalamide anion binding sites linked via a three carbon chain was shown to have a higher affinity for anions than either of its homoaromatic congeners.18 The authors attribute this to the syn–syn conformation adopted by the 2,6-dicarboxamidopyridine enforcing the same conformation in the isophthalamide. Thus these systems do not contain intramolecular hydrogen bonding interactions as seen in compound 5.
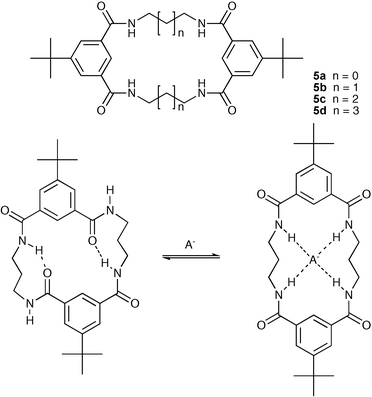 | ||
| Scheme 2 Anion complexation results in a conformational change in receptor 5b. | ||
Costero and co-workers have studied macrocycles 6a–d as part of a colorimetric displacement assay for sensing inorganic anions such as F−, AcO− and H2PO4−.19 Complexation of nitrophenolate (log K = 4.55 ± 0.03, 3.22 ± 0.22 and 4.09 ± 0.02 M−1 by compounds 6a, 6b and 6c respectively in CH3CN at 25 °C) results in a partially discoloured complex. Addition of competitive anions displaces the nitrophenolate anion and restores the yellow colour. Competition studies have been performed using UV-vis spectroscopic techniques, revealing that the most basic anions (F−, AcO− and H2PO4−) displace the nitrophenolate anion whilst other halides had little effect on the nitrophenolate complex.
Gupta and co-workers have synthesised two novel isophthalamide-based receptors N,N′-bis(phenyl)isophthalohydrazide (7) and N,N′-bis(2,4-dinitrophenyl)isophthalohydrazide (8) and studied their anion binding abilities by UV-vis spectroscopic methods in acetonitrile solution.20 These two compounds displayed selectivity for carbonate (CO32− ≫ HPO42− > F− > Cl− > SO42−). As a consequence of this selectivity, PVC based membranes containing these receptors have been prepared and investigated as carbonate selective ion selective electrodes (ISEs ), which showed a selective response to the presence of other lipophilic anions such as salicylate, ClO4− and SCN−. Thus, these sensors can be used for the selective determination of carbonate. The analytical utility of these ISEs has been demonstrated by measuring the total inorganic carbon (TCO2) in water samples.
Jeong and co-workers have synthesised a series of large 44-membered macrocycles 9a–e which can display two different diagonal binding modes.21 The unsubstituted macrocycle 9a binds naphthalene-2,6-dicarboxylate strongly through hydrogen bonds (Ka = 4500 M−1) in 40% (v/v) CD3CN–CDCl3. Introduction of an electron-withdrawing substituent (Cl) at all four corners increases the binding affinity (22000 M−1 for 9b), while that of an electron-donating substituent (pyrrolidinyl) greatly decreases it (150 M−1 for 9c). The same propensity has been observed by the authors with macrocycles 9d and 9e, bearing different substituents at two diagonal corners, with NMR experiments showing the anion favouring binding to the most electron deficient corners in these cases (Scheme 3).
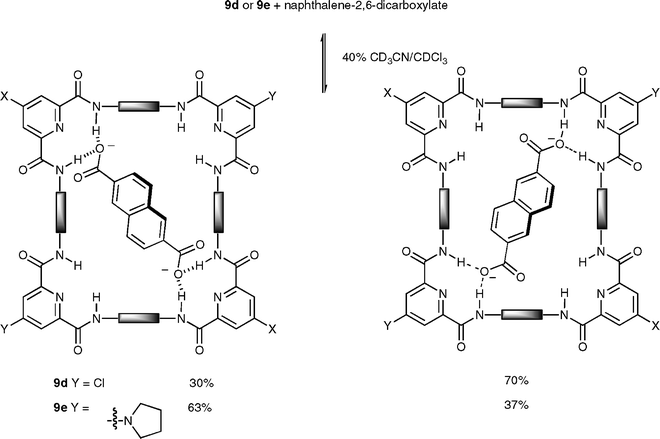 | ||
| Scheme 3 Relative populations of two different binding modes of complexes between macrocycles 9d and 9e, and naphthalene-2,6-dicarboxylate in 40% CD3CN–CDCl3 at 24 ± 1 °C. | ||
The same research group has also studied the anion binding properties of new molecular clefts based on a 2,2′-biindolyl scaffold (10a,b and 11a,b) that contain both indole and amide hydrogen bond donor groups.22 The binding properties of the compounds were evaluated in CH3CN solution by UV-vis spectroscopy, showing a dramatic increase in the association constants of up to 40-fold when the biindolyl was rigidified (for example, stability constants with bromide in CH3CN at 22 °C were found to be 2.1 × 102 M−1 with 10a and 8.7 × 103 M−1 with 11a). Stability constants were also found to be higher for the phenyl amides vs. the methyl amides , presumably due to electronic effects.
Hiratani and co-workers have investigated the molecular recognition properties of a series of crownophanes containing two hydroxy groups and two amide groups (12–13 and 15) and model compound 14 with anions such as halides, dihydrogen phosphate and acetate.23 The anion coordination ability of these species was measured by 1H NMR techniques in CDCl3 solution, and it was found that amidocrownophanes 12 and 13 bind anions with moderate affinity with the following order of selectivity: H2PO4− > F− > CH3COO− > Cl− ≫ Br− and I− (e.g. compound 12 binds H2PO4− with a stability constant of 4.42 × 102 M−1 under these conditions), whilst compounds 14, which possesses no hydroxyl groups, and 15 had no affinity for anions under these conditions. This lack of affinity must presumably be due to intramolecular hydrogen bonding interactions in the phthalamide derivatives 14 and 15 that are not present in the isophthalamide derivatives 12 and 13.
Beer and co-workers continued their work on calix[4]diquinone systems24,25 and have described the synthesis and binding properties of a novel heteroditopic calix[4]diquinone receptor 16.26 This compound demonstrates a dramatic enhancement of anion binding in the presence of a co-bound cation (Table 1) and, in some cases, strong binding with associated ion pairs where no affinity for the free ions is observed. This is the first example of a receptor where the compound displays no discernible affinity for either one of the free ions, but which binds the associated ion pair species strongly. A possible reason for this phenomenon could be the self-inhibition of the cation and anion binding sites of the molecule by intramolecular hydrogen bonding of the quinone unit by the isophthalamide, which is disrupted only by a suitable ion-pair .
He and co-workers have reported the synthesis of two fluorescent chiral receptors 17a,b containing amide units and L-tryptophan.27 Proton NMR and fluorescence studies have shown that the compounds exhibit enantioselective recognition properties with D- and L-bis(tetrabutylammonium) dibenzoyl tartrate, forming a 1 : 1 complex between host and guest . For example, compound 17a was found to bind D-dibenzoyl tartrate in DMSO with a stability constant of (7.93 ± 0.04) × 104 M−1vs. (1.28 ± 0.02) × 104 M−1 for L-dibenzoyl tartrate. Most interestingly, compound 17b bound the D-enantiomer with a stability constant of (2.61 ± 0.05) × 105 M−1 whilst the stability constant of the complex formed with the L-enantiomer was too small to calculate.
Kang and Kim have followed their previous work on diphenyl glycoluril scaffolds28,29 producing receptor 18 that contains four pendant naphthyl groups.30 This host shows selectivity for bromide, binding this anion more strongly than the other halides (Br− > Cl− > F− > I−) with a stability constant 1.2 × 105 ± 1.4 × 104 M−1 in acetonitrile. The authors suggest this selectivity is due to the complementarity of size between the receptor and bromide anion.
The same group has described the synthesis of a new enantioselective receptor 19 by incorporation of chiral building blocks onto a glycoluril core.31 Binding studies carried out using 1H NMR spectroscopy in CD3CN revealed that the receptor 19 possesses moderate enantioselectivity with a general preference for D-amino acids. The highest enantioselectivity was observed with the D- and L-enantiomers of N-Boc–LeuCO2− amongst the amino acids investigated (8.9 × 102 M−1 for the D-enantiomer vs. 2.5 × 102 M−1 for the L-).
B. D. Smith and co-workers have continued their work on ion-pair receptor systems32,33 with a detailed study of nitrate inclusion by receptor 20.34 Detailed X-ray analysis of a series of nitrate salts bound in the receptor together with solution NMR binding data in CDCl3 suggests that anisotropy in the nitrate anion may act to shield NH protons bound to it in certain geometries (when binding nitrate salts, upfield shifts of the amide NH resonances of between 0.05–0.22 ppm were observed). The authors calculated the shielding surface around nitrate and found that it is deshielding around the peripheral plane of the anion and shielding in a region above the central nitrogen.
Shinkai and co-workers have described a new dynamic bicyclic receptor 21 which has a diethynyl tetrafluorophenyl axis.35 This host displays allosteric binding, controlled by the central tetrafluorophenyl platform which functions as a molecular ‘turnstile’. Initially, the receptor adopts a closed state with the central ring in the plane of the rest of the receptor preventing the approach of guest anions . However, should one acetate bind to the interior of the receptor this forces the central ring to be perpendicular to the receptor so opening up the second binding site (Scheme 4). For example, in THF-d8–DMSO-d6 (5 : 1, v/v) solution acetate is bound with K1 = 144 ± 2 M−1 and K2 = 66 ± 3 M−1 with a Hill parameter n of 1.4, indicating cooperative binding.
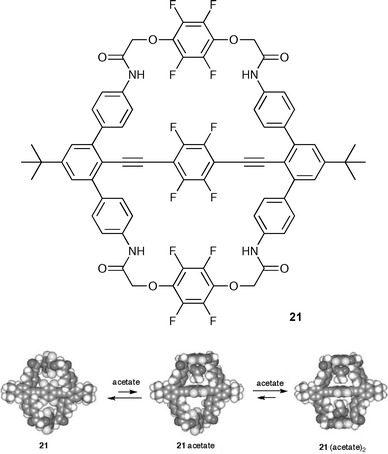 | ||
| Scheme 4 Cooperative acetate binding to 21 with the energy-minimised structures of free 21, 21·acetate 1 : 1 complex and 21·(acetate)2 1 : 2 complex. Reproduced with permission from ref. 35. Copyright © 2005 Royal Society of Chemistry. | ||
Bis-amide 22a and thioamide 22b, derivatives of azulene-5,7-dicarboxylic acid, were synthesised by Jurczak and co-workers.36 The seven-membered azulene ring gives access to a new geometry for ‘Crabtree-type isophthalamide’ analogues in syn–syn conformations. Thioamide 22b was found to bind anions about twice as strongly as the amide analogue 22a with the same selectivity (Table 2). The anion affinities of the two azulene derivatives were compared to Jurczak's dicarboxamidopyrrole 2337 which showed that the azulenes generally had a lower affinity for anions than compound 23 (excepting chloride).
He and co-workers have synthesised two chiral fluorescent macrocycles 24 and 25 containing naphthalene and amino acid units.38 The enantioselective recognition properties of these macrocycles for amino acid anions were explored using fluorescence and 1H NMR spectrometry in DMSO and DMSO-d6, respectively, indicating good enantioselectivity of compound 24. For example, 24 binds N-protected phenylalanine anions with a ratio K(D-Phe)/K(L-Phe) = 4.94.
Following on from their earlier work,39 Kubik, Otto and co-workers have presented structural and thermodynamic data on the binding properties of anion receptors composed of cyclopeptides 26–29 that complex sulfate and iodideanions with micromolar affinity in aqueous solution.40 Specifically, they showed how hydrophobic interactions between two covalently linked peptide rings that do not directly involve the guest contribute to the complexation of iodide and sulfate by two synthetic bis-cyclopeptide-based anion receptors 28 and 29. Evidence comes from X-ray structure data, the solvent dependence of the anion affinity and previous observations of the binding behaviour of structurally related monomeric cyclopeptides.39
Yang, Wu and co-workers have synthesised cyclic hexapeptide 30 which contains alternating D-α-amino and D-α-aminoxy acids.41 In dichloromethane solution, this macrocycle displays a high selectivity for chloride anions amongst the anions studied (Table 3).
| Anion | K a/M−1b | Δδmax(O–NH)c | Δδmax(NH)c |
|---|---|---|---|
| a Anions were added as concentrated CD2Cl2 solutions of Ph4PCl, Ph4PBr, Bu4NI, or Bu4NNO3. To account for dilution effects, these anion solutions also contained receptor 30 at its initial concentration (2–4 mM). b Determined by following the changes that occurred to the resonances of the aminoxy amide NH protons. c Estimated maximum change in chemical shift (ppm). d Cannot be estimated from the titration curve. | |||
| Cl− | 15000 ± 1500 | 2.39 | −1.39 |
| Br− | 910 ± 43 | 1.70 | −1.07 |
| I− | 51 ± 3 | d | d |
| NO3− | 440 ± 42 | 1.43 | −0.55 |
Chen, Huang and co-workers have synthesised the novel upper-rim anthracene bridged calix[4]arene 31.42 The close proximity of the anthracene group to the anion binding site effectively couples the fluorescence of this system to its guest binding properties. It was found that the fluorescence of the anthracene group was quenched upon addition of basic anions such as AcO− and F− in acetonitrile solution (Fig. 1). Stability constants were found to be 3200 M−1 and 800 M−1 for AcO− and F−, respectively.
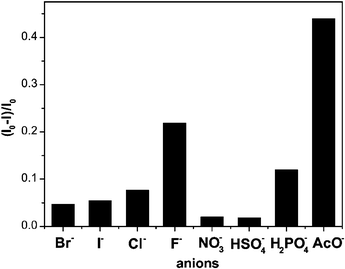 | ||
| Fig. 1 Fluorescence quenching ratio (Io − I)/Io of compound 31 with various anions (added as tetrabutylammonium salts) in CH3CN (0.4% v/v CHCl3) at λex = 370 nm. Reproduced with permission from ref. 42. © 2005 Elsevier. | ||
Receptors based on tris(2-aminoethyl)amine (tren) have been explored by a number of groups since Reinhoudt et al.'s seminal paper on these systems in 1993.43 A novel C3v-symmetrical N7-hexahomotriazacalix[3]cryptand (32) has recently been reported by Pulpoka and co-workers (Scheme 5).44 The binding abilities of 32 were evaluated by 1H NMR spectroscopy and UV-vis spectrometry in CDCl3 and DMSO solutions, respectively, showing that the receptor selectively binds halide anions (Cl− > Br−) over CH3COO−, PhCOO−, NO3−, PF6−, and ClO4−, forming complexes with 1 : 1 stoichiometry with all the anions except carboxylates (see Table 4). Presumably access to the interior of this capsule is restricted in this case, diminishing the affinity of the receptor for the more basic yet larger carboxylates. The anion complexation ability of a zinc complex of 32 was also studied by the authors.
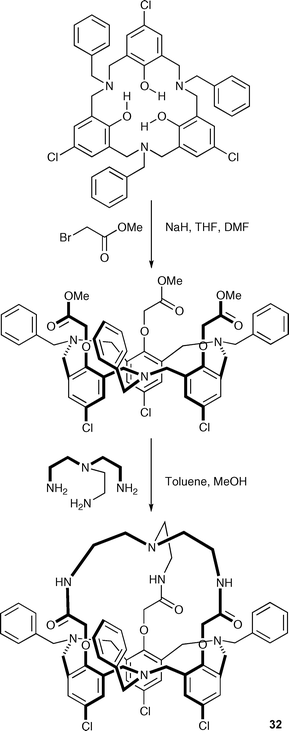 | ||
| Scheme 5 Synthesis of compound 32. | ||
| Anion | log β/M−1a |
|---|---|
| a Mean values of n ≥ 3 independent determinations, with standard deviation σn−1 on the mean in parentheses. | |
| F− | 2.78 (0.01) |
| Cl− | 4.55 (0.03) |
| Br− | 3.97 (0.01) |
| I− | 2.72 (0.01) |
| NO3− | 1.77 (0.01) |
| ClO4− | Undetermined |
| CH3COO− | β 1 = 2.92 (0.01), β2 = 6.06 (0.01) |
| PhCOO− | β 1 = 2.36 (0.06), β2 = 6.26 (0.01) |
De Namor and co-workers have studied the complexation ability of a partially lower-rim substituted calix[4]arene hydroxyamide derivative, 25,27-bis[N-(2-hydroxy-1,1-bishydroxymethylethyl)aminocarbonylmethoxy]calix[4]arene-26,28-diol (33) for cations and anions through 1H NMR spectroscopy, conductometry, spectrophotometry, and calorimetry in dipolar aprotic media.45 These studies suggest that this receptor is able to interact with divalent cations through the carbonyl, the phenolic, and the hydroxyl oxygens of the pendant arms and with inorganic anions (fluoride, dihydrogen phosphate, and pyrophosphate) through hydrogen bond formation to the amide groups.
Continuing their seminal work on amide based cryptand hosts for anionic species,46 Bowman-James and co-workers have synthesised a tricyclic receptor 34 consisting of two monocyclic macrocycles joined by two bridging ethylene linkages.47 Binding studies of 34 with anions by using 1H NMR techniques revealed remarkably selective binding and high affinity for the bifluorideFHF− ion (Ka = 5500 M−1) followed by H2PO4− (Ka = 740 M−1), N3− (Ka = 340 M−1) and CH3COO− (Ka = 100 M−1), with negligible binding for HSO4−, Cl−, Br−, I−, NO3− and ClO4− ions in DMSO-d6. The crystal structure of the bifluoride complex (nBu4N[1(FHF)]·3H2O) was also elucidated (Fig. 2), and shows that the FHF− anion is encapsulated within the receptor in the solid state, bound by four amide NH groups.
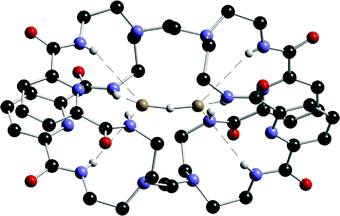 | ||
| Fig. 2 The X-ray crystal structure of the bifluoride complex of 34. | ||
Beer and co-workers have continued their work on anion templated assembly and with a detailed study of the effect of anion template, strength of ion-pair thread association and macrocyclic ring size on pseudorotaxane formation (Fig. 3). A series of different threads and macrocycles were studied, including macrocycles containing isophthalamide groups 35a–c and threads 36–40.48 Proton NMR studies have demonstrated that the penetration of the ion pair into the macrocycle and the stability of the pseudorotaxane depends critically on the nature of the halide anion template (chloride was the optimum template), π–π stacking interactions between macrocycle and thread, CH hydrogen bonding, size effects and also, importantly, on the strength of the ion-pairing between the anion template and the cationic thread. For example, the association of macrocycles 35a, 35b and 35c with threads 36 and 37 have been measured with the results shown in Table 5. A variety of crystal structures confirmed pseudorotaxane formation in the solid state in a number of cases. Further examples of anion templated assembly are discussed later in this review.
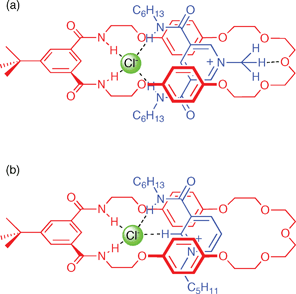 | ||
| Fig. 3 (a) 3,5-Dicarboxamidopyridinium-based pseudorotaxane assembly. (b) Nicotinamide pseudorotaxane assembly. | ||
| Thread | Macrocycle | ||
|---|---|---|---|
| 35a | 35b | 35c | |
| 36a | 9500 | 2400 | 980 |
| 37a | 1900 | 950 | 320 |
| 36b | 610 | 700 | 120 |
| 37b | 200 | 240 | 180 |
| 36c | <50 | 65 | <50 |
| 37c | 65 | <50 | 50 |
Zhang and Echegoyen have immobilised a tris-amide receptor functionalised cyclotriveratrylene on a gold surface forming a self-assembled monolayer .49 Proton NMR titrations and UV-vis spectroscopy showed that the receptor is selective for acetate from amongst the anions studied (Cl−, Br−, NO3−, HSO4−, H2PO4−) in CDCl3. The cyclotriveratrylene thioctic ester 41 was immobilised on a gold support and impedance spectroscopy used to study anion complexation using Fe(CN)63−/4− as a redox probe. Impedance responses employing both negatively and positively charged redox couples have confirmed acetate was bound selectively on the modified surface in aqueous media.
