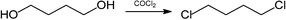Applications of ionic liquids in the chemical industry
Natalia V. Plechkovaa and Kenneth R. Seddonab
aQUILL, The Queen's University of Belfast, Stranmillis Road, Belfast, Northern Ireland UK BT9 5AG. E-mail: quill@qub.ac.uk
bInstituto de Tecnologia Química e Biológica, ITQB 2, Universidade Nova de Lisboa, Apartado 127, 2780-901, Oeiras, Portugal
First published on 30th November 2007
Abstract
In contrast to a recently expressed, and widely cited, view that “Ionic liquids are starting to leave academic labs and find their way into a wide variety of industrial applications”, we demonstrate in this critical review that there have been parallel and collaborative exchanges between academic research and industrial developments since the materials were first reported in 1914 (148 references)
 Natalia V. Plechkova | Natalia Plechkova was born in Moscow in 1979, and graduated from the Russian Mendeleev University of Chemical Technology, Moscow, with both BSc and MSc degrees in Chemistry. She studied for her PhD in QUILL, at the Queen's University of Belfast, and recently submitted her thesis. She has published over a dozen papers and patents on ionic liquids, and is currently a PDRA and project manager within QUILL, organising a team of 24 scientists. During her PhD, she also studied in research laboratories in Lisbon, Tokyo and Cambridge. |
 Kenneth R. Seddon | Kenneth Seddon was born in Liverpool in 1950, and graduated from Liverpool University with a first class BSc(Hons) and a PhD, whence he moved to a research Fellowship at St Catherine's College, Oxford, and later to a Lectureship in Experimental Chemistry at the University of Sussex. In 1993, he was appointed to the Chair in Inorganic Chemistry at the Queen's University of Belfast, where he is also a co-director of QUILL (Queen's University Ionic Liquids Laboratories), an industrial–academic consortium which was awarded the 2006 Queen's Anniversary Prize for Higher and Further Education. He is a Professor Catedrático Visitante at ITQB (New University of Lisbon), holds an honorary chair at the DICP in Dalian, China (