Tucker and co-workers have synthesised two tripodal receptors 42a and 42b and evaluated their anion binding properties with phosphate and vanadateanions in different organic solvents.50 They found that in CD3CN solution vanadate was bound by receptor 42a in a similar fashion to its interaction with dihydrogen phosphate. However, receptor 42b in CD3CN–CD2Cl2 produced a very different response with vanadate by 1H NMR titration , with an upfield shift of the amide NH protons observed. The authors suggest that this is indicative of the formation of a N–V bond between the receptor and anion .
The application of receptors as membrane transport agents for anions with potential future application in the treatment of channelopathies such as cystic fibrosis (CF) is attracting increasing interest.51 J. T. Davis and co-workers have continued their studies on the transport of anions across lipid bilayer membranes; studies with tripodal amides 43a and 43b in egg yolk phosphatidylcholine (EYPC) liposomes showed that receptor 43a selectively transports NO3− over Cl− in a H+–NO3− cotransport process (for examples of H+–Cl− cotransport please see below).52 This is the first example of a synthetic transporter displaying such selectivity and in the future will allow a new approach to inducing pH changes in cells containing NO3−/Cl− gradients.
J. T. Davis and co-workers have also continued their work on calixarene and oligophenoxyacetamide-based53,54 membrane transport agents for chloride and HCl. Previous work had shown that tetraamide calix[4]arenes in the 1,3-alternate conformation form channels across liposomal membranes that allow chloride to pass through. Tetraamide functionalised calixarenes 44a and 44b were synthesised in the partial cone conformation.55 Both compounds were shown to bind chloride in CDCl3 solution, albeit with low stability constants (Ka = 10–20 M−1). However, studies in dipalmitoyl phosphatidylcholine (DPPC) liposomes at 43 °C showed that only compound 44a transports chloride anions. By lowering the temperature to 37 °C the lipid undergoes a phase change to a gel phase, conditions under which compound 44a shows a non-linear dependence of chloride transport activity with concentration, a result consistent with the formation of membrane active aggregates. Solid state evidence shows that ‘inverted’ amide NH in 44a forms an intramolecular hydrogen bond with the ether oxygen atom in the same chain whilst in 44b the equivalent amide NH forms intermolecular hydrogen bonds giving a significantly more staggered arrangement of calixarenes in the solid state. The authors hypothesise that the dramatically different packing arrangement is also reflected in aggregate formation by these species in membranes. Interestingly, it was found that chloride transport by 44a was inhibited upon addition of 44b.
Gokel and co-workers have pioneered the application of chloride transport by synthetic molecules in lipid bilayer membranes. In a series of recent papers56–59 this group has shown that amphiphilic peptide chains of general structure (R1)2NCOCH2OCH2CO–(Gly)3Pro(Gly)3–OR can render lipid bilayer membranes permeable to chloride. In important recent work, this group has studied the effects of these compounds on epithelial cells.60 Specifically, the biological activity of (H37C18)2NCOCH2OCH2CO–(Gly)3Pro(Gly)3–OCH2Ph57 was compared with that of model compound (H37C18)2NCOCH2OCH2CO–(Gly)3Leu(Gly)3–OCH2Ph (used as a control as it is six times less effective as a chloride transporter than the proline containing analogue). Studies showed that the proline containing peptide functioned as a chloride transporter in the epithelial cells whereas the model compound did not, demonstrating that synthetic compounds have real potential in the treatment of diseases such as CF.
Sulfonamide based anion receptors
Sulfonamides have also been widely employed as anion binding groups in hydrogen bond donor organic based receptors. These groups are more acidic than the analogous secondary amides and hence are expected to form more stable complexes with anions .Atmospheric pressure chemical ionisation mass spectrometry (APCI-MS) was employed by Kavallieratos and co-workers to identify anion complexes (M–Cl−, M–Br−, M–I−, M–NO3−) formed by sulfonamide receptors 45a, 45b and 46 in dichloromethane.61 This method offers the advantage that multiply charged species are not detected. Additionally, larger supramolecular ions are generally not detected meaning that species that are detected by this method are generally the more stable supramolecular complexes. Kavallieratos showed that complexes with the above anions could easily be detected using this technique.
Berryman, Johnson and co-workers have designed and synthesised a pair of sulfonamide -based receptors 47a and 47b that differ only by the substituents on their aromatic rings.62 Proton NMR spectroscopic data obtained from each receptor with an array of halides (Table 6) show a trend in stability constant strength for receptor 47a and the halides chloride, bromide and iodide that cannot solely be explained by the strength of the hydrogen bond with each anion , suggesting that there is an attractive interaction between the electron deficient aromatic ring in receptor 47a and the anionic guests underscoring the hypothesis that electron deficient aromatics can be used as a component of a design strategy to target anions in solution.
Pyrrole and indole based anion receptors
Amongst neutral hydrogen bond donor groups used to bind anions , pyrroles and indoles differ from the amides and sulfonamides discussed above and the urea derivatives discussed below as they do not contain a hydrogen bond acceptor group.Arguably the simplest pyrrole based anion receptor is meso-octamethylcalix[4]pyrrole48 which can be synthesised in one step by the acid catalysed condensation of pyrrole with acetone. In 1996, Sessler and co-workers demonstrated that this macrocycle binds fluoride anions selectively in CD2Cl2 solution.63 More recently, evidence began to emerge that the selectivity of calix[4]pyrrole for anions was very dependent on the nature of the solvent64 and that fluoride selectivity could be lost under certain conditions. A detailed study by Sessler, Schmidtchen, Gale et al. with several chloride salts in solution by isothermal titration calorimetry (ITC) and 1H NMR spectroscopic titrations and in the solid state by X-ray crystallography65 showed no dependence of stability constant on method of measurement. However, the resulting stability constants were found to be highly dependent on the choice of solvent with Kas ranging from 102–105 M−1. When dichloromethane was used as solvent, a strong dependence on the countercation was also seen, with the Kas for the interaction with chloride ranging from 102–104 M−1, demonstrating that the choice of countercation can be significant.
Direct evidence for anion pair complexation by compound 48 came from solution state and solid state studies of compound 48 with a variety of caesium and imidazolium salts. Moyer, Sessler, Gale et al. demonstrated66 that anion complexes of 48 can include large charge diffuse cations such as caesium and imidazolium in the anion -induced calix[4]pyrrole cup. Shielding of the imidazolium CH hydrogen atoms in CD2Cl2 solution in the presence of calixpyrrole and an anion which binds to calixpyrrole was presented as evidence of solution complexation of ion pairs . This work was extended to show that N-ethylpyridinium cations also bind within the calix[4]pyrrole cavity in anion complexes (Fig. 4).67
![N-Ethylpyridinium chloride–meso-octamethylcalix[4]pyrrole complex (solvent omitted for clarity). The macrocycle is rendered transparent and the pyridinium carbons and hydrogens in red to illustrate the degree of penetration of the cation into the calixpyrrole cup shaped cavity. Reproduced with permission from ref. 67 (Fig. 2). Copyright © 2006 The Royal Society of Chemistry.](/image/article/2008/CS/b715825d/b715825d-f4.gif) | ||
| Fig. 4 N-Ethylpyridinium chloride–meso-octamethylcalix[4]pyrrole complex (solvent omitted for clarity). The macrocycle is rendered transparent and the pyridinium carbons and hydrogens in red to illustrate the degree of penetration of the cation into the calixpyrrole cup shaped cavity. Reproduced with permission from ref. 67 (Fig. 2). Copyright © 2006 The Royal Society of Chemistry. | ||
N-Confused calix[4]pyrrole 49 is formed in moderate yield during the acid catalysed condensation of pyrrole and acetone to form compound 48. The first crystal structures of imidazolium chloride complexes of 49 show that compound 49 employs three NH groups to bind chloride in the solid state (Fig. 5).68 Solution state studies by Anzenbacher and co-workers show that the β-CH on the inverted pyrrole ring is involved in hydrogen bonding to bound anion guests in solution.69,70
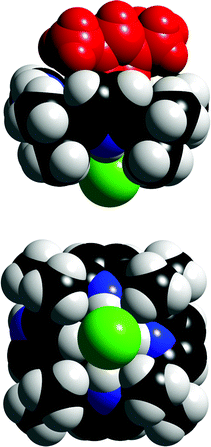 | ||
| Fig. 5 Space filling representations of the side and bottom views of the ethylmethylimidazolium chloride complex of compound 49. The bottom view of the complex illustrates the displacement of chloride towards the NH groups. Reproduced with permission from ref. 68 (Fig. 2). Copyright © 2006 The Royal Society of Chemistry. | ||
Sessler and co-workers have continued their work on fluorinated calixpyrroles. The use of 3,4-difluoropyrrole in the condensation reaction allows access to kinetic products with calix[4–8]pyrroles being isolated in the yields shown in Scheme 6.71
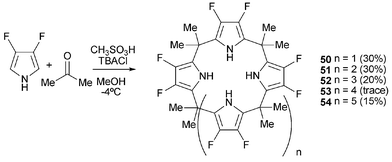 | ||
| Scheme 6 The synthesis of fluorinated calixpyrroles. | ||
Detailed binding studies by ITC in CH3CN and DMSO show size effects play a major role in the selectivity of this set of compounds (Table 7).
| Anion | Solvent | 48 | 50 | 51 | 52 |
|---|---|---|---|---|---|
| a The host (macrocycle ) solution was titrated with the guest (anion ) solution unless otherwise indicated. b Stability constant too low to be determined by ITC. c A good fit of the data to a 1 : 1 binding profile could not be made. d The guest solution was titrated with the host solution (reverse titration ). | |||||
| Cl− | CH3CN | 140000 | 53000072 | 41000 | 280000 |
| DMSO | 130072 | 150072 | |||
| Br− | CH3CN | 340072 | 850072 | 4500 | 110000 |
| I− | CH3CN | b | b | b | 610 |
| CH3CO2− | CH3CN | 29000073 | 1900000 | c | c |
| CH3CN d | 350000 | 2400000 | 520000 | 1000000 | |
| DMSO | 6100 | 48000 | |||
| C6H5CO2− | CH3CN | 12000073 | 1200000 | 52000 | c |
| CH3CN d | 170000 | 1400000 | 83000 | 580000 | |
| H2PO4− | DMSO | 5100 | 17000 | 9600 | 15000 |
| H2PO2− | DMSO | b | 3300 | 13000 | 35000 |
An alternative approach to the synthesis of expanded calix[4]pyrroles is to construct them from bipyrrole.74 Recently, Sessler has shown that macrocycle 56 has a particularly high affinity for chloride (Ka = 2900000 M−1 in acetonitrile).75 Solid state evidence shows that this macrocycle can adopt a V-shaped conformation when binding this anion , so employing all eight hydrogen bond donor NH groups (Fig. 6), which is presumably the reason for the high affinity in solution. The calix[3]bipyrrole analogue of this receptor (55) has a lower affinity for chloride (Ka = 110000 M−1 in acetonitrile). In addition to this work, this group has also synthesised calixpyrrole analogues containing other heterocyclic rings.76
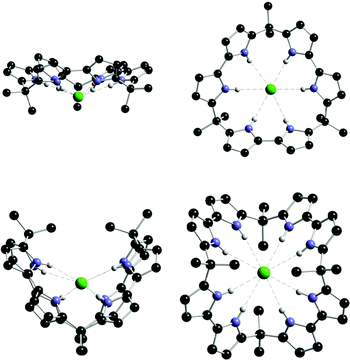 | ||
| Fig. 6 Side and top views of the X-ray crystal structures of the chloride complexes of 55 (top) and 56 (bottom). | ||
Lee, Sessler and co-workers have synthesised a variety of ‘strapped’ calixpyrroles including the isophthalamide strapped system 57.77 This bridge was found to increase the binding affinity for halide anions in acetonitrile solution but failed to show an appreciable size-based selectivity amongst the anions (Br−, Cl− and I−) due to the tilt of the strap to one side of the receptor, allowing the formation of 2 : 1 (receptor : anion ) complexes. Fluorescent analogues of this strapped system have been synthesised that contain coumarin and function as fluorescent sensors for anionic guests .78
Kałedkowski and Trochimczuk have synthesised a number of calixpyrrole based resins including resin 58, prepared by reacting the calix[4]pyrrole prepared from 4-hydroxyphenylmethylketone and pyrrole79 as a mixture of isomers with formaldehyde and sodium hydroxide.80 This material was shown to selectively extract fluoride anions from dry acetonitrile solution, complexing up to 96.5% of fluorides from 10−3 M solutions.
Continuing on from earlier work,81 Sessler, Jeppesen and co-workers have described the synthesis of two new tetrathiafulvalene (TTF)-calix[4]pyrroles, namely monotetrathiafulvalene-calix[4]pyrrole 59a and bis(tetrathiafulvalene)-calix[4]pyrrole 59b, as well as their anion binding properties using 1H NMR spectroscopic and ITC techniques in CD2Cl2, acetone-d6 and CD3CN.82 These studies provide support for the notion that the incorporation of one or more electron rich TTF subunits into the calix[4]pyrrole backbone improves the anion binding abilities of the receptors, while also affecting their selectivity. Halide anion complexation was also monitored by cyclic voltammetry studies in 1,2-dichloroethane, which provided evidence of an anion -dependent electrochemical response with Cl− and Br− ions.
The same researchers have studied the treatment of a solution of a tetrathiafulvalene-functionalised calixpyrrole (60) with chloride anions in dichloromethane, affording a bowl-like receptor that is able to encapsulate C60 in a 2 : 1 (calixpyrrole–chloride : C60) barrel-like manner.83 As was observed with cation inclusion in the calixpyrrole cup, initial anion binding in the present ensemble serves as a “trigger” for the crucial reorganisation of the calix[4]pyrrole receptor needed to produce a “cone” conformation, wherein multiple TTF units are appropriately arranged to allow for fullerene binding. The binding constants were determined by UV-vis and fluorescence titration experiments in dichloromethane as solvent, leading to the formation of the putative 2 : 1 complex between 60·Cl− and C60 with K1 = 2.3 × 103 M−1 and K2 = 1.3 × 104 M−1 reflecting the formation of C60⊂60·Cl− and C60⊂(60·Cl−)2, respectively. Moreover, the dramatic colour change that accompanies the C60 binding event allows the recognition process to be followed easily, and thus the presence of this fullerene to be readily “sensed”.
Dehaen and co-workers have reported the first example of substitution reaction in the free α-position of N-confused calix[4]pyrroles 61a–e84 by azo-coupling with various arenediazonium salts. The stabilities of anion complexes formed with α-arylazo-N-confused calix[4]pyrroles were found to be slightly higher than those of unsubstituted N-confused calix[4]pyrrole.
Knoevenagel condensations of 2-formyl-octamethylcalix[4]pyrrole with selected 1,3-indanedione derivatives to yield calix[4]pyrrole anion sensors (62a–d) with push–pull chromophores displaying strong intramolecular charge transfer have been performed by Anzenbacher and Nishiyabu.85 Visual inspection of solutions of these sensors in CH2Cl2 or DMSO with 0.5% water, before and after the addition of anion salts, showed a dramatic change in colour in the case of fluoride, acetate and, to a lesser extent, dihydrogen phosphate, suggesting strong binding, whereas the addition of chloride, bromide, or nitrate resulted in weak or no changes in colour. The 1H NMR titrations in DMSO-d6 with 0.5% water show that the sensors within the series show increasing affinity for anions and dramatically enhanced selectivity for acetatevs. chloride (see Table 8).
In a continuation of their earlier work,86 Anzenbacher and co-workers have described the synthesis and anion binding properties of chromogenic octamethylcalix[4]pyrroles 63a and 64a (OMCPs) as well as their N-confused octamethylcalix[4]pyrrole isomers 63b and 64b (NC–OMCPs).69 The chromogenic NC–OMCPs showed significantly stronger anion -induced colour changes compared to the corresponding chromogenic OMCPs, and the absorption spectroscopy titrations in DMSO indicated that chromogenic OMCPs (F− > HP2O73− > AcO− > H2PO4− > Cl−) and NC–OMCPs (AcO− > F− > HP2O73− > H2PO4− > Cl−) also possess different anion binding selectivity (see Table 9). Moreover, preliminary colorimetric microassays using chromogenic calixpyrroles embedded in partially hydrophilic polyurethane matrices allow for observation of analyte-specific changes in colour when the anions are administered in the form of their aqueous solutions and in the presence of weakly competing anions .
| Anion a | 63a | 63b | 64a | 64b |
|---|---|---|---|---|
| a Anions were used in the form of their Bu4N+ (TBA) salts. The errors in all fits are <15%. b Association constants were calculated on the assumption that pyrophosphate forms a dimer in DMSO. | ||||
| F− | >106 | 7240 | >106 | >106 |
| Cl− | 741 | <50 | 1370 | 319 |
| AcO− | 8540 | 16600 | 242000 | >106 |
| H2PO4− | 3330 | 430 | 5230 | 810000 |
| HP2O73− b | 92200 | 5650 | 584000 | N. D. |
| K AcO− /KCl− | 12 | 330 | 176 | >10000 |
| K AcO− /KH2PO4− | 3 | 39 | 46 | 25 |
Black, Colbran and co-workers have synthesised two new meso-indanyl-substituted calix[4]pyrrole receptors 65 and 6687 in order to study the effect of meso-substitution on anion affinity. In CD3CN (0.04% w/w H2O), 65 has shown a higher affinity for chloride ion than 48 (Ka respectively 5.2 (± 0.8) × 104 M−1 and 2.6 (± 1.4) × 105 M−1). Interestingly, the authors found mass spectroscopic evidence from negative ion ESI-FTICR experiments that compound 65 may be deprotonated by fluoride.
De Namor and co-workers have described the synthesis of two isomeric meso-tetramethyltetrakis(3-hydroxyphenyl)calix[4]pyrroles, 67-ααββ and 68-αβαβ and have investigated their anion binding properties by 1H NMR and conductometric titrations in acetonitrile and N,N-dimethylformamide solutions.88 These investigations revealed that 67-ααββ shows selectivity for H2PO4− in acetonitrile while its isomer 68-αβαβ is selective for fluoride. Moreover, using thermodynamic, conductometric and calorimetric data this paper demonstrates that the enthalpy parameter may be a suitable reporter of the number of hydrogen bonds formed when calix[4]pyrrole and its derivatives interact with the dihydrogen phosphateanion in acetonitrile, and highlight the importance of the medium effect on the stability of the complex reflecting the inherent nature of the solvent and its highly significant involvement in the complexation process.
Acyclic pyrrole-based anion receptors have also been the subject of intense interest recently,89,90 as these systems may have potential application in the transport of chloride across lipid bilayers. The prodigiosins are a family of naturally occurring pyrrole alkaloids 91 produced by organisms such as Streptomyces and Serratia.92,93 They have the general structure 69 and show a range of useful biological activities including induction of tumour cell apoptosis and toxicity against fungi, bacteria and the malaria parasite. There is evidence that these species promote the co-transport of HCl across lipid bilayer membranes94—however, it is not clear whether this ability is the cause of their biological activity. This has prompted a number of groups to study the chloride transport ability of the prodigiosins and their analogues.
In a very interesting recent study, J. T. Davis and Seganish have shown that prodigiosin 69 (R1 = H, R2 = C5H11, R3 = Me) can also transport chloridevia an antiport mechanism, i.e. exchanging anions across a lipid bilayer, in addition to functioning as an H+/Cl−symporter.95
Gale, B. D. Smith and co-workers recently reported the synthesis of amidopyrroles 70 and 71.96 Like the prodigiosins, these compounds contain two hydrogen bond donor groups and a basic site that may be protonated. The crystal structure of the HCl complex of compound 70 shows that the receptor forms a “2 + 2” complex with HCl with two chloride anions bound, each by three NH hydrogen bonds, one each from the imidazolium ring of one receptor and the amide and pyrrole NH groups of the other receptor in the dimer (Fig. 7).
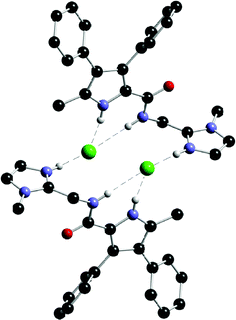 | ||
| Fig. 7 Crystal structure of the HCl complex of 70 showing the formation of a ‘2 + 2’ hydrogen bonded dimer in the solid state. | ||
Transport studies, conducted using POPC (1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine)/cholesterol vesicles showed that, in the presence of a pH gradient with low pH (high HCl concentration) inside the vesicle, receptor 70 can complex, cotransport across the vesicle membrane and release HCl much more efficiently than pyridyl analogue 71 (Scheme 7). A chloride selective electrode was used to monitor chloride release whilst release of protons from the vesicle was confirmed by the use of a pH sensitive dye (Oregon Green). Presumably the difference in transport ability between 70 and 71 is due to a combination of pKa and geometry effects. Interestingly, in the absence of a pH gradient, compound 70 could still release chloride from the vesicles, although at a slower rate than in the presence of a pH gradient.
 | ||
| Scheme 7 | ||
Sessler and co-workers have investigated the propensity of linear bi- and tripyrrolic fragments to complex and transport chloride.97Dipyrromethenes 72 and 73 were compared with prodigiosins 74, 75 and 76.
The X-ray crystal structure of the protonated chloride complex of prodigiosin 75 revealed that the protonated species binds chloride with three hydrogen bonds (Fig. 8).
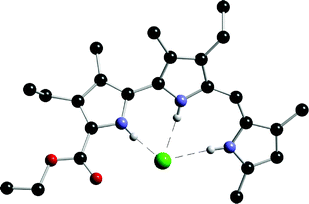 | ||
| Fig. 8 The X-ray crystal structure of the complex formed between monoprotonated prodigiosin 75 and chloride. | ||
Experiments in POPC/POPS (1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-L-serine) liposomes were conducted monitoring chloride efflux triggered by these receptors as a function of time (Fig. 9). The anticancer properties of the receptors were measured using a cell proliferationassay . pH changes demonstrated that the receptors were functioning as H+/Cl−symporters whilst the cell assays showed that compounds 72, 73, 74 and 76 all exhibited significant cytotoxic activity with 100% cancer cells killed at a concentration of 40 µM in the cell lines studied.
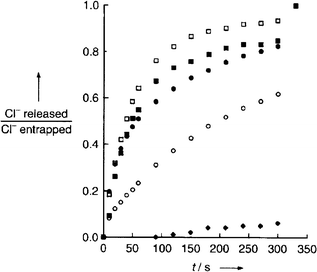 | ||
| Fig. 9 Time dependent efflux of chloride ion from 200 nm vesicles loaded with a solution of NaCl (500 mM) and suspended in a solution of NaNO3 (500 mM) and triethylsilane (5 mM) adjusted to pH 7.4. Prodigiosins are indicated by filled markers (● 76; ■ 74; ◆ 75); dipyrromethenes are indicated by open markers (□ 72; ○ 73). Reproduced with permission from ref. 97. Copyright © Wiley-VCH 2005. | ||
A new bis(pyrrol-2-yl)-2,5-diamidopyrrole (77) has been synthesised by Sessler, Gale and co-workers, and its binding abilities studied by 1H NMR titration techniques in DMSO-d6 solution.98 The authors found that receptor 77 showed a significantly higher affinity for oxo-anions such as acetate (Ka = 10300 M−1) than previous generation 2,5-dicarboxamidopyrroles (78; Ka = 560 M−1 measured in DMSO-d6–0.5% water),99 results that can be rationalised by the presence of the two new pyrrole rings in 77 and hence a beneficial increase in the number of hydrogen bond donor sites as compared to ‘parent’ compound 78.
Following on from their earlier work,100 Sun and co-workers have reported the synthesis of compounds 79 and 80 featuring the dipyrrole carboxamide moiety for anion recognition.101 The ability of 79 and 80 to complex anions was explored with UV-vis absorption and fluorescence spectrometry using a CH3CN–H2O mixture (90 : 10, v/v) as solvent, showing that both 79 and 80 responded to only cyanide anion resulting in a colour change from colourless to yellow and the fluorescence change for compound 79 from blue to green.
Sun and co-workers have synthesised a series of dipyrrolylquinoxaline (DPQ)-containing monomer (81a) and polymers (81b and 81c) which were employed as chromogenic and fluorescent chemosensors for inorganic anions .102Anion binding studies were conducted using UV-vis, fluorescence and 1H NMR spectroscopic techniques in CH2Cl2 and DMSO-d6 solution, respectively, and the following binding affinity order for all the compounds examined was found: F− > HP2O7−3 > CN− > AcO− > H2PO4− ≈ Cl− ≈ Br− ≈ I− ≈ NO3−. The sensitivity of the DPQ-based chemosensor was found to display a 34-fold enhancement by incorporation into the conjugated polymer. However, in the presence of fluoride or pyrophosphate, the colorimetric responses and fluorescence quenching observed in chemosensors 81a–c were found to be due to deprotonation of the N–H proton of the pyrrole units, so the anion selectivity is primarily determined by the relative basicity of the anions .
Anzenbacher and co-workers have synthesised DPQ analogues 82a and 82b103 containing quinone groups which combine colorimetric and electrochemical responses to anionic guests into single receptors. As each receptor has effectively two output channels, comparison of the colorimetric and electrochemical data can be used to determine the nature of the anionic substrate present, as opposed to systems with a single output channel which may not provide a unique response to each anionic guest .
The synthesis, characterisation and ion binding studies of 2,3-di(1H-2-pyrrolyl)pyrido[2,3-b]pyrazine (83) have been described by Samanta and co-workers.104Titration studies of compound 83 with different cations and anions were carried out in CH3CN using UV-vis spectroscopic techniques, revealing a binding constant for fluoride of 4.9 × 103 M−1. Fluoride also triggers a colour change in acetonitrile solutions of 83 from yellowish green to red.
Cheng and co-workers have synthesised a new pyrrole-based tripodal anion receptor 84 and studied its anion binding properties by X-ray crystallography, 1H NMR spectroscopy, and ESI-MS, revealing a selectivity for the more basic H2PO4− and F− ions.105
Maeda and Kusunose have synthesised a variety of receptors based upon dipyrrolylketone difluoroboron complexes (85a and 85b).106 These receptors employ both NH and CH interactions107 to bind anionic guests . Stability constants of compound 85b in CH2Cl2 at room temperature determined by UV-vis titration techniques with tetrabutylammonium anion salts revealed a selectivity for acetate,108 with this anion bound with a stability constant of 110000 M−1vs. 81000 M−1 for fluoride, 13000 M−1 for H2PO4− and 2000 M−1 for chloride. Analogues containing 3,4-difluoropyrrole units were shown to have enhanced anion affinities.
Beer and co-workers have synthesised a series of simple preorganised indolo[2,3-a]carbazole derivatives 86a–d.109 Proton NMR titrations in acetone with these receptors show that all the compounds are selective for benzoate followed by H2PO4− > F− > Cl− > HSO4−. In acetone solution, a significant fluorescence enhancement was observed upon addition of fluoride, chloride and dihydrogen phosphate. The crystal structure of the fluoride complex of 86b is shown in Fig. 10 showing the formation of an unusual 2 : 1 receptor : anion stoichiometry complex in the solid state.
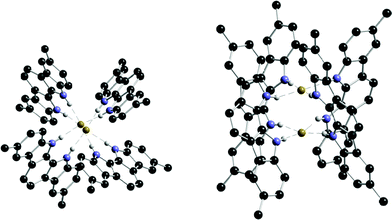 | ||
| Fig. 10 Top (left) and side (right) views of the crystal structure of the 2 : 1 complex of TBAF with receptor 86b. Countercation and non-acidic hydrogen atoms omitted for clarity. | ||
Jeong and co-workers have employed indoles and indolocarbazoles in the very rigid alkyne linked macrocyclic receptors 87a and 87b.110Anion affinity was measured by UV-vis titration techniques in acetonitrile. The results, shown in Table 10, reveal that both 87a and 87b have a high affinity for anions , binding fluoride selectively under these conditions. The crystal structure of the chloride complex of 87b (Fig. 11) shows chloride perched above the plane of the macrocyclic ring. Presumably, the smaller fluoride anion can fit more snugly in the macrocyclic cavity and hence is bound with higher affinity. Interestingly, in CD3CN solution, slow exchange was observed on the NMR timescale at ambient temperature upon addition of all the putative anionic guests , including the weakly bound Br− and I− anions.
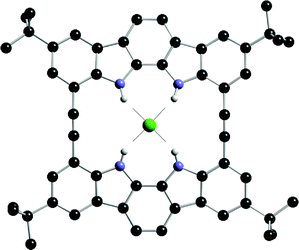 | ||
| Fig. 11 The crystal structure of the chloride complex of receptor 87b. Non-acidic hydrogen atoms and countercation omitted for clarity. | ||
| Anion | Stability constant (Ka)/M−1 | |
|---|---|---|
| Receptor 87a | Receptor 87b | |
| a The stability constants between the macrocycles and fluoride anions were determined by competition experiments with chloride. | ||
| F− a | 2.0 × 108 | 5.6 × 108 |
| AcO− | 5.9 × 106 | 6.5 × 106 |
| H2PO4− | 2.1 × 106 | 3.2 × 106 |
| Cl− | 1.5 × 106 | 2.1 × 106 |
| N3− | 8.8 × 105 | 9.1 × 105 |
| HSO4− | 6.5 × 105 | 6.8 × 105 |
| NO3− | 3.9 × 105 | 3.9 × 105 |
| CN− | 6.5 × 104 | 7.5 × 104 |
| Br− | K 1 = 1.9 × 103 | K 1 = 1.9 × 103 |
| K 2 = 10 | K 2 = 14 | |
| I− | K 1 = 3.1 × 102 | K 1 = 3.0 × 102 |
| K 2 = 6 | K 2 = 5 | |
Kwon and Jeong have prepared a dihydrogen phosphate receptor 88 containing both hydrogen bond donors and acceptors by incorporating two pyridyl units into a preorganised biindole scaffold.111 Receptor 88 strongly and selectively binds dihydrogen phosphatevia multiple hydrogen bonds with an association constant of 1.1 × 105 M−1 in CH3CN as determined by UV-vis spectroscopy. The high selectivity toward the target anion over other anions is presumably due to two additional hydrogen bonds between the phosphate hydroxyl groups and the pyridyl nitrogens that can be formed when binding this diprotic anion .
Sessler and co-workers have reported the synthesis of a new series of 2,3-diindol-3′-yl quinoxalines (89a,b) and studied their anion recognition properties by UV-vis absorption titrations in dichloromethane solution.112 These new indole-based receptors proved to have a particular affinity for dihydrogen phosphate, binding this anion selectively with stability constants of 6800 and 20000 M−1, for compounds 89a and 89b, respectively. Moreover, receptor 89b allows for the visual detection of this anion via an anion -triggered colour change (Fig. 12).
 | ||
| Fig. 12 Colour changes observed upon the addition of anions (10 equiv.) to otherwise identical solutions of receptor 89b (4.34 × 10−5 M in dichloromethane. From left to right: F− + 89, Cl− + 89, BzO− + 89, HSO4− + 89, H2PO4− + 89, 89. Reproduced with permission from ref. 112. Copyright © 2006 ACS. | ||
Urea and thiourea based anion receptors
Ureas and thiourea groups are excellent receptors for oxoanions such as carboxylates and phosphates to which they can donate two hydrogen bonds. Their synthetic accessibility has allowed their inclusion in a wide variety of anion receptors with much effort still devoted to the synthesis and study of these effective receptor systems.5Fabbrizzi and co-workers have synthesised two chiral receptors using the R,R or S,S enantiomeric forms of trans-1,2-cyclohexane by reaction with 4-nitrophenylisocyanate.113 The S,S enantiomer 90 forms a hydrogen-bonded complex with the biologically important D-2,3-diphosphoglycerate anion , with a stability constant twice that formed with the R,R enantiomer in DMSO-d6.
Fabbrizzi and co-workers have also conducted a number of studies on anion triggered deprotonation of ureas and thioureas. In organic solution, basic anions such as fluoride and acetate have been shown to deprotonate a variety of neutral hydrogen bond donor receptor systems.89,114,115 Deprotonation processes are often driven by the formation of a particularly stable species such as HF2−. Fabbrizzi and co-workers have compared the anion complexation properties of urea 91a and thiourea 91b.116 Thiourea 91b is more acidic than urea 91a and deprotonates in the presence of anions such as fluoride, carboxylates and dihydrogen phosphate whereas the less acidic receptor 91a is deprotonated only by the most basic anion studied, fluoride in DMSO solution.
Deprotonation processes can be used to colorimetrically sense anions .117,118 Fabbrizzi and co-workers have synthesised chemosensor 92 containing a urea group substituted with two chromophoric electron-withdrawing naphthalenimide subunits.119,120 In DMSO, anions such as acetate and dihydrogen phosphate were found to induce a single deprotonation of the receptor (Scheme 8), whilst more basic fluoride and hydroxide induced double deprotonation. The deprotonated species were shown to be red (LH−) and blue (L2−) respectively (Fig. 13).
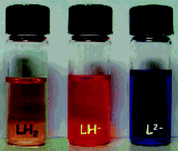 | ||
| Fig. 13 Colour changes observed upon addition of TBAF to a DMSO solution of receptor 92 (= LH2). Left to right: free receptor (dominant species LH2); plus 5 equiv. TBAF (dominant species LH−); plus 40 equiv. TBAF (dominant species L2−) Reproduced with permission from ref. 119. Copyright © 2005 ACS. | ||
Similar studies with (benzylideneamino)thiourea receptors 93a–g in acetonitrile showed fluoride triggered monodeprotonation whilst oxo-anions such as acetate formed 1 : 1 complexes with the receptors.121
Matthews, Gunnlaugsson and Quinlan have reported the synthesis of amidourea-based colorimetric anion sensors 94 and 95.122 The evaluation of these compounds as colorimetric sensors was studied using UV-vis spectroscopy with anions such as acetate, fluoride, dihydrogen phosphate and hydrogen pyrophosphate (HP2O73−) in DMSO. Whilst both sensors gave rise to red shifts in their absorption spectra in the presence of anions , addition of F− or HP2O73− gave rise to significant changes in the UV-vis spectra of the receptors with concomitant colour changes from yellow to purple, which were visible to the naked eye. The authors attribute the colour changes to a combination of anion complexation and deprotonation.
He and co-workers have reported the synthesis of two calix[4]arene-based chiral chromogenic receptors (96a and 96b) which contain both thiourea and amino acid binding units.123 Their chiral anion -binding abilities have been evaluated by UV-vis absorption and 1H NMR spectroscopy in DMSO and CDCl3, respectively. Table 11 shows that receptor 96a has a higher degree of enantioselective discrimination relative to 96b, with the most significant difference being obtained with enantiomers of α-phenylglycine anions (96a: K(L)/K(D) = 4.76; 96b: K(D)/K(L) = 2.84).
| Anion a | Receptor 96a | Receptor 96b |
|---|---|---|
| K ass/M−1b,c | K ass/M−1b,c | |
| a Anions were used as their tetrabutylammonium salts. b Values of Kass were calculated from UV-vis titrations in DMSO. c All error values were obtained by non-linear curve fitting. | ||
| L-α-Phenylglycine | (2.34 ± 0.03) × 105 | (2.95 ± 0.01) × 103 |
| D-α-Phenylglycine | (4.91 ± 0.02) × 104 | (8.40 ± 0.02) × 103 |
| L-Mandelate | 413.64 ± 2.70 | 194.16 ± 1.81 |
| D-Mandelate | 166.94 ± 2.53 | 337.51 ± 3.04 |
| Dibenzoyl L-tartrate | 1560.27 ± 5.52 | 1104.47 ± 4.41 |
| Dibenzoyl D-tartrate | 1654.36 ± 4.87 | 501.94 ± 3.91 |
Albrecht, and co-workers have synthesised fluorescent receptor 97 consisting of a quinoline substituted with an amide in the 2-position and a urea group in the 8-position.124 This system may be regarded as a semi-rigid version of a 2,6-dicarboxamidopyridine. As with the pyridine systems, two hydrogen bonds (between the quinoline nitrogen and the amide NH and adjacent urea NH) preorganise the binding site into a syn–syn conformation. NMR and fluorescence titration experiments in chloroform at 296 K revealed that the receptor has a high affinity for small halides; the highest affinity was observed for F− (Ka = 14400 M−1 determined by fluorescence titration ). Fluoride binding was accompanied by a strong fluorescence enhancement (Fig. 14).
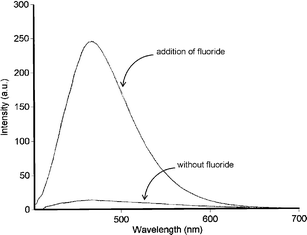 | ||
| Fig. 14 Emission spectrum of 97 (1 µM) in chloroform at 296 K in the absence and presence of excess fluoride ions (excitation at λ = 403 nm). Figure reproduced with permission from ref. 124. Copyright © 2005 Georg Thieme. | ||
There have been a number of recent accounts of amidourea compounds functioning as colorimetric anion sensors.125 In order to investigate the interactions of this family of compounds with anions , Gale, Quesada and co-workers synthesised a series of pyrrolylamidothiourea derivatives 98a–d126 and 99a–c127 with a range of NH acidities. The anion binding abilities of these new receptors were studied by means of UV-vis and 1H NMR titration experiments using DMSO and DMSO-d6 as solvent, respectively, revealing that compound 98b and 99a–c are capable of binding anions , however, with little selectivity (Table 12). However, interestingly, the more acidic derivatives 98c and 98d deprotonate upon addition of only one equivalent of anion (e.g.benzoate, acetate, dihydrogen phosphate or fluoride), a process accompanied by a distinctive colour change from yellow to red in DMSO. In the case of compound 98d an X-ray crystal structure that was obtained using a synchrotron X-ray source confirmed deprotonation at the urea NH adjacent to the amide group.
Gale and co-workers have also synthesised a series of anion receptors 100–102 based upon an ortho-phenylenediamine core.128 Compound 102 had previously been synthesised by Cheng and co-workers and its anion binding properties studied with a variety of anions excluding carboxylates.129 Gale et al. found that the most effective receptor in this series is the bis-urea 101, which shows selectivity for carboxylates (Table 13). In the solid state, an X-ray crystal structure revealed that the receptor forms four hydrogen bonds to a bound benzoate anion (Fig. 15).
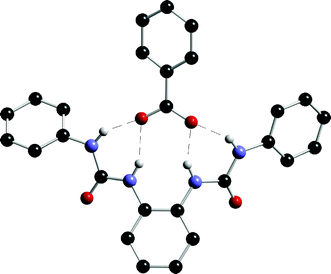 | ||
| Fig. 15 The X-ray crystal structure of the benzoate complex of receptor 101. Non-acidic hydrogen atoms and tetrabutylammonium countercation have been omitted for clarity. | ||
By attaching electron-withdrawing groups to this bis-urea skeleton (e.g. compounds 103 and 104), Gale and co-workers have increased the affinity of this class of receptor for anions in DMSO-d6–0.5% water solution.130 In particular compound for example receptor 103 binds acetate with a stability constant of 8080 M−1 under the same conditions. Moreover, carboxylate/dihydrogen phosphate selectivity can be tuned by functionalising the bis-urea skeleton or by converting the urea groups to thioureas.
The same research group has also studied the anion binding properties of a new hybrid amide –urea macrocycle 105 (Scheme 9) with a variety of putative anionic guests .131 This compound shows a high selectivity for carboxylate anions over dihydrogen phosphate and chloride, binding acetate approximately 100 times more strongly than dihydrogen phosphate both in DMSO-d6–0.5% water and in DMSO-d6–5% water solution (Table 14). Interestingly, crystals of 105 were grown by slow evaporation of a solution of the receptor in DMSO in the presence of tetrabutylammonium fluoride. Rather than a fluoride complex, a mixed carbonate, fluoride complex was obtained with carbonate (presumably arising from CO2 fixation by the fluoride solution) bound within the macrocycle and hydrated fluoride bound outside the macrocyclic cavity. The crystal structure of the carbonate complex is shown in Fig. 16.
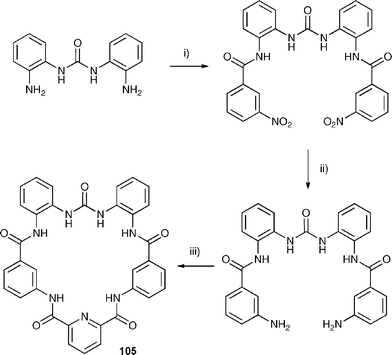 | ||
| Scheme 9 The synthesis of macrocycle 105. (i) 3-Nitrobenzoic acid, benzotriazol-1-yl-oxytripyrrolidinophosphonium hexafluorophosphate (PyBOP), Et3N, 1-hydroxybenzotriazole (HOBt), DMF (anhydrous). (ii) NH2NH2·H2O, Pd/C 10% cat., EtOH. (iii) 2,6-pyridine dicarbonylchloride, tetrabutylammonium acetate, Et3N, DMAP, CH2Cl2. | ||
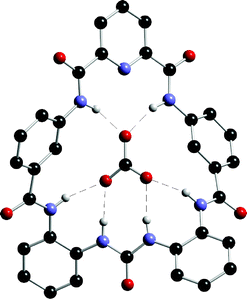 | ||
| Fig. 16 The X-ray crystal structure of carbonate included within macrocycle 105. Other components of the structure and non-acidic hydrogen atoms in the structure have been omitted for clarity. | ||
| Anion | Stability constants/M−1 | |
|---|---|---|
| DMSO-d6–0.5% water | DMSO-d6–5% water | |
| a Due to NH broadening titration was conducted by following the shift of an ArH proton. b This value is greater than 104 M−1. As such the stability constant is at the upper limit that can be determined by this technique and should be treated with caution. | ||
| Cl− | 194 | 42 |
| Br− | 10 | — |
| HSO4− | 115 | — |
| H2PO4− | 142a | 51 |
| NO3− | <10 | — |
| CH3CO2− | 16500b | 5170 |
| C6H5CO2− | 6430 | 1830 |
| Selectivity | ||
| K (CH3CO2−)/K(H2PO4−) | 116 | 101 |
Gunnlaugsson and co-workers have described a family of fluorescent photoinduced electron transfer (PET) chemosensors 106a–c for bis-anions .132 The general structure for these sensors is based on the motif: receptor–spacer–fluorophore–spacer–receptor. This family of receptors is able to bind and sense simple inorganic anions such as F−, AcO− and H2PO4−, but the most interesting result is with bis-anions such as dicarboxylates. In this case, they have observed the formation of a 1 : 1 or a 1 : 2 (receptor : anion ) complex depending on the length of the spacer separating the two carboxylate moieties and the nature of the receptor. A variety of other thiourea containing fluorescent receptors have been synthesised by Ghosh and Adhikari,133 Swamy, Yoon and co-workers,134 and Kondo and Sato.135
Gunnlaugsson, Kruger and co-workers have also developed thiourea-based sensors 107a and b containing naphthalimide groups.136 Receptor 107a proved to be capable of sensing anions in 1 : 1 EtOH–H2Oimidazole–HBr buffered solutions at pH 7.15. Significant colour changes were observed upon addition of acetate or dihydrogen phosphate with NMR and UV-vis experiments providing evidence of a binding rather than a deprotonation process.136 Pfeffer and co-workers have described the synthesis of receptor 108. This receptor employs the naphthalimide NH group when binding dihydrogen phosphate in DMSO-d6 but not when binding acetate, an interaction that should allow for future selective sensing of this anion .137
Pfeffer, Gunnlaugsson, Kruger and co-workers have described the use of a polynorbornane scaffold in the construction of anion receptors 109a and 109b.138 Most interestingly, these systems form 2 : 1 receptor : anion complexes with pyrophosphate (H2P2O72−) anions in DMSO-d6 opening up the prospect of using these systems as precursors to anion templated catenane systems.
Pappalardo, Parisi and co-workers have reported the synthesis of the heterotetratopic receptor 110 composed of two calix[5]arene units (cation binding sites) linked via the upper rim by a 1,4-bis(ureido)phenylene spacer.139 This compound has been shown to bind α,ω-alkanediammonium salts in the calixarene cups whilst binding the counteranions (chloride or acetate) to the urea groups. Depending on the length of carbon chain in the bis-ammonium salt, the receptor either encapsulates both ammonium groups (longer carbon chains) or only a single ammonium group from each cation (for shorter salts with carbon chains containing less than 10 carbon atoms).
Lang, Lhotak and co-workers have synthesised a series of calix[4]arenes substituted at the upper rim with 4-nitrophenylurea groups. 1,3-, 1,2- And monosubstituted derivatives were prepared (compounds 111a–c respectively).140 Interestingly, it was found that while the 1,3- and monosubstituted derivatives form 1 : 1 complexes with a variety of anionic guests in dichloromethane solution, the 1,2-substituted derivative forms 2 : 1 receptor : anion complexes with a variety of anionic guests (Table 15), forming 1 : 1 complexes at high anion concentrations. The authors have also studied the anion binding properties of urea appended thiacalixarenes.141
| Anion a | 111a | 111b b | 111c |
|---|---|---|---|
| a Anions were used as tetrabutylammonium salts. Estimated error is 15%. b In all cases the Job plots indicate the stoichiometry of 2 : 1. | |||
| Cl− | >106 | ≥105, β21 ≥ 1011 | 5.3 × 104 |
| Br− | >106 | β 21 ≥ 1010 | 1.2 × 104 |
| I− | 1.7 × 105 | 5 × 103, β21 = 5 × 109 | 1.2 × 103 |
| NO3− | 4.1 × 105 | 1 × 104, β21 ≥ 1010 | 6.9 × 103 |
| BzO− | >106 | 1 × 105, β21 ≥ 1010 | 6.2 × 105 |
| AcO− | >106 | β 21 ≥ 109 | 4.3 × 105 |
The cyclen receptor 112 has been designed by Sidorov and co-workers to bind anionic pyranine dyes (sulfonated pyrene derivatives) and functions under near physiological conditions.142 The base of the receptor is a cyclen tetraester that is able to bind physiologically abundant Na+ cations. Four thiourea groups are connected to this platform that can bind the pyrazine sulfonates. The terminal naphthalenes can interact and stabilise the pyranine's aromatic core by π-stacking. This cyclen receptor shows a high affinity for pyranine and is finding application as a new membrane leakage assay .
Steed and co-workers have reported an enantiomerically pure tris-urea 113 which is able to act as a low molecular weight gelator (LMWG) in addition to binding chloride anions (with a stability constant of 1154 M−1 in DMSO-d6 solution with 1 : 1 stoichiometry).143 The self-association and anion interactions are in competition with the formation of the helical gel fibres partially inhibited by addition of chloride anions (a process which triggers full crystallisation of 113).
Continuing their work on tripodal receptors for anions , Steed and co-workers have also reported the synthesis of two tripodal tris-urea pyridinium containing receptors 114a,b with p-tolyl or octyl substituents, respectively. In a very elegant report the association behaviour with anionic guests has been studied using several methods including solid-state, solution and computational studies.144,145 The studies reveal that the most stable conformation for the chloride complex of 114a is an up, up, up conformation wherein the anion is encapsulated within the three arms of the receptor, whilst for 114b, the most stable conformation is a 2-up, 1-down arrangement with the anion bound by two urea groups (Fig. 17). This group has also synthesised amidopyridinium calix[4]arenes and studied the interaction of these related species with anionic guests .146
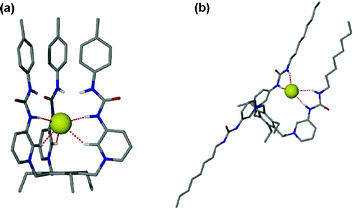 | ||
| Fig. 17 (a) DFT-minimised structure of the 114a–Cl− complex showing the manner in which the anion is bound. (b) DFT-minimised structure of the 2-up, 1-down structure of 114b–Cl−. Reproduced with permission from ref. 144. Copyright © 2006 ACS. | ||
Following on from their earlier work,147,148 Costero and co-workers have reported the preparation of two new cyclohexane based ligands 115a,b, both as racemic mixtures, and their utility in the selective recognition of maleatevs.fumarate anions.149 Compound 115a can act as a selective fluorescent sensor for maleatevs.fumarate in DMSO even in the presence of 5% water. The selectivity is due to a conformational change from chair to boat in the cyclohexane moiety induced by the 1 : 1 complexation with maleate (both carboxylate groups can then be bound by the receptor). Fumarate, with a trans configuration, is able to be complexed by the ligand but the complexation does not cause a conformational change.
The synthesis of the simple pyridyl thioureas 116a–c and their anion binding properties has been investigated by Kilburn and co-workers.150 An intramolecular hydrogen bond between the urea NH group and pyridine nitrogen atom decreases the affinity of these receptors for anions . However, protonation of the pyridine nitrogen releases the urea or thiourea group, allowing more efficient anion complexation. They found that the neutral thioureas bind acetate, but not the more weakly basic chloride or bromide, whereas the protonated thioureas bind strongly to chloride or bromide (Fig. 18), but are deprotonated by acetate in CD3CN solution. Chloride : receptor 2 : 1 complexation occurs at higher chloride concentrations due to a second anion binding to the protonated pyridine NH.
Roussel and co-workers have described the synthesis of a limited series of non-racemic atropisomeric 1-(2-(4-methyl-2-thioxothiazol-3(2H)-yl)phenyl)-3-(hetero)aryl-(thio)ureas 117a–h which present two (or more) hydrogen bond accepting sites and two (or more) hydrogen bond donating sites.151 The binding constants for some of these optically pure (thio)ureas with the enantiomers of N-protected amino acid tetrabutylammonium salts of N-Ac–Phe–COO−, N-Ac–Val–COO−, N-Ac–Leu–COO−, N-Ac–Trp–COO− and naproxenate were determined in CD3CN using 1H NMR titration experiments, showing moderate binding affinities (Kass = 330–3900 M−1) and discrimination. Contrary to what was expected on the basis of the NH acidity of the thiourea vs. urea group, the stability constants were lower with the thiourea than with the corresponding urea (i.e.117avs. 117i).
A polymer-supported ureidopyridyl ligand (118), capable of simultaneous anion and cation binding, has been reported by Steed and co-workers.152 Exposure of this polymer to increasing concentrations of CuCl2, Cu(NO3)2, Co(NO3)2 and Cr(NO3)3 results in the polymer taking on the characteristic colour of the metal ion (green for Cu(II), pink for Co(II) and yellowish brown for Cr(III)). The maximum uptake, determined by elemental analysis of the isolated products, corresponded to a ratio of 0.5 metal ions to pyridyl units for Cu(NO3)2 and 0.65 for CuCl2.
Rurack, Martínez-Mañez and co-workers have designed an optical sensor material for the selective recognition of long-chain carboxylates in water.153 A spacer substituted 7-urea-phenoxazin-3-one was employed as a signalling unit and was bound to a mesoporous trimethylsilylated UVM-7 (MCM-41 type) solid support material to afford 119. The hydrophobic nature of the surface allowed this material to selectively sense long chain carboxylates over short chain analogues and to function in water.
Receptors that employ a steroid backbone have been pioneered by A. P. Davis and co-workers and have found application as very high affinity chloride receptors and transport agents.154 Davis coined the term ‘cholapod’ to describe this class of anion receptor as they are derived from cholic acid. Davis, B. D. Smith et al. have recently published an extensive study of geometric effects in these systems.155 They studied a wide variety of different systems with different types and numbers of hydrogen bond donors and with a range of anion affinities and found that the more powerful receptors also have the widest range of binding free energies with different anions and hence a higher degree of selectivity. For example, compound 120 was found to bind chloride (as the tetraethylammonium salt) in water saturated chloroform with a stability constant of 1.8 × 1011 M−1 and bromide with a stability constant of 4.3 × 1010 M−1.
Davis and co-workers have recently prepared macrocycles 121a,b that contain a quaternary ammonium centre at C3.156 The receptors were used in anion phase transfer experiments, comparing their anion extraction ability from water to chloroform to the previously reported157 non-macrocyclic receptor 122. It was found that receptor 121b overcomes Hofmeister bias to a remarkable degree with an order of extractabilities of Br− > I− ≈ Cl− > NO3− > PF6− > AcO− ≈ EtSO3−.
Davis and co-workers have prepared the tris-urea 123 as the first example of an “allocholapod” anion receptor i.e. the steroid has a ‘5-α’ skeleton rather than the more common ‘5-β’ configuration.158 Tris-urea 123 was synthesised in a single step from a “triaza-analogue” of an allocholic acid derivative through treatment with phenyl isocyanate (Scheme 10). The tris-amine described has the potential to be derivatised to form a whole new family of steroid based anion receptors. The anion complexation ability of compound 123 was measured in water saturated chloroform by extraction of salts and compared to the 5-β analogue 124 (Table 16).
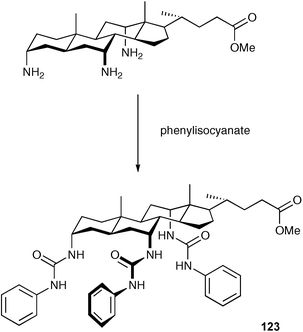 | ||
| Scheme 10 The synthesis of compound 123 in THF. | ||
Chan and co-workers have reported a number of fluorescent steroid based anion sensors containing urea or thiourea groups.159,160 For example chiral fluorescent receptor 125 based on cholic acid was designed and synthesised by Chan and co-workers.161 The enantioselective recognition ability of this receptor for mandelate anion was studied by fluorescence and 1H NMR spectroscopy in CH3CN and DMSO-d6, respectively, indicating that 125 exhibited a good enantioselectivity for the (S)-mandelate anion (Ka = 3.43 × 103 M−1) vs.(R)-mandelate anion(Ka too small to determine) in CH3CN.
The same research group has reported the synthesis of two similar charge neutral fluorescent sensors 126a and b bearing two pyrene signalling subunits at C7 and C12 and two binding groups at C3 and C24 of cholic acid, respectively.162 The binding abilities of these compounds to dicarboxylates were determined using fluorescence, UV-vis and 1H NMR spectroscopic techniques in acetonitrile. Compound 126a and b were shown to exhibit a highly sensitive response to long-chain dicarboxylates (Table 17), with very strong binding between host and guest rendering the detection of dicarboxylates possible even at the concentration of 10−8 M.
| Anion c | 126a | 126b | ||
|---|---|---|---|---|
| K a a | K a b | K a a | K a b | |
| a The values were calculated from the change of the fluorescence spectra. b The values were calculated from the change of the UV-vis spectra. c Anions were used as their tetrabutylammonium salts. d The value is too large to calculate. | ||||
| Glutarate | (8.22 ± 1.51) × 105 | (1.08 ± 0.09) × 106 | (1.68 ± 0.17) × 106 | (1.60 ± 0.11) × 105 |
| Adipate | (1.27 ± 0.12) × 106 | (1.29 ± 0.14) × 106 | (5.56 ± 0.42) × 106 | (1.87 ± 0.14) × 107 |
| Suberate | (1.64 ± 0.14) × 106 | (1.03 ± 0.06) × 106 | (2.10 ± 0.19) × 107 | (3.19 ± 0.10) × 107 |
| Sebacate | (2.27 ± 0.23) × 107 | (2.30 ± 0.23) × 107 | >108d | >108d |
| L-Glutamate | (1.18 ± 0.13) × 106 | (2.21 ± 0.13) × 106 | (2.35 ± 0.11) × 106 | (2.03 ± 0.22) × 106 |
| D-Glutamate | (1.81 ± 0.11) × 106 | (1.81 ± 0.19) × 106 | (4.21 ± 0.23) × 106 | (5.23 ± 0.11) × 106 |
| N-Acetyl-L-glutamate | (1.29 ± 0.19) × 106 | (1.09 ± 0.10) × 106 | (1.63 ± 0.21) × 106 | (8.35 ± 1.30) × 105 |
Ammonium based anion receptors
The earliest synthetic anion receptor systems were comprised of ammonium based cryptand -like receptors capable of encapsulating halide anions .163 Today, research in anion complexation by ammonium based receptors continues—reflecting the ability of these species to function as anion receptors in aqueous solution.Two tren-based macrocyclic receptors containing three [12]aneN4 (127) or [14]aneN4 (128) have been synthesised by Bencini and co-workers and their binding properties with benzene tricarboxylate isomers studied in detail.164 These receptors have a large bowl-shaped cavity in which it is possible to control the degree of protonation of the cyclic amines . The stability constants of 127 and 128 in various protonation states with the tricarboxylates are shown in Table 18. Whilst these data are complex, it is possible to see that both systems bind the symmetrical 1,3,5-benzene tricarboxylate selectively over the pH range studied.
| Reaction | Log K | |||||
|---|---|---|---|---|---|---|
| 127 | 128 | |||||
| 1,2,3-BTC | 1,2,4-BTC | 1,3,5-BTC | 1,2,3-BTC | 1,2,4-BTC | 1,3,5-BTC | |
| H2L2+ + A3− ⇌ H2LA− | 3.18(5) | 3.12(6) | 4.51(6) | 4.20(5) | ||
| H3L3+ + A3− ⇌ H3LA | 3.35(6) | 3.30(7) | 5.02(3) | 2.83(4) | 3.70(5) | 4.34(9) |
| H4L4+ + A3− ⇌ H4LA+ | 4.27(6) | 4.02(5) | 5.90(5) | 2.85(9) | 3.75(9) | 4.89(6) |
| H5L5+ + A3− ⇌ H5LA2+ | 3.80(8) | 4.05(9) | 5.85(5) | 3.08(7) | 4.08(9) | 5.28(9) |
| H6L6+ + A3− ⇌ H6LA3+ | 4.47(5) | 4.11(4) | 6.23(5) | 3.54(4) | 4.15(9) | 5.64(7) |
| H7L7+ + A3− ⇌ H7LA4+ | 6.28(5) | 5.24(5) | 7.73(5) | 4.21(5) | 4.85(8) | 6.28(8) |
| H7L7+ + HA2− ⇌ H8LA5+ | 6.27(7) | 5.00(3) | 7.70(6) | |||
| H8L7+ + A3− ⇌ H8LA5+ | 5.19(2) | 5.42(7) | 6.72(7) | |||
| H8L8+ + HA2− ⇌ H9LA6+ | 5.91(9) | 4.62(6) | 7.52(6) | |||
| H9L9+ + A3− ⇌ H9LA6+ | 5.50(3) | 5.71(9) | 7.02(9) | |||
| H8L8+ + H2A− ⇌ H10LA7+ | 5.48(7) | 4.09(7) | 7.26(6) | |||
| H10L10+ + A3− ⇌ H10LA7+ | 6.00(2) | 6.41(6) | 7.40(7) | |||
| H8L8+ + H3A ⇌ H11LA8+ | 5.17(8) | 3.61(9) | 6.65(5) | |||
| H10L10+ + HA2− ⇌ H11LA8+ | 5.57(2) | 6.10(7) | 7.54(7) | |||
| H11L11+ + HA2− ⇌ H12LA9+ | 4.87(2) | 5.48(8) | 7.08(8) | |||
| H11L11+ + H2A− ⇌ H13LA10+ | 5.24(8) | 6.95(7) | ||||
| H11L11+ + H3A ⇌ H14LA11+ | 4.88(9) | 6.37(8) | ||||
Continuing their studies of ammonium based receptors,165–167 Bianchi and co-workers have described the binding properties of two macrobicyclic polyamines 129 and 130, which contain N7 and N6O donor set of atoms respectively, towards halide anions by means of potentiometric titrations in aqueous media.168 Interestingly, while compound 129 forms complexes with halides in various states of protonation [129HnX]n−1 (n = 2–5 for F− and Cl−, n = 1–5 for Br−) only [130H4X]3+ (X = F−, Cl−, Br−) complexes are formed by 130. Molecular modelling studies were used to rationalise binding selectivities.
The same research group has also studied169 the synthesis and characterisation of a new bis([9]aneN3) ligand 131 containing two [9]aneN3 macrocyclic moieties separated by a 2,6-dimethylenepyridine unit.170 The coordination properties of these receptors toward Cu(II), Zn(II), Cd(II) and Pb(II) were studied by means of potentiometric and UV spectrophotometric measurements. Moreover, a potentiometric and 1H NMR study of the coordination of halide anions by 131 and its structural analogue 132,169 in aqueous solution at different pH values and at ambient temperature, shows that bromide is selectively recognised by 131, while chloride is selectively bound by 132.
Delgado, Félix and co-workers have studied the molecular recognition ability of the ditopic receptor (H6Me2[30]pbz2N6)6+ (133) towards a variety of aliphatic and aromatic carboxylate anions , which differ in size, shape, rigidity and electronic properties.171 The binding studies were carried out by potentiometry and 1H NMR spectroscopy in aqueous (or D2O) solution. The molecular recognition processes in water of the receptor 133 with the three aromatic anions , phthalate (ph2−), isophthalate (iph2−), and terephthalate (tph2−), were theoretically evaluated by MD simulations. The NMR and the theoretical studies suggest that the receptor has a clear preference for the tph2− anion forming an inclusion supermolecule, while for the remaining anions the binding occurs outside of the macrocyclic cavity.
A highly enantioselective molecular recognition of the malate dianion by the synthetic receptor (R,R)-134 in aqueous solution has been studied by Alfonso, Gotor, and co-workers using potentiometric titrations , mass spectrometry (ESI-MS), diffusion measurements (PGSE NMR) and molecular modelling.172 This combination of techniques revealed that the receptor (R,R)-134 forms more stable complexes with (S)-malate than with the R enantiomer (KS > KR). This selectivity persists over the pH range tested, being very high at basic pH (pH = 10, KS/KR = 11.50) but lower when the pH was decreased (pH = 2, KS/KR = 3.89). Interestingly, at neutral pH the interaction is also highly enantioselective (pH = 6.5, KS/KR = 6.76). Thus significant enantioselective molecular recognition occurs with malate enantiomers under physiological conditions.
A detailed theoretical study of the association of triazine cage (135) and its cyanuric acid (136) and boroxine (137) analogues with the fluoride and chloride ions has been performed by Mascal.173 Using the density functional B3LYP method, the author proposed that these novel receptors take advantage of the high conformational stability of the cylindrophane framework, which effectively discriminates guests by size. Based on these electron-deficient s-triazine, cyanuric acid, and boroxine ring systems, the theoretical analysis of the binding energies of chloride and fluoride ions in macrocycles 135–137 suggests a high level of selectivity for fluoride both in the gas phase and in a water solvent model.
Using DFT and ab initio calculations Frontera, Morey, Anslyn and co-workers have designed a quaternary ammonium containing squaramido functionalised tripodal receptor 138 (synthesised using “green chemistry”) that is able to form stable complexes with tricarboxylate anions .174 The anion complexation properties of this receptor have been determined with several mono-, di-, and tricarboxylate salts (139–143) using microcalorimetry experiments in very highly competitive media (H2O–EtOH (1 : 3)). Stability constants in the range 104–105 M−1 were obtained with 139–141 whilst guest 143 was bound with a stability constant of 2.8 × 102 M−1. Receptor 138 was used as part of a displacement assay with fluorescein to determine the citrate concentration in commercial toothpaste.
Guanidinium based anion receptors
The combination of a positive charge and two hydrogen bond donor groups in bicyclic guanidinium species makes this group a particularly effective receptor for carboxylates and phosphates.175Continuing their detailed thermodynamic studies of the interactions of anion receptors with guests , Jadhav and Schmidtchen have studied the interaction of receptors 144 and 145 with guests 146–151 as tetrabutylammonium salts using isothermal calorimetry in acetonitrile. The authors found large entropy differences between the two receptors and the set of guests which were attributed to variations in the ‘stiffness’ and number of mutual binding modes rather than from desolvation processes. Binding studies show compound 144 has a higher affinity for anions than 145 due to a lower degree of ‘structural definition’ present in complexes with 145 resulting in a more favourable entropic component to binding rather than being due to the presence of extra hydrogen bond donor groups.176
Following on from their earlier work,177 Jadhav and Schmidtchen have synthesised the macrocycle 152 containing two chiral guanidinium anchor groups linked via four urea groups in an effort to address the order–disorder distinction in the diastereomeric complexes formed from a chiral macrocyclic host and enantiomeric carboxylates.178 The complexation characteristics of the bis-guanidinium macrocycle 152 with a series of simple chiral carboxylates of varying dimensions were evaluated by isothermal titration calorimetry in acetonitrile solutions and at ambient temperature. Significant differences were found in the entropy of binding between enantiomers in the case of tartrate with a smaller difference observed upon aspartate binding.
Over the last few years Schmuck and co-workers have demonstrated that guanidiniocarbonylpyrroles e.g.153 are highly effective carboxylate receptors.179 Recently, Schmuck and Machon have compared the binding ability of these types of receptor with guanidiniocarbonylpyridine (154 and 155) and benzene analogues (156).180 They found that complexes formed by the pyridine receptors with amino acid carboxylates in aqueous DMSO were generally weaker than complexes formed by the analogous pyrrole containing receptor (or benzene analogue) presumably due to repulsion between the pyridine lone pair and the guest species. For example, Ac–L-Phe–O− is bound with a stability constant of 1200 M−1 in water–DMSO-d6 (40% v/v) at 25 °C by receptor 153 and 230 M−1 by receptor 154. Similar studies with 156 showed anion affinities lying between those of the pyrrole and pyridine analogues.
Continuing this work on analogues of these receptors, Schmuck and Machon have synthesised a series of cationic 2-(guanidiniocarbonyl)furans 157–160 and studied their anion binding properties by UV-vis spectroscopy in water–DMSO (1 : 1).181 The high acidity of the (guanidiniocarbonyl)furans 157–160 (pKa ≈ 5.5) means that these receptors can bind only weakly basic anions such as hydrogen sulfate (Ka = 600 M−1). Hence anion complexation can only occur in acidic solutions below pH 5, ruling out complexation of more basic anions such as carboxylates.
Schmuck and Schwegmann employed the guanidiniocarbonylpyrrole motif in tripodal receptor 161 that was employed as an Anslyn-type182 displacement assay with carboxyfluorescein.183,184 This system allowed remarkably selective, naked eye detection of citrate with respect to other substrates such as malate or tartrate in water.
Continuing their earlier work,185 Schmuck and Heil have probed the substrate selectivity of the ‘one-armed’ receptor 162a for binding the polar tetrapeptide N-Ac–D-Ala–D-Ala–L-Lys–D-Glu–OH (AAKE) 162b in buffered water (Fig. 19).186 This substrate is efficiently bound by the library of receptors 162a, with Ka ≈ 6000 M−1 by the library members with highest affinity, as determined both by a quantitative on-bead binding assays and by 1H NMR, UV-vis and fluorescence binding studies in water solution. However, tetrapeptide 162b is in general bound two or three times less efficiently than its inverse N-Ac–D-Glu–L-Lys–D-Ala–D-Ala–OH (EKAA) (Ka ≈ 17000 M−1),185 even though the complexation mainly involves long-range electrostatic interactions and both the receptor and substrate are flexible. Molecular modelling and ab initio calculations were used to rationalise the selectivity and analyse the binding interactions in the complexes.
 | ||
| Fig. 19 Schematic representation of complex formation between the receptor library 162a and the dansylated tetrapeptide substrate 162b. | ||
Subsequently, Schmuck and Wich have also reported that receptor 162a shows not only significant substrate selectivity but also a remarkable sequence dependent stereoselectivity in the binding of polar tetrapeptides in water.187
Following on from an earlier work,188 Schmuck and co-workers have also synthesised receptors 163a and 163b, which contain hydrophobic cavities and are specifically designed to bind alanine-containing dipeptide carboxylates, and have evaluated their complexation properties using UV-vis and fluorescence titration studies.189 They observed that these receptors are able to bind the deprotonated dipeptide Ac–D-Ala–D-Ala–OH in buffered water with Ka = 33100 M−1, whilst dipeptides such as Ac–Gly–Gly–OH or Ac–D-Val–D-Val–OH had significantly lower affinities (Ka < 3000 M−1). Molecular modelling studies revealed that this efficient binding and the pronounced sequence selectivity are the result of a combination of strong electrostatic contacts and size-discriminating hydrophobic interactions .
Imidazolium based anion receptors
Imidazolium groups provide both a positive charge and relatively acidic CH group with which to bind anionic species. The use of these groups in anion complexation chemistry was pioneered by Alcalde et al. in a number of important papers over the past decade.190,191 A number of groups are now exploring the molecular recognition properties of receptors containing this group.192Hwang, Kim and co-workers have reported the simple and elegant synthesis and binding properties of a calix[4]imidazolium[2]pyridine.193 The receptor 164 contains an array of positively charged imidazolium groups and was found to form a 1 : 1 complex with fluoride in solution and in the solid state (Fig. 20) and 1 : 2 (receptor : anion ) complexes with Cl−, Br−, CH3COO− and HSO4−, binding the anions via CH⋯A− hydrogen bonding interactions. In DMSO-d6, this positively charged macrocycle shows the highest affinity with F− as shown in Table 19.
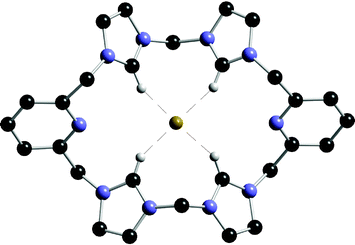 | ||
| Fig. 20 The fluoride complex of receptor 164. Countercations and non-acidic hydrogen atoms have been omitted for clarity. | ||
Beer and co-workers have synthesised tetrakis(imidazolium) (165) and benzimidazolium (166a–c) macrocyclic receptors.194 In a competitive solvent mixture (9 : 1 CD3CN–H2O), macrocycles 166a and 166c show a marked selectivity for fluoride, binding this anion in a 1 : 1 stoichiometry (Table 20) as measured by 1H NMR titration techniques. In the case of benzoate, a 1 : 2 complex (receptor : anion ) was observed with the four macrocycles 165 and 166a–c. A size effect was clearly shown with iodide; the strongest complex was obtained with the largest macrocycle 166b.
| Anion | 165 | 166a | 166b | 166c |
|---|---|---|---|---|
| a Solvent = CD3CN–H2O 9 : 1, temperature = 295 K and errors are ±10%. b Mixed host –guest binding stoichiometry prevented the calculation of a sensible stability constant value. c Precipitation occurred during titration . d The stoichiometry of receptor–benzoate binding is 1 : 2. K1 and K2 are quoted in the table. | ||||
| F− | b | >104 | c | >104 |
| Cl− | b | 1100 | c | 710 |
| Br− | b | 1050 | c | 500 |
| I− | 370 | 560 | 900 | 470 |
| BzO− d | K 1 1800 | K 1 1070 | K 1 1700 | K 1 2700 |
| K 2 470 | K 2 1000 | K 2 940 | K 2 600 | |
Kang and co-workers have synthesised a wide variety of imidazolium containing anion receptor species.
Kang and In have synthesised the methylene-bridged bis-imidazolium receptors 167a and b.195Anion binding studies for these compounds were carried out using 1H NMR spectroscopy in 10% DMSO-d6 in CD3CN and revealed that they displayed high affinities for acetate, with the more acidic derivative showing the higher affinity (Ka(167a) = 1.6 × 103 M−1; Ka(167b) = 2.6 × 104 M−1), and bound spherical halide anions only weakly.
Receptor 168, which contains four imidazolium groups appended to a naphthalene scaffold, was synthesised by Kang and Kim as shown in Scheme 11.196 Receptor 168 was found to bind iodide selectively over bromide by 1H NMR titration techniques in 10% DMSO-d6 in CD3CN solution, with a stability constant of 1600 ± 220 M−1 found for iodidevs. 140 ± 7.0 M−1 found for bromide. Addition of iodide was also found to quench the fluorescence of the receptor. Kang and co-workers have also synthesised a number of other imidazolium containing anion receptors including systems appended to a glycouril scaffold,197 and systems based on 1,3-functionalised phenyl scaffolds.198,199
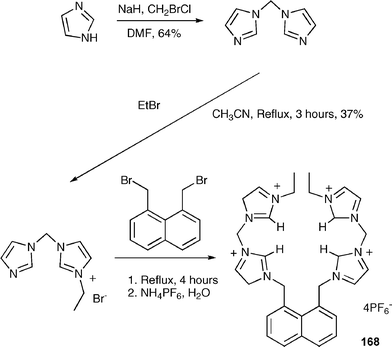 | ||
| Scheme 11 Synthesis of receptor 168. | ||
Schatz and co-workers have prepared a series of supramolecular imidazolium salts based on benzene (169a,b and 170a–d) and calix[4]arenes (171a–d) that are easily accessible and efficient receptor molecules for small inorganic anions (H2PO4−, HSO4−, Cl− and Br−).200 Receptors 169b and 171d showed selective dihydrogen phosphate binding whilst the other receptors did not show a high degree of selectivity amongst the anions studied (Table 21).
Following on from their earlier work,201,202 Kim, Yoon and co-workers have prepared two new fluorescent anion receptors (172 and 173) bearing two imidazolium groups at the 9,10-positions of anthracene and 2,2′-positions of the binaphthyl ring, respectively.203 These receptors form strong (C–H)+⋯X hydrogen bonds with anions and, as theoretical calculations predict, selectively recognise pyrophosphate and phosphate over other halides in acetonitrile. Binding can be monitored through 1H NMR titration experiments and also via fluorescence quenching of the anthracene moiety. Using this method, association constants were found to be 3.58 × 106 M−1 (HP2O73−) and 6.31 × 105 M−1 (H2PO4−) for 172 and 6.76 × 106 M−1 (HP2O73−) and 4.21 × 105 M−1 (H2PO4−) for 173.
Novel deoxycholic acid-based cyclic receptors, 174 and 175, containing two imidazolium groups and m-xylene and p-xylene as spacers have been synthesised by Pandey and co-workers.204 The anion binding properties of these compounds were studied using standard 1H NMR methods in CDCl3 solutions, and revealed that the receptor with m-xylene shows a moderate selectivity for fluoride ion (Ka = 2400 M−1) whereas the receptor with p-xylene, and hence a larger spacing between the imidazolium groups, exhibits high affinity and selectivity towards chloride (Ka = 12000 M−1).
Receptors containing hydroxyl groups
Hydroxyl groups have rarely been used in synthetic anion receptor systems. This may be because of their propensity to deprotonate in the presence of basic anions in organic solution. However, recently a number of groups have begun to explore the use of this moiety in anion complexation.205 A series of sulfonamide receptors (176–178b) with pendant hydroxy groups were prepared by Kondo and co-workers.206Anion binding studies were limited to chloride, bromide and iodide in acetonitrile, which showed that compounds 178a and 178b had a particularly high affinity for chloride (Table 22).207D. K. Smith and co-workers have reported how some simple and commercial available dihydroxybenzenes such as 179–181 can exhibit relatively high degrees of tuneable anion selectivity using several methods including 1H NMR spectroscopy and UV-vis spectrometry and electrochemical studies.208
Stability constants in CD3CN solution were determined for compounds 179 and 180 with halide anions . It was found that catechol179 forms the strongest complex, with a stability constant of 1570 M−1, with chloridevs. 110 M−1 for the chloride complex of compound 180. Basic anions such as dihydrogen phosphate were found to deprotonate receptor 181, whilst fluoride was found to selectively deprotonate catechol179 in acetonitrile solution giving rise to a blue coloration due to deprotonation followed by oxidative degradation of the receptor. Linked catechol groups have also been shown to have appreciable anion affinity.209
The fluorescent chemosensor 182, prepared by Zhang, Wu and co-workers, employs anion triggered deprotonation as a novel sensing mechanism.210 The fluorescence of the receptor was studied in the presence of different anions (F−, H2PO4−, AcO−, HSO4−, NO3−, Cl−, Br−, and I−) in acetonitrile solution and shows a fluoride selective response. The fluorescence intensity of compound 182 at 360 nm decreases dramatically and a new broad emission band peaked at 455 nm emerges with increasing intensity upon addition of fluoride (Fig. 21). The authors propose that the aminonaphthol hydroxyl OH group is involved in intramolecular hydrogen bonding that holds the receptor in a rigid conformation (Scheme 12), however in the presence of fluoride this hydrogen bond is removed as the OH group deprotonates, allowing the molecule to fold up into a conformation allowing exciplex formation.
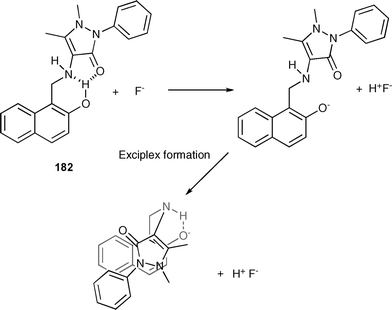 | ||
| Scheme 12 Fluoride triggered deprotonation and conformational rearrangement by receptor 182. | ||
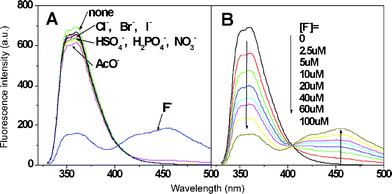 | ||
| Fig. 21 Fluorescence spectra of compound 182 (10 µM) in acetonitrile upon addition of (A) different anions (F−, H2PO4−, AcO−, HSO4−, NO3−, Cl−, Br−, I−) (100 µM) and (B) different concentrations of F−. Excitation wavelength: 320 nm Reproduced with permission from ref. 210 (Fig. 2). Copyright © 2005 ACS. | ||
A series of di- and tetrafunctionalised calixarenes have been prepared by Casnati and co-workers with pendant 2,2,2-trifluoroethanol binding groups at the upper rim.211 The tetrapropoxy bis-trifluoroethanol calix[4]arenes 183a (R,R+S,S) and 183b (R,S) show selectivity for carboxylates and H2PO4− over HSO4−, Br− and CN−anions in chloroform solution, whereas calixarene 184, bearing a crown ether at the lower rim and trifluoroethanol groups at the upper rim, behaves as a ditopic receptor, simultaneously binding the cation and the anion counterparts of ion pairs . Moreover, this calixcrown-4 derivative 184 shows a five-fold increase in the binding of acetate anion when a sodium ion is complexed in the polyether bridge.
Anion templation and anion -directed assembly
Examples of anions directing the formation of molecular architectures were, until a few years ago, difficult to find. However, this situation has now changed, and reports of the templating influence of anions are now becoming widespread.212,213Following on from their earlier work,214 Beer and co-workers have described the anion templated synthesis of three new [2]rotaxanes containing positively charged pyridinium axles and neutral isophthalamide macrocyclic components 185a–c.215 Removal of the chloride anion template from the cavity of the [2]rotaxanes using silver hexafluorophosphate yielded the corresponding hexafluorophosphate [2]rotaxane salts 186a–c (Scheme 13). Simple modification of just one component can improve both rotaxane assembly yields and anion binding strengths significantly. In this instance the incorporation of electron-withdrawing substituents, such as a nitro group, into the 5-position of an isophthalamide anion binding motif increases the acidity of the amide protons, which leads to impressive yields of up to 60% for [2]rotaxane formation, concomitant with the rotaxane binding domain both augmenting thermodynamic stability and conserving selectivity for chloride, the templating anion , as indicated the 1H NMR titration studies of compounds 186a–c in a mixture of CD3OD–CDCl3 (1 : 1).
Beer and co-workers have also described the first example of an anion directed interweaving of two identical acyclic positively charged anion binding receptors. Mixing one equivalent of 187a and one equivalent of 187b resulted in the formation of an orthogonal assembled structure around the single chloride anion which, upon a subsequent double cyclisation using ring closing metathesis methods, produces a novel [2]catenane 188(Cl−)(PF6−) in 78% yield.216 Proton NMR spectroscopy, electrospray mass spectrometry and single crystal X-ray analysis (see Fig. 22) all provide evidence for the formation of this catenane . After anion exchange, the anion binding properties of the catenane 188(PF6−)2 with TBA salts of F−, Cl−, Br−, H2PO4− and OAc− in CDCl3–acetone-d6 1 : 1 were investigated, revealing that the catenane binds chloride much more strongly (K11 = 9240 M−1K12 = 160 M−1) than acetate (K11 = 420 M−1K12 = 40 M−1) or bromide (K11 = 790 M−1K12 = 40 M−1).
![The crystal structure of the [2]catenane 188 (Cl−)(PF6−) with a chloride ion in the binding cavity. Hydrogen atoms have been omitted except for those involved in hydrogen bonding.](/image/article/2008/CS/b715825d/b715825d-f22.gif) | ||
| Fig. 22 The crystal structure of the [2]catenane 188 (Cl−)(PF6−) with a chloride ion in the binding cavity. Hydrogen atoms have been omitted except for those involved in hydrogen bonding. | ||
Verboom and co-workers have described the first example of the use of triple-ion interactions for the construction of novel [2 + 4] cavitand -containing capsules 189 in methanol and water.217 The capsules employ four monovalent anions (bromide, nitrate, acetate or tosylate) to bring together two tetrakis(pyridiniummethyl)tetramethyl cavitands by pyridinium–anion –pyridinium interactions (Scheme 14). This work has been followed by work on [2 + 3] systems218 and capsule formation with tetracationic and tetraanionic cavitands .219
Sessler and co-workers have reported the anion -induced synthesis of a new class of 2,6-diamidopyridine-bipyrrole macrocyclic receptor.220 The macrocycles were obtained by reaction of diformylbipyrrole 190 and diamine 191 in methanol under acidic conditions. The choice of the acid used (HCl, HBr, CH3CO2H, CF3CO2H, H3PO4, H2SO4, HNO3) was shown to play a critical role in defining the distribution of products from this condensation reaction. HCl and HBr afford the formation of oligomers with high molecular weights (m/z > 3000) and a small amount of the unstable [1 + 1] macrocycle . The use of CH3CO2H, CF3CO2H and H3PO4 led to the formation of the [2 + 2] macrocycle 192 contaminated by uncharacterised oligomers. HNO3 affords only oligomeric species with high molecular weights. The use of H2SO4 led to the formation of the kinetic product 192·2H2SO4 under stirring with the free macrocycle being liberated by suspension of the H2SO4 complex in dichloroethane and treatment with triethylamine (Scheme 15). Macrocycle 192 interacts strongly with tetrahedral anions HSO4− (1 : 1; Ka = 63500 ± 3000 M−1) and H2PO4− (2 : 1; Ka1 = 191000 ± 15400 M−1; Ka2 = 60200 ± 6000 M−1), less strongly with acetate (1 : 1, Ka = 26000 ± 2400 M−1), and not at all with the Cl−, Br− and NO3−.
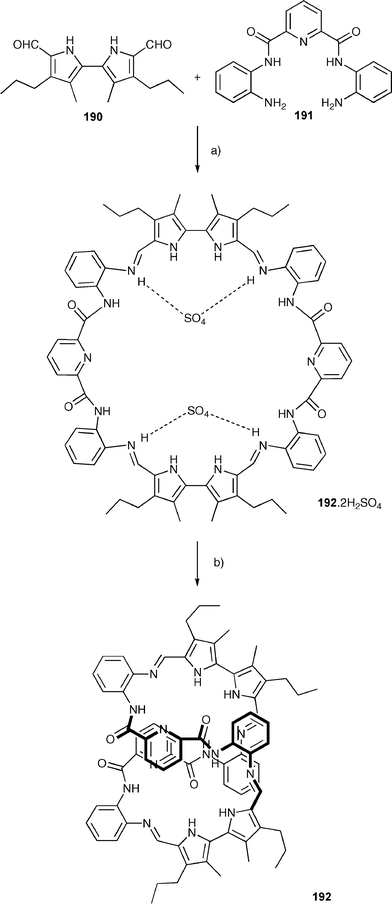 | ||
| Scheme 15 (a) Conc. H2SO4 (2.2 equiv.), 48 h, rt; (b) dichloroethane, Et3N (excess), MeOH–CH2Cl2. | ||
If compound 192 was dissolved in acetonitrile and allowed to stand for 5 days in the presence of TBAHSO4 or TBAH2PO4, it was found to rearrange to give the [3 + 3] analogue 193 (Scheme 16). However, if the reaction mixture was subject to stirring, the [3 + 3] macrocyclic product was formed only in trace amounts and instead the [2 + 2] macrocycle 192 was formed as the H2SO4 complex. Thus it seems that in this case the kinetics and thermodynamics of this reaction are in fine balance and depend on subtle changes in reaction conditions. Under stirring, the H2SO4 complex of 192 forms quickly and precipitates. In the absence of stirring the precipitation process is slow allowing isolation of 193 as the thermodynamic product.
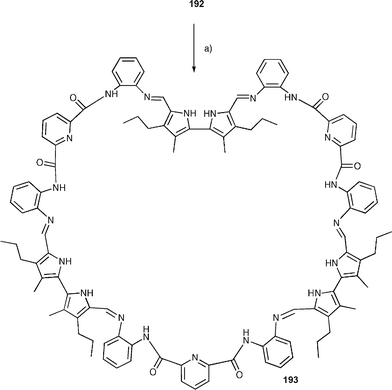 | ||
| Scheme 16 (a) TBAHSO4, MeCN, 5 days, room temperature without stirring followed by Et3N; or TBAH2PO4, MeCN, 5 days, room temperature without stirring. | ||
Modelling studies
Hay and co-workers have continued their work on theoretical insights into anion complexation and anion receptor design. Bryantsev and Hay have combined theoretical calculations, an examination of the Cambridge Crystallographic Database and experimental results for binding energies to analyse whether C–H groups are significant hydrogen bond donor groups in anion receptors.221 They studied in detail complexes of benzene with chloride, nitrate and perchlorate at the MP2/aug-cc-pVTZ level of theory with benzene interacting with these anions in several different binding geometries (Fig. 23). The authors found that even in the absence of electron-withdrawing groups, simple arenes can form hydrogen bonds that exceed 50% of the strength of those formed by OH or NH hydrogen bond donor groups. In another publication, the influence of substituents on the aromatic ring on hydrogen bond strength was evaluated.222 Hay and co-workers have also conducted an extensive modelling study in order to find the optimal arrangement of urea groups to bind Cl−, NO3− and ClO4−223 and with Johnson and co-workers, in a seminal contribution dated 2007 but published in late 2006, a detailed study of the interaction of halides with electron-deficient arenes ,224 an area attracting much current interest.225 | ||
| Fig. 23 Structures and ΔE values (kcal mol−1) obtained after geometry optimisation at the MP2/aug-cc-pVTZ level of theory. When the two hydrogen bonds are not equivalent, 195, 196, 201, the weaker interaction is indicated by the thinner dashed line. Reproduced with permission from ref. 221. Copyright © 2005 ACS. | ||
Conclusions
The years 2005 and 2006 saw continuing interest and effort devoted to the production of new anion receptors. The development of imidazolium based receptors continues apace whilst the first examples of synthetic indole containing receptors have been reported. Applications of receptors as lipid -bilayer membrane transport agents for chloride or HCl are attracting growing interest. Synthetic molecular shuttles and channels have been developed which show effective chloride transport ability in model systems and in the case of Gokel’s peptide based transport agents in epithelial cells. New approaches to chloride transport are also being developed, including revolutionary systems such as Matile’s anion –π slides226 (Fig. 24) based on electron deficient oligo(p-phenylene)-N,N-naphthalenediimide (O-NDI) rods. Potential application of these systems as future treatments for cystic fibrosis and other channelopathies continue to drive this research forward.Acknowledgements
We would like to thank the EPSRC for a postdoctoral fellowship (JG). SEGG would like to thank the Ministerio de Educacion y Ciencia of Spain for the award of a Postdoctoral grant.References
- J. L. Sessler, P. A. Gale and W.-S. Cho, Anion Receptor Chemistry, Royal Society of Chemistry, Cambridge, 2006 Search PubMed.
- R. Quesada and P. A. Gale, Coord. Chem. Rev., 2006, 250, 3219 CrossRef CAS.
- Supramolecular Chemistry of Anions, ed. A. Bianchi, K. Bowman-James and E. García-España, Wiley-VCH, New York, 1997 Search PubMed.
- P. D. Beer and P. A. Gale, Angew. Chem., Int. Ed., 2001, 40, 486 CrossRef.
- P. A. Gale, in The Encyclopedia of Supramolecular Chemistry, ed. J. L. Atwood and J. W. Steed, Dekker, New York, 2004, p. 31 Search PubMed.
- K. Kavallieratos, S. R. de Gala, D. J. Austin and R. H. Crabtree, J. Am. Chem. Soc., 1997, 119, 2325 CrossRef CAS.
- K. Kavallieratos, C. M. Bertao and R. H. Crabtree, J. Org. Chem., 1999, 64, 1675 CrossRef CAS.
- B. D. Smith and M. P. Hughes, J. Org. Chem., 1997, 62, 4492 CrossRef CAS.
- S.-I. Kondo, Y. Hiraoka, N. Kurumatani and Y. Yano, Chem. Commun., 2005, 1720 RSC.
- S. J. Coles, J. G. Frey, P. A. Gale, M. B. Hursthouse, M. E. Light, K. Navakhun and G. L. Thomas, Chem. Commun., 2003, 568 RSC.
- S. J. Brooks, L. S. Evans, P. A. Gale, M. B. Hursthouse and M. E. Light, Chem. Commun., 2005, 734 RSC.
- S. J. Brooks, P. R. Birkin and P. A. Gale, Electrochem. Commun., 2005, 7, 1351 CrossRef CAS.
- M. J. Chmielewski and J. Jurczak, Tetrahedron Lett., 2004, 45, 6007 CrossRef CAS.
- M. J. Chmielewski, A. Szumna and J. Jurczak, Tetrahedron Lett., 2004, 45, 8699 CrossRef CAS.
- M. J. Chmielewski and J. Jurczak, Chem.–Eur. J., 2005, 11, 6080 CrossRef CAS.
- M. J. Chmielewski, L. Dobrzycki, J. Jurczak and K. Wozniak, Cryst. Growth Des., 2005, 5, 1339 CrossRef CAS.
- M. J. Chmielewski and J. Jurczak, Chem.–Eur. J., 2006, 12, 7652 CrossRef CAS.
- M. J. Chmielewski and J. Jurczak, Tetrahedron Lett., 2005, 46, 3085 CrossRef CAS.
- A. M. Costero, M. J. Banuls, M. J. Aurell and M. C. R. De Arellano, J. Inclusion Phenom. Macrocyclic Chem., 2006, 54, 61 Search PubMed.
- A. K. Jain, V. K. Gupta and J. R. Raisoni, Electrochim. Acta, 2006, 52, 951 CrossRef CAS.
- M. K. Chae, G.-Y. Cha and K.-S. Jeong, Tetrahedron Lett., 2006, 47, 8217 CrossRef CAS.
- K.-J. Chang, M. K. Chae, C. Lee, J.-Y. Lee and K.-S. Jeong, Tetrahedron Lett., 2006, 47, 6385 CrossRef CAS.
- S. Naher, K. Hiratani and S. Ito, J. Inclusion Phenom. Macrocyclic Chem., 2006, 55, 151 Search PubMed.
- P. D. Beer, Z. Chen, M. G. B. Drew and P. A. Gale, Chem. Commun., 2004, 2207 Search PubMed.
- P. D. Beer, P. A. Gale, Z. Chen, M. G. B. Drew, J. A. Heath, M. I. Ogden and H. R. Powell, Inorg. Chem., 1997, 36, 5880 CrossRef CAS.
- M. D. Lankshear, A. R. Cowley and P. D. Beer, Chem. Commun., 2006, 612 RSC.
- K.-X. Xu, Y.-B. He, H.-J. Qin, G.-Y. Qing and S.-Y. Liu, Tetrahedron: Asymmetry, 2005, 16, 3042 CrossRef CAS.
- J. Kang, H.-K. Ju and J.-H. Jo, Supramol. Chem., 2004, 16, 175 CrossRef CAS.
- J. Kang, J.-H. Jo and S. In, Tetrahedron Lett., 2004, 45, 5225 CrossRef CAS.
- J. Kang and J. Kim, Tetrahedron Lett., 2005, 46, 1759 CrossRef CAS.
- H. Kim and J. Kang, Bull. Korean Chem. Soc., 2006, 27, 1791 CAS.
- J. M. Mahoney, A. M. Beatty and B. D. Smith, J. Am. Chem. Soc., 2001, 123, 5847 CrossRef CAS.
- M. J. Deetz, M. Shang and B. D. Smith, J. Am. Chem. Soc., 2000, 122, 6201 CrossRef CAS.
- J. M. Mahoney, K. A. Stucker, H. Jiang, I. Carmichael, N. R. Brinkmann, A. M. Beatty, B. C. Noll and B. D. Smith, J. Am. Chem. Soc., 2005, 127, 2922 CrossRef CAS.
- O. Hirata, M. Takeuchi and S. Shinkai, Chem. Commun., 2005, 3805 RSC.
- T. Zieliński, M. Kędziorek and J. Jurczak, Tetrahedron Lett., 2005, 46, 6231 CrossRef CAS.
- T. Zieliński and J. Jurczak, Tetrahedron, 2005, 61, 4081 CrossRef CAS.
- H. Qin, Y. He, G. Qing, C. Hu and X. Yang, Tetrahedron: Asymmetry, 2006, 17, 2143 CrossRef CAS.
- S. Otto and S. Kubik, J. Am. Chem. Soc., 2003, 125, 7804 CrossRef CAS.
- Z. Rodriguez-Docampo, S. I. Pascu, S. Kubik and S. Otto, J. Am. Chem. Soc., 2006, 128, 11206 CrossRef CAS.
- D. Yang, X. Li, Y. Sha and Y.-D. Wu, Chem.–Eur. J., 2005, 11, 3005 CrossRef CAS.
- R. Miao, Q.-Y. Zheng, C.-F. Chen and Z.-T. Huang, Tetrahedron Lett., 2005, 46, 2155 CrossRef CAS.
- S. Valiyayeettil, J. F. J. Engbersen, W. Verboom and D. N. Reinhoudt, Angew. Chem., Int. Ed. Engl., 1993, 32, 900 CrossRef.
- C. Kaewtong, S. Fuangswasdi, N. Muangsin, N. Chaichit, J. Vicens and B. Pulpoka, Org. Lett., 2006, 8, 1561 CrossRef CAS.
- A. F. D. de Namor, J. K. Chaaban and I. Abbas, J. Phys. Chem. A, 2006, 110, 9575 CrossRef CAS.
- K. Bowman-James, Acc. Chem. Res., 2005, 38, 671 CrossRef CAS.
- S. O. Kang, D. Powell, V. W. Day and K. Bowman-James, Angew. Chem., Int. Ed., 2006, 45, 1921 CrossRef CAS.
- M. R. Sambrook, P. D. Beer, J. A. Wisner, R. L. Paul, A. R. Cowley, F. Szemes and M. G. B. Drew, J. Am. Chem. Soc., 2005, 127, 2292 CrossRef CAS.
- S. Zhang and L. Echegoyen, J. Am. Chem. Soc., 2005, 127, 2006 CrossRef CAS.
- S. Tapper, J. A. Littlechild, Y. Molard, I. Prokes and J. H. R. Tucker, Supramol. Chem., 2006, 18, 55 CrossRef CAS.
- W. E. Allen and J. L. Sessler, CHEMTECH, 1999, 29, 16 CAS.
- P. V. Santacroce, O. A. Okunola, P. Y. Zavalij and J. T. Davis, Chem. Commun., 2006, 3246 RSC.
- V. Sidorov, F. W. Kotch, G. Abdrakhmanova, R. Mizani, J. C. Fettinger and J. T. Davis, J. Am. Chem. Soc., 2002, 124, 2267 CrossRef CAS.
- V. Sidorov, F. W. Kotch, J. L. Kuebler, Y.-F. Lam and J. T. Davis, J. Am. Chem. Soc., 2003, 125, 2840 CrossRef CAS.
- J. L. Seganish, P. V. Santacroce, K. J. Salimian, J. C. Fettinger, P. Zavalij and J. T. Davis, Angew. Chem., Int. Ed., 2006, 45, 3334 CrossRef CAS.
- P. H. Schlesinger, R. Ferdani, J. Liu, J. Pajewska, R. Pajewski, M. Saito, H. Shabany and G. W. Gokel, J. Am. Chem. Soc., 2002, 124, 1848 CrossRef CAS.
- P. H. Schlesinger, R. Ferdani, J. Pajewska, R. Pajewski and G. W. Gokel, New J. Chem., 2003, 27, 60 RSC.
- N. Djedovič, R. Ferdani, E. Harder, J. Pajewska, R. Pajewski, M. E. Weber, P. H. Schlesinger and G. W. Gokel, New J. Chem., 2005, 29, 291 RSC.
- R. Pajewski, R. Ferdani, J. Pajewska, N. Djedovič, P. H. Schlesinger and G. W. Gokel, Org. Biomol. Chem., 2005, 3, 619 RSC.
- R. Pajewski, R. Garcia-Medina, S. L. Brody, W. M. Leevy, P. H. Schlesinger and G. W. Gokel, Chem. Commun., 2006, 329 RSC.
- K. Kavallieratos, A. J. Sabucedo, A. T. Pau and J. M. Rodriguez, J. Am. Soc. Mass Spectrom., 2005, 16, 1377 CrossRef CAS.
- O. B. Berryman, F. Hof, M. J. Hynes and D. W. Johnson, Chem. Commun., 2006, 506 RSC.
- P.A. Gale, J.L. Sessler, V. Král and V. Lynch, J. Am. Chem. Soc., 1996, 118, 5140 CrossRef CAS.
- S. Camiolo and P. A. Gale, Chem. Commun., 2000, 1129 RSC; F. P. Schmidtchen, Org. Lett., 2002, 4, 431 CrossRef CAS.
- J. L. Sessler, D. E. Gross, W.-S. Cho, V. M. Lynch, F. P. Schmidtchen, G. W. Bates, M. E. Light and P. A. Gale, J. Am. Chem. Soc., 2006, 128, 12281 CrossRef CAS.
- R. Custelcean, L. H. Delmau, B. A. Moyer, J. L. Sessler, W.-S. Cho, D. Gross, G. W. Bates, S. J. Brooks, M. E. Light and P. A. Gale, Angew. Chem., Int. Ed., 2005, 44, 2537 CrossRef CAS.
- G. W. Bates, P. A. Gale and M. E. Light, CrystEngComm, 2006, 8, 300 RSC.
- G. W. Bates, M. Kostermans, W. Dehaen, P. A. Gale and M. E. Light, CrystEngComm, 2006, 8, 444 Search PubMed.
- R. Nishiyabu, M. A. Palacios, W. Dehaen and P. Anzenbacher, Jr., J. Am. Chem. Soc., 2006, 128, 11496 CrossRef CAS.
- P. Anzenbacher, Jr., R. Nishiyabu and M. A. Palacious, Coord. Chem. Rev., 2006, 250, 2929 CrossRef.
- J. L. Sessler, W.-S. Cho, D. E. Gross, J. A. Shriver, V. M. Lynch and M. Marquez, J. Org. Chem., 2005, 70, 5982 CrossRef CAS.
- C. H. Lee, H.-K. Na, D.-W. Yoon, H. Miyagi, W.-S. Cho, V. M. Lynch, S. V. Shevchuk and J. L. Sessler, J. Am. Chem. Soc., 2003, 125, 7301 CrossRef CAS.
- J. L. Sessler, D. An, W.-S. Cho and V. Lynch, J. Am. Chem. Soc., 2003, 125, 13646 CrossRef CAS.
- J. L. Sessler, D. An, W.-S. Cho and V. Lynch, Angew. Chem., Int. Ed., 2003, 42, 2278 CrossRef CAS.
- J. L. Sessler, D. An, W.-S. Cho, V. Lynch and M. Marquez, Chem. Commun., 2005, 540 RSC.
- J. L. Sessler, D. An, W.-S. Cho, V. Lynch, D.-W. Yoon, S.-J. Hong and C.-H. Lee, J. Org. Chem., 2005, 70, 1511 CrossRef CAS.
- C.-H. Lee, J.-S. Lee, H.-K. Na, D.-W. Yoon, H. Miyaji, W.-S. Cho and J. L. Sessler, J. Org. Chem., 2005, 70, 2067 CrossRef CAS.
- H. Miyaji, H.-K. Kim, E.-K. Sim, C.-K. Lee, W.-S. Cho, J. L. Sessler and C.-H. Lee, J. Am. Chem. Soc., 2005, 127, 12510 CrossRef CAS.
- P. Anzenbacher, Jr., K. Jursíkova, V. M. Lynch, P. A. Gale and J. L. Sessler, J. Am. Chem. Soc., 1999, 121, 11020 CrossRef.
- A. Kałedkowski and A. W. Trochimczuk, React. Funct. Polym., 2006, 66, 740 CrossRef CAS.
- K. A. Nielsen, W.-S. Cho, J. O. Jeppesen, V. M. Lynch, J. Becher and J. L. Sessler, J. Am. Chem. Soc., 2004, 126, 16296 CrossRef CAS.
- K. A. Nielsen, W.-S. Cho, J. Lyskawa, E. Levillain, V. M. Lynch, J. L. Sessler and J. O. Jeppesen, J. Am. Chem. Soc., 2006, 128, 2444 CrossRef CAS.
- K. A. Nielsen, W.-S. Cho, G. H. Sarova, B. M. Petersen, A. D. Bond, J. Becher, F. Jensen, D. M. Guldi, J. L. Sessler and J. O. Jeppesen, Angew. Chem., Int. Ed., 2006, 45, 6848 CrossRef CAS.
- R. Gu, S. Depraetere, J. Kotek, J. Budka, E. Wagner-Wysiecka, J. F. Biernat and W. Dehaen, Org. Biomol. Chem., 2005, 3, 2921 RSC.
- R. Nishiyabu and P. Anzenbacher, Jr., Org. Lett., 2006, 8, 359 CrossRef CAS.
- R. Nishiyabu and P. Anzenbacher, Jr., J. Am. Chem. Soc., 2005, 127, 8270 CrossRef CAS.
- X. K. Ji, D. S. Black, S. B. Colbran, D. C. Craig, K. M. Edbey, J. B. Harper and G. D. Willett, Tetrahedron, 2005, 61, 10705 CrossRef CAS.
- A. F. D. de Namor, M. Shehab, I. Abbas, M. V. Withams and J. Zvietcovich-Guerra, J. Phys. Chem. B, 2006, 110, 12653 CrossRef.
- P. A. Gale, Acc. Chem. Res., 2006, 39, 467.
- P. A. Gale, Chem. Commun., 2005, 3761 RSC.
- A. Fürstner, Angew. Chem., Int. Ed., 2003, 42, 3582 CrossRef.
- N. N. Gerber, Crit. Rev. Microbiol., 1974, 3, 469 Search PubMed.
- J. W. Bennett and R. Bentley, Adv. Appl. Microbiol., 2000, 47, 1 Search PubMed.
- S. Ohkuma, T. Sato, M. Okamoto, K. Matsuya, K. Arai, T. Kataoka, K. Nagai and H. H. Wasserman, Biochem. J., 1998, 334, 731 CAS.
- J. L. Seganish and J. T. Davis, Chem. Commun., 2005, 5781 RSC.
- P. A. Gale, M. E. Light, B. McNally, K. Navakhun, K. E. Sliwinski and B. D. Smith, Chem. Commun., 2005, 3773 RSC.
- J. L. Sessler, L. R. Eller, W.-S. Cho, S. Nicolaou, A. Aguilar, J. T. Lee, V. M. Lynch and D. J. Magda, Angew. Chem., Int. Ed., 2005, 44, 5989 CrossRef CAS.
- J. L. Sessler, G. D. Pantos, P. A. Gale and M. E. Light, Org. Lett., 2006, 8, 1593 CrossRef CAS.
- P. A. Gale, S. Camiolo, G. J. Tizzard, C. P. Chapman, M. E. Light, S. J. Coles and M. E. Hursthouse, J. Org. Chem., 2001, 66, 7849 CrossRef CAS.
- Z. Yin, Z. Li, A. Yu, J. He and J.-P. Cheng, Tetrahedron Lett., 2004, 45, 6803 CrossRef CAS.
- C.-L. Chen, Y.-H. Chen, C.-Y. Chen and S.-S. Sun, Org. Lett., 2006, 8, 5053 CrossRef CAS.
- C.-Y. Wu, M.-S. Chen, C.-A. Lin, S.-C. Lin and S.-S. Sun, Chem.–Eur. J., 2006, 12, 2263 CrossRef CAS.
- P. Anzenbacher, Jr., M. A. Palacios, K. Jursíkova and M. Marquez, Org. Lett., 2005, 7, 5027 CrossRef.
- T. Ghosh, B. G. Maiya and A. Samanta, Dalton Trans., 2006, 795 RSC.
- Z. Yin, Y. Zhang, J. He and J.-P. Cheng, Tetrahedron, 2006, 62, 765 CrossRef CAS.
- H. Maeda and Y. Kusunose, Chem.–Eur. J., 2005, 11, 5661 CrossRef CAS.
- C. Fujimoto, Y. Kusunose and H. Maeda, J. Org. Chem., 2006, 71, 2389 CrossRef CAS.
- H. Maeda and Y. Ito, Inorg. Chem., 2006, 45, 8205 CrossRef CAS.
- D. Curiel, A. Cowley and P. D. Beer, Chem. Commun., 2005, 236 RSC.
- K.-J. Chang, D. Moon, M. S. Lah and K.-S. Jeong, Angew. Chem., Int. Ed., 2005, 44, 7926 CrossRef CAS.
- T. H. Kwon and K.-S. Jeong, Tetrahedron Lett., 2006, 47, 8539 CrossRef CAS.
- J. L. Sessler, D.-G. Cho and V. Lynch, J. Am. Chem. Soc., 2006, 128, 16518 CrossRef CAS.
- V. Amendola, M. Boiocchi, D. Esteban-Gómez, L. Fabbrizzi and E. Monzani, Org. Biomol. Chem., 2005, 3, 2632 RSC.
- T. Gunnlaugsson, P. E. Kruger, P. Jensen, F. M. Pfeffer and G. M. Hussey, Tetrahedron Lett., 2003, 44, 8909 CrossRef CAS.
- V. Amendola, D. Esteban-Gómez, L. Fabbrizzi and M. Licchelli, Acc. Chem. Res., 2006, 39, 343 CrossRef CAS.
- D. E. Gómez, L. Fabbrizzi, M. Licchelli and E. Monzani, Org. Biomol. Chem., 2005, 3, 1495 RSC.
- S. Camiolo, P. A. Gale, M. B. Hursthouse and M. E. Light, Org. Biomol. Chem., 2003, 1, 741 RSC.
- X. He, S. Hu, K. Liu, Y. Guo, J. Xu and S. Shao, Org. Lett., 2006, 8, 333 CrossRef CAS.
- D. Esteban-Gómez, L. Fabbrizzi and M. Licchelli, J. Org. Chem., 2005, 70, 5717 CrossRef CAS.
- D. Esteban-Gómez, L. Fabbrizzi, M. Licchelli and D. Sacchi, J. Mater. Chem., 2005, 15, 2670 RSC.
- M. Bonizzoni, L. Fabbrizzi, A. Taglietti and F. Tiengo, Eur. J. Org. Chem., 2006, 3567 CrossRef CAS.
- E. Quinlan, S. E. Matthews and T. Gunnlaugsson, Tetrahedron Lett., 2006, 47, 9333 CrossRef CAS.
- G.-Y. Qing, Y.-B. He, Y. Zhao, C.-G. Hu, S.-Y. Liu and X. Yang, Eur. J. Org. Chem., 2006, 1574 CrossRef CAS.
- M. Albrecht, Triyanti, M. de Groot, M. Bahr and E. Weinhold, Synlett, 2005, 2095 CrossRef CAS.
- F.-Y. Wu, Z. Li, L. Guo, X. Wang, M.-H. Lin, Y.-F. Zhao and Y.-B. Jiang, Org. Biomol. Chem., 2006, 4, 624 RSC.
- L. S. Evans, P. A. Gale, M. E. Light and R. Quesada, Chem. Commun., 2006, 965 RSC.
- L. S. Evans, P. A. Gale, M. E. Light and R. Quesada, New J. Chem., 2006, 30, 1019 RSC.
- S. J. Brooks, P. A. Gale and M. E. Light, Chem. Commun., 2005, 4696 RSC.
- Z. Yin, Z. Li, A. Yu, J. He and J. P. Cheng, Tetrahedron Lett., 2004, 45, 6803 CrossRef CAS.
- S. J. Brooks, P. R. Edwards, P. A. Gale and M. E. Light, New J. Chem., 2006, 30, 65 RSC.
- S. J. Brooks, P. A. Gale and M. E. Light, Chem. Commun., 2006, 4344 RSC.
- T. Gunnlaugsson, A. P. Davis, J. E. O'Brien and M. Glynn, Org. Biomol. Chem., 2005, 3, 48 RSC.
- K. Ghosh and S. Adhikari, Tetrahedron Lett., 2006, 47, 8165 CrossRef CAS.
- E. J. Jun, K. M. K. Swamy, H. Bang, S.-J. Kim and J. Yoon, Tetrahedron Lett., 2006, 47, 3103 CrossRef CAS.
- S.-i. Kondo and M. Sato, Tetrahedron, 2006, 62, 4844 CrossRef CAS.
- T. Gunnlaugsson, P. E. Kruger, P. Jensen, J. Tierney, H. D. P. Ali and G. M. Hussey, J. Org. Chem., 2005, 70, 10875 CrossRef CAS.
- F. M. Pfeffer, A. M. Buschgens, N. W. Barnett, T. Gunnlaugsson and P. E. Kruger, Tetrahedron Lett., 2005, 46, 6579 CrossRef.
- F. M. Pfeffer, T. Gunnlaugsson, P. Jensen and P. E. Kruger, Org. Lett., 2005, 7, 5357 CrossRef CAS.
- D. Garozzo, G. Gattuso, A. Notti, A. Pappalardo, S. Pappalardo, M. F. Parisi, M. Perez and I. Pisagatti, Angew. Chem., Int. Ed., 2005, 44, 4892 CrossRef CAS.
- K. Lang, P. Cuřínová, M. Dudič, P. Prošková, I. Stibor, V. Št'astný and P. Lhoták, Tetrahedron Lett., 2005, 46, 4469 CrossRef CAS.
- P. Lhoták, J. Svoboda and I. Stibor, Tetrahedron, 2006, 62, 1253 CrossRef CAS.
- C. A. Winschel, A. Kalidindi, I. Zgani, J. L. Magruder and V. Sidorov, J. Am. Chem. Soc., 2005, 127, 14704 CrossRef CAS.
- C. E. Stanley, N. Clarke, K. M. Anderson, J. A. Elder, J. T. Lenthall and J. W. Steed, Chem. Commun., 2006, 3199 RSC.
- D. R. Turner, M. J. Paterson and J. W. Steed, J. Org. Chem., 2006, 71, 1598 CrossRef CAS.
- J. W. Steed, Chem. Commun., 2006, 2637 RSC.
- M. H. Filby, T. D. Humphries, D. R. Turner, R. Kataky, J. Kruusma and J. W. Steed, Chem. Commun., 2006, 156 RSC.
- A. M. Costero, J. P. Villarroya, S. Gil, M. J. Aurell and C. Ramirez de Arellano, Tetrahedron, 2002, 58, 6729 CrossRef CAS.
- A. M. Costero, J. P. Villarroya, S. Gil, P. Gaviña and C. Ramírez de Arellano, Supramol. Chem., 2003, 15, 403 CrossRef CAS.
- A. M. Costero, M. Colera, P. Gaviña and S. Gil, Chem. Commun., 2006, 761 RSC.
- S. Rashdan, M. E. Light and J. D. Kilburn, Chem. Commun., 2006, 4578 RSC.
- C. Roussel, M. Roman, F. Andreoli, A. Del Rio, R. Faure and N. Vanthuyne, Chirality, 2006, 18, 762 CrossRef CAS.
- J. M. Russell, A. D. M. Parker, I. Radosavljevic-Evans, J. A. K. Howard and J. W. Steed, Chem. Commun., 2006, 269 RSC.
- A. B. Descalzo, K. Rurack, H. Weisshoff, R. Martínez-Mañez, M. D. Marcos, P. Amorós, K. Hoffmann and J. Soto, J. Am. Chem. Soc., 2005, 127, 184 CrossRef CAS.
- A. P. Davis, Coord. Chem. Rev., 2006, 250, 2939 CrossRef CAS.
- J. P. Clare, A. J. Ayling, J.-B. Joos, A. L. Sisson, G. Magro, M. N. Pérez-Payán, T. N. Lambert, R. Shukla, B. D. Smith and A. P. Davis, J. Am. Chem. Soc., 2005, 127, 10739 CrossRef CAS.
- A. L. Sisson, J. P. Clare and A. P. Davis, Chem. Commun., 2005, 5263 RSC.
- A. L. Sisson, J. P. Clare, L. H. Taylor, J. P. H. Charmant and A. P. Davis, Chem. Commun., 2003, 2246 RSC.
- K. M. Bhattarai, V. del Amo, G. Magro, A. L. Sisson, J.-B. Joos, J. P. H. Charmant, A. Kantacha and A. P. Davis, Chem. Commun., 2006, 2335 RSC.
- S.-Y. Liu, L. Fang, Y.-B. He, W.-H. Chan, K.-T. Yeung, Y.-K. Cheng and R.-H. Yang, Org. Lett., 2005, 7, 5825 CrossRef CAS.
- L. Fang, W.-H. Chan, Y.-B. He, D. W. J. Kwong and A. W. M. Lee, J. Org. Chem., 2005, 70, 7640 CrossRef CAS.
- S. Y. Liu, K. Y. Law, Y. B. He and W. H. Chan, Tetrahedron Lett., 2006, 47, 7857 CrossRef CAS.
- S.-Y. Liu, Y.-B. He, W. H. Chan and A. W. M. Lee, Tetrahedron, 2006, 62, 11687 CrossRef CAS.
- C. H. Park and H. E. Simmons, J. Am. Chem. Soc., 1968, 90, 2431 CrossRef CAS.
- C. Bazzicalupi, A. Bencini, A. Bianchi, L. Borsari, C. Giorgi, B. Valtancoli, C. Anda and A. Llobet, J. Org. Chem., 2005, 70, 4257 CrossRef CAS.
- C. Bazzicalupi, A. Bencini, A. Bianchi, V. Fusi, P. Paoletti and B. Valtancoli, J. Chem. Soc., Chem. Commun., 1994, 881 RSC.
- A. Bencini, A. Bianchi, P. Dapporto, V. Fusi, E. Garcia-España, M. Micheloni, P. Paoletti, P. Paoli, A. Rodriguez and B. Valtancoli, Inorg. Chem., 1993, 32, 2753 CrossRef CAS.
- A. Bencini, A. Bianchi, E. Garcia-España, V. Fusi, M. Micheloni, P. Paoletti, J. A. Ramirez, A. Rodriguez and B. Valtancoli, J. Chem. Soc., Perkin Trans. 2, 1992, 1059 RSC.
- C. Bazzicalupi, A. Bencini, A. Bianchi, A. Danesi, C. Giorgi, M. A. Martinez Lorente and B. Valtancoli, New J. Chem., 2006, 30, 959 RSC.
- M. Arca, A. Bencini, E. Berni, C. Caltagirone, F. A. Devillanova, F. Isaia, A. Garau, C. Giorgi, V. Lippolis, A. Perra, L. Tei and B. Valtancoli, Inorg. Chem., 2003, 42, 6929 CrossRef CAS.
- C. Bazzicalupi, A. Bencini, E. Faggi, A. Garau, C. Giorgi, V. Lippolis, A. Perra and B. Valtancoli, Dalton Trans., 2006, 1409 RSC.
- S. Carvalho, R. Delgado, N. Fonseca and V. Félix, New J. Chem., 2006, 30, 247 RSC.
- A. González-Álvarez, I. Alfonso, P. Díaz, E. García-España and V. Gotor, Chem. Commun., 2006, 1227 RSC.
- M. Mascal, Angew. Chem., Int. Ed., 2006, 45, 2890 CrossRef CAS.
- A. Frontera, J. Morey, A. Oliver, M. N. Piña, D. Quiñonero, A. Costa, P. Ballester, P. M. Deyà and E. V. Anslyn, J. Org. Chem., 2006, 71, 7185 CrossRef CAS.
- P. Blondeau, M. Segura, R. Pérez-Fernández and J. de Mendoza, Chem. Soc. Rev., 2007, 36, 198 RSC.
- V. D. Jadhav and F. P. Schmidtchen, Org. Lett., 2005, 7, 3311 CrossRef CAS.
- A. Gleich, F. P. Schmidtchen, P. Mikulcik and G. Müller, J. Chem. Soc., Chem. Commun., 1990, 55 RSC.
- V. D. Jadhav and F. P. Schmidtchen, Org. Lett., 2006, 8, 2329 CrossRef CAS.
- C. Schmuck, Coord. Chem. Rev., 2006, 250, 3053 CrossRef CAS.
- C. Schmuck and U. Machon, Chem.–Eur. J., 2005, 11, 1109 CrossRef CAS.
- C. Schmuck and U. Machon, Eur. J. Org. Chem., 2006, 4385 CrossRef CAS.
- S. L. Wiskur, H. Ait-Haddou, J. J. Lavigne and E. V. Anslyn, Acc. Chem. Res., 2001, 34, 963 CrossRef CAS.
- C. Schmuck and M. Schwegmann, J. Am. Chem. Soc., 2005, 127, 3373 CrossRef CAS.
- C. Schmuck and M. Schwegmann, Org. Biomol. Chem., 2006, 4, 836 RSC.
- C. Schmuck, M. Heil, J. Scheiber and K. Baumann, Angew. Chem., Int. Ed., 2005, 44, 7208 CrossRef CAS.
- C. Schmuck and M. Heil, Chem.–Eur. J., 2006, 12, 1339 CrossRef CAS.
- C. Schmuck and P. Wich, Angew. Chem., Int. Ed., 2006, 45, 4277 CrossRef CAS.
- C. Schmuck and L. Geiger, J. Am. Chem. Soc., 2004, 126, 8898 CrossRef CAS.
- C. Schmuck, D. Rupprecht and W. Wienand, Chem.–Eur. J., 2006, 12, 9186 CrossRef CAS.
- E. Alcalde, N. Mesquida, L. Pérez-García, C. Alvarez-Rúa, S. García-Granda and E. García-Rodriguez, Chem. Commun., 1999, 295 RSC.
- E. Alcalde, S. Ramos and L. Pérez-García, Org. Lett., 1999, 1, 1035 CrossRef CAS.
- J. Yoon, S. K. Kim, N. J. Singh and K. S. Kim, Chem. Soc. Rev., 2006, 35, 355 RSC.
- K. Chellappan, N. J. Singh, I.-C. Hwang, J. W. Lee and K. S. Kim, Angew. Chem., Int. Ed., 2005, 44, 2899 CrossRef CAS.
- W. W. H. Wong, M. S. Vickers, A. R. Cowley, R. L. Paul and P. D. Beer, Org. Biomol. Chem., 2005, 3, 4201 RSC.
- S. In and J. Kang, J. Inclusion Phenom. Macrocyclic Chem., 2006, 54, 129 Search PubMed.
- H. Kim and J. Kang, Tetrahedron Lett., 2005, 46, 5443 CrossRef CAS.
- S. In and J. Kang, Tetrahedron Lett., 2005, 46, 7165 CrossRef CAS.
- S. In, S. J. Cho and J. Kang, Supramol. Chem., 2005, 17, 443 CrossRef CAS.
- S. In, S. J. Cho, K. H. Lee and J. Kang, Org. Lett., 2005, 7, 3993 CrossRef CAS.
- T. Fahlbusch, M. Frank, J. Schatz and H. Schmaderer, Eur. J. Org. Chem., 2006, 1899 CrossRef CAS.
- S. K. Kim, N. J. Singh, S. J. Kim, H. G. Kim, J. K. Kim, J. W. Lee, K. S. Kim and J. Yoon, Org. Lett., 2003, 5, 2083 CrossRef CAS.
- J. Yoon, S. K. Kim, N. J. Singh, J. W. Lee, Y. J. Yang, K. Chellappan and K. S. Kim, J. Org. Chem., 2004, 69, 581 CrossRef CAS.
- S. K. Kim, N. J. Singh, J. Kwon, I.-C. Hwang, S. J. Park, K. S. Kim and J. Yoon, Tetrahedron, 2006, 62, 6065 CrossRef CAS.
- V. K. Khatri, S. Upreti and P. S. Pandey, Org. Lett., 2006, 8, 1755 CrossRef.
- H. Miyaji and J. L. Sessler, Angew. Chem., Int. Ed., 2001, 40, 154 CrossRef CAS.
- S.-i. Kondo, T. Suzuki, T. Toyama and Y. Yano, Bull. Chem. Soc. Jpn., 2005, 78, 1348 CrossRef CAS.
- S. Kondo, T. Suzuki and Y. Yano, Tetrahedron Lett., 2002, 43, 7059 CrossRef CAS.
- K. J. Winstanley, A. M. Sayer and D. K. Smith, Org. Biomol. Chem., 2006, 4, 1760 RSC.
- K. Ito, M. Nishiki and Y. Ohba, Chem. Pharm. Bull., 2005, 53, 1352 CrossRef CAS.
- J.-S. Wu, J.-H. Zhou, P.-F. Wang, X.-H. Zhang and S.-K. Wu, Org. Lett., 2005, 7, 2133 CrossRef CAS.
- A. Casnati, A. Sartori, L. Pirondini, F. Bonetti, N. Pelizzi, F. Sansone, F. Ugozzoli and R. Ungaro, Supramol. Chem., 2006, 18, 199 CrossRef CAS.
- M. S. Vickers and P. D. Beer, Chem. Soc. Rev., 2007, 36, 211 RSC.
- P. D. Beer, M. R. Sambrook and D. Curiel, Chem. Commun., 2006, 2105 RSC.
- J. A. Wisner, P. D. Beer, M. G. B. Drew and M. R. Sambrook, J. Am. Chem. Soc., 2002, 124, 12469 CrossRef CAS.
- M. R. Sambrook, P. D. Beer, M. D. Lankshear, R. F. Ludlow and J. A. Wisner, Org. Biomol. Chem., 2006, 4, 1529 RSC.
- K.-Y. Ng, A. R. Cowley and P. D. Beer, Chem. Commun., 2006, 3676 RSC.
- G. V. Oshovsky, D. N. Reinhoudt and W. Verboom, J. Am. Chem. Soc., 2006, 128, 5270 CrossRef CAS.
- G. V. Oshovsky, D. N. Reinhoudt and W. Verboom, J. Org. Chem., 2006, 71, 7441 CrossRef CAS.
- G. V. Oshovsky, D. N. Reinhoudt and W. Verboom, Eur. J. Org. Chem., 2006, 2810 CrossRef CAS.
- E. A. Katayev, G. D. Pantos, M. D. Reshetova, V. N. Khrustalev, V. M. Lynch, Y. A. Ustynyuk and J. L. Sessler, Angew. Chem., Int. Ed., 2005, 44, 7386 CrossRef CAS.
- V. S. Bryantsev and B. P. Hay, J. Am. Chem. Soc., 2005, 127, 8282 CrossRef CAS.
- V. S. Bryantsev and B. P. Hay, Org. Lett., 2005, 7, 5031 CrossRef CAS.
- B. P. Hay, T. K. Firman and B. A. Moyer, J. Am. Chem. Soc., 2005, 127, 1810 CrossRef CAS.
- O. B. Berryman, V. S. Bryantsev, D. P. Stay, D. W. Johnson and B. P. Hay, J. Am. Chem. Soc., 2007, 129, 48 CrossRef CAS.
- C. Garau, D. Quinonero, A. Frontera, P. Ballester, A. Costa and P. M. Deya, J. Phys. Chem. A, 2005, 109, 9341 CrossRef CAS.
- V. Gorteau, G. Bollot, J. Mareda, A. Perez-Velasco and S. Matile, J. Am. Chem. Soc., 2006, 128, 14788 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2008 |

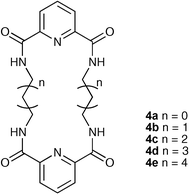
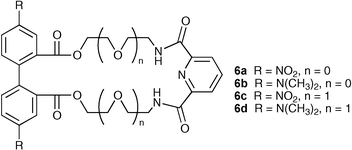
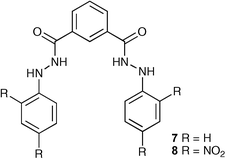
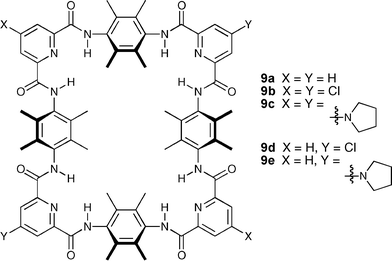
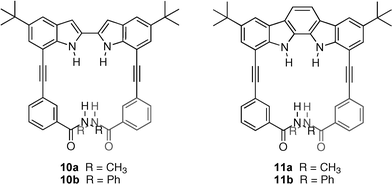
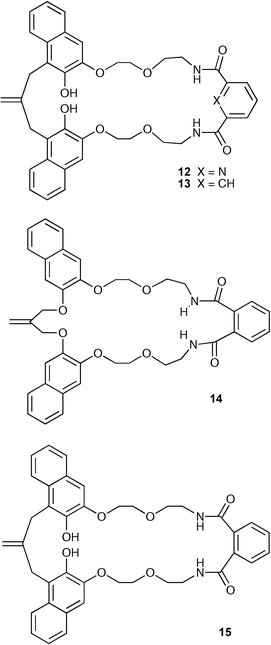
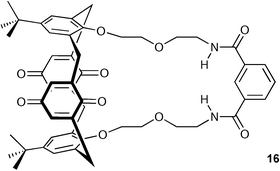
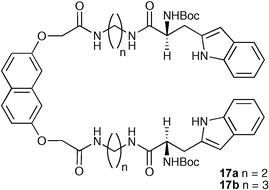
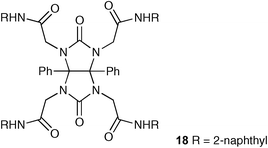
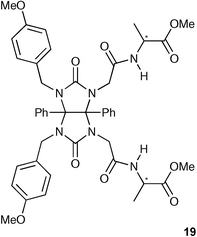
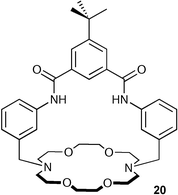
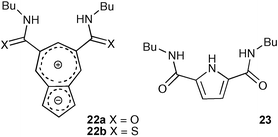
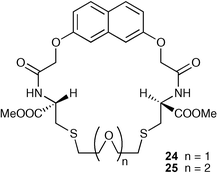
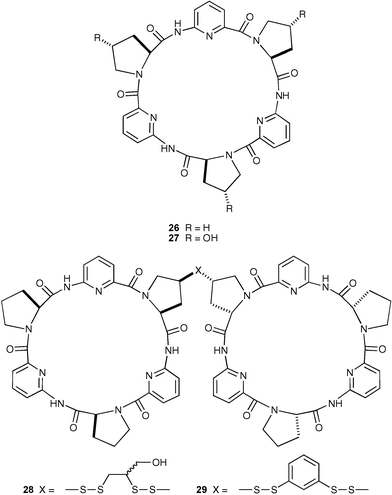
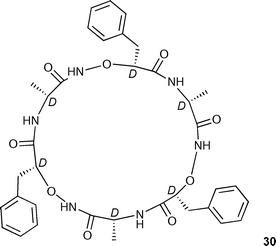
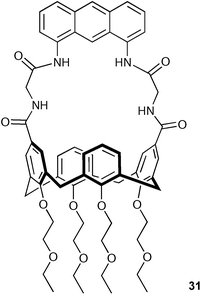
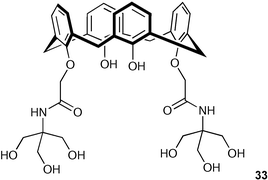
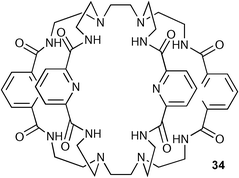
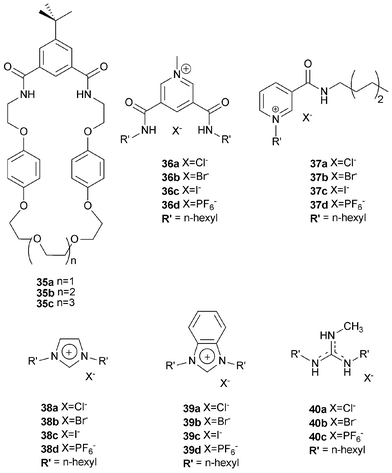
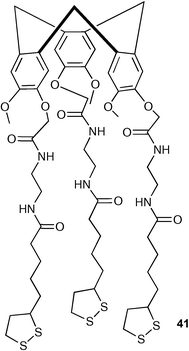
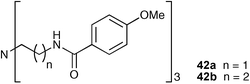
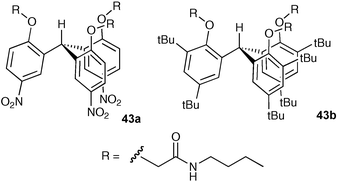
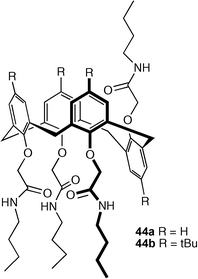
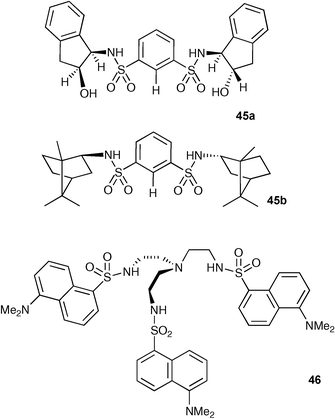
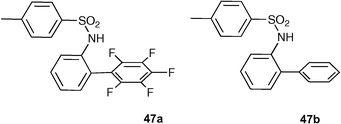
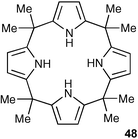
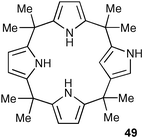
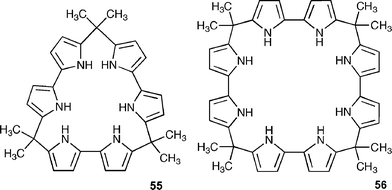
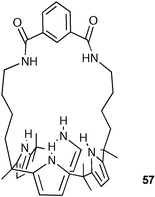
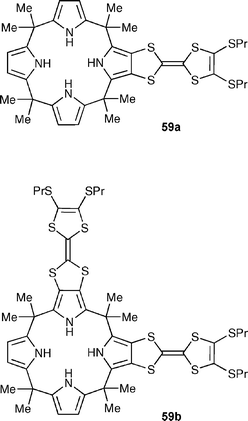
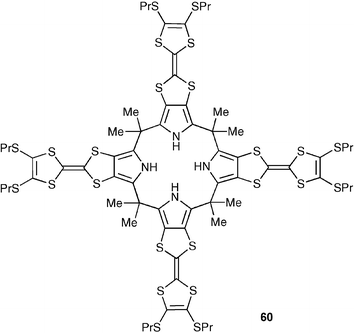
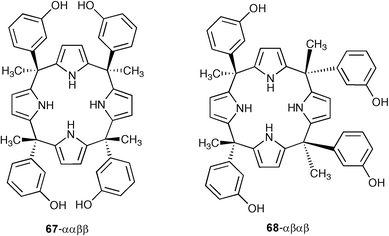
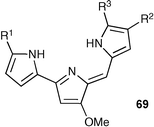
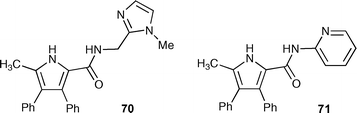
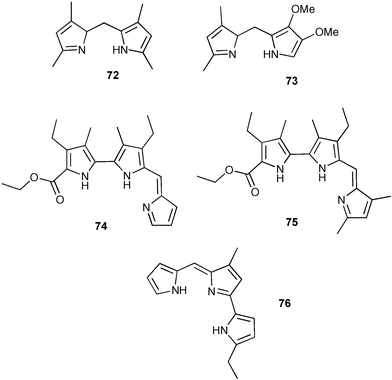
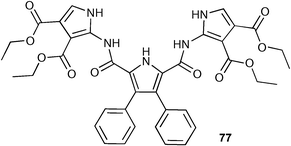
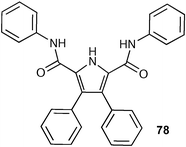
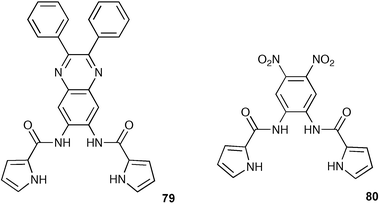
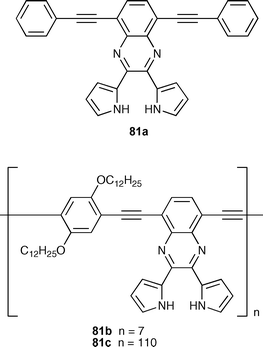
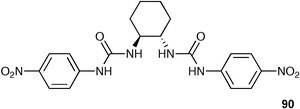
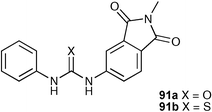
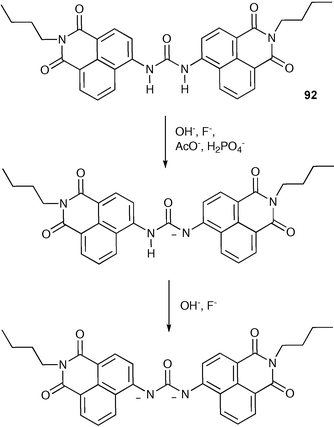
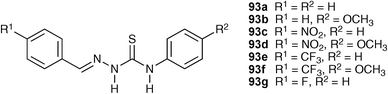
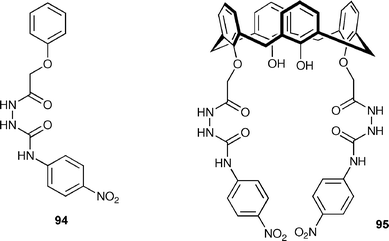
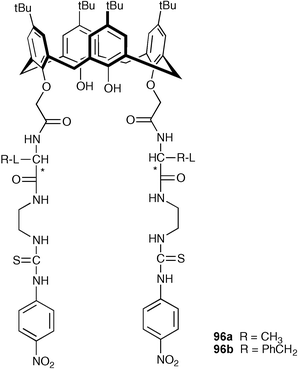
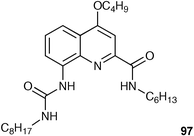
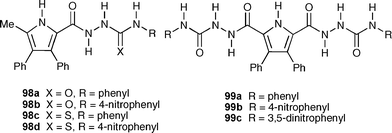
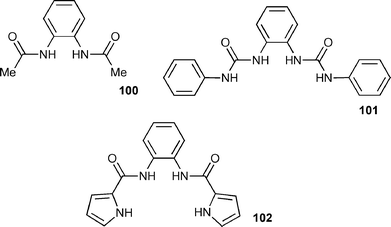
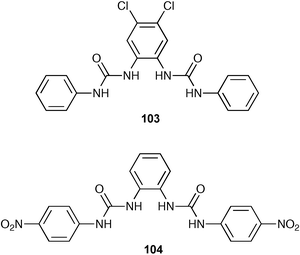
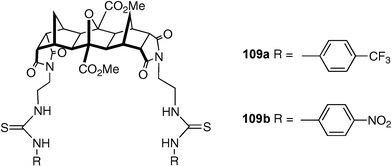
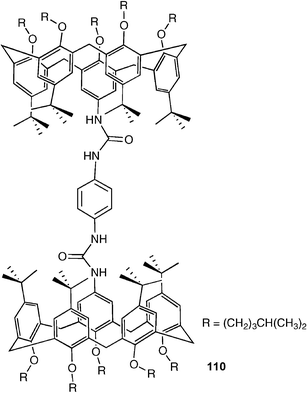
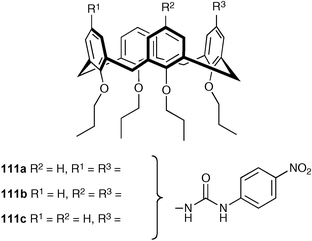
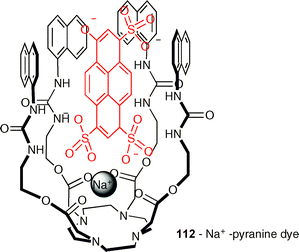
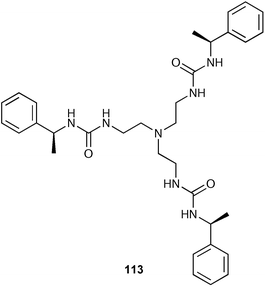
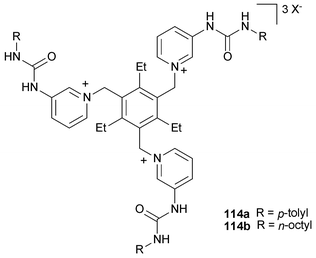
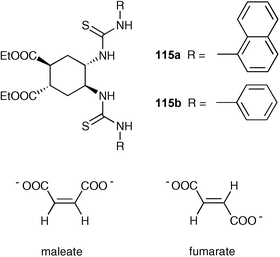
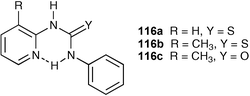
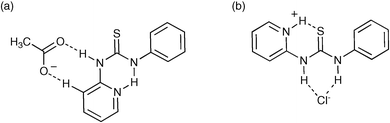
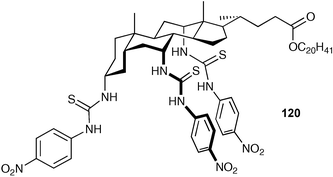
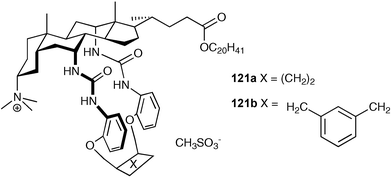
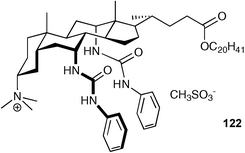
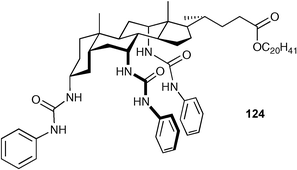
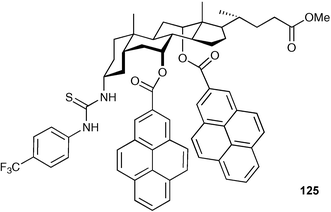
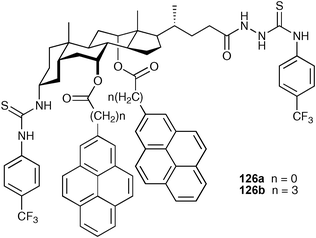
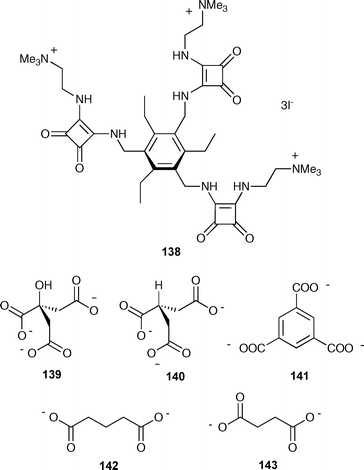
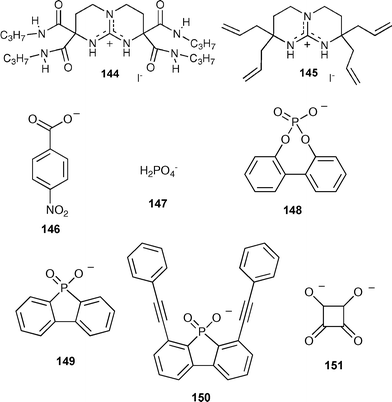
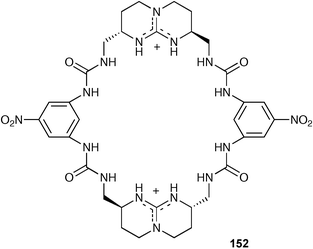
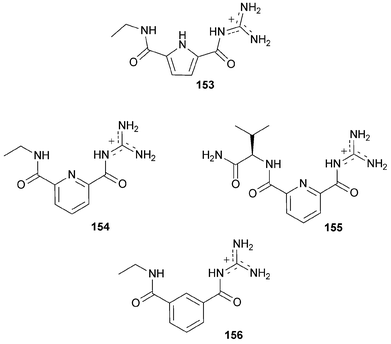
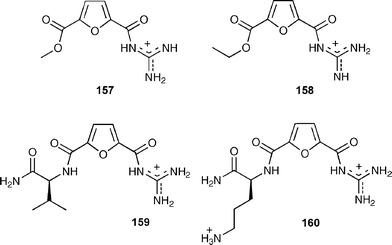
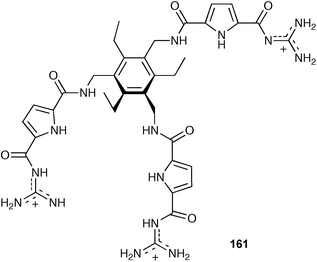
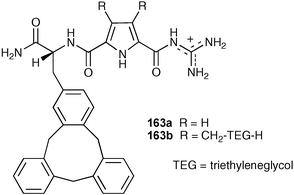
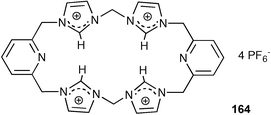
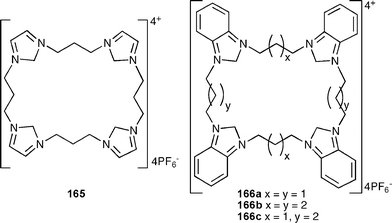
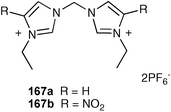
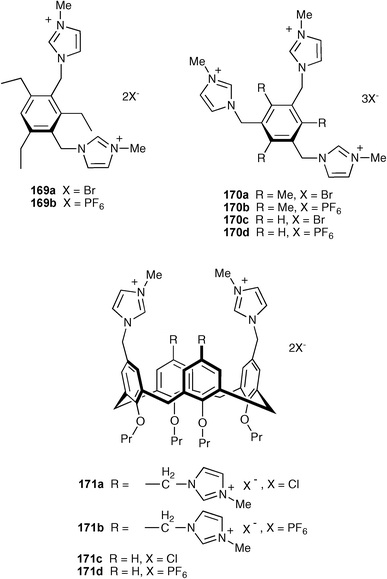
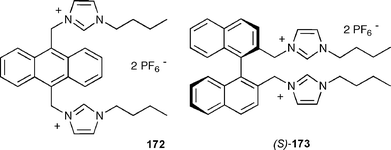
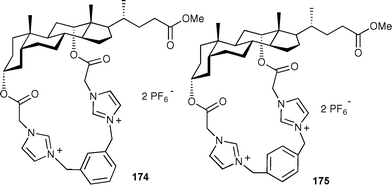
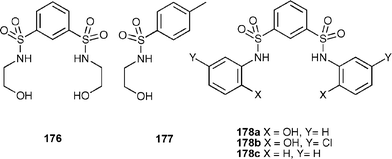
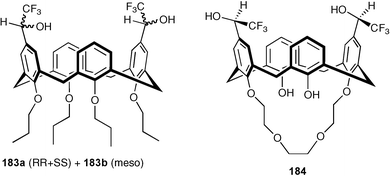
![[2]Rotaxane synthesis. (i) AgPF6, CH2Cl2.](/image/article/2008/CS/b715825d/b715825d-s13.gif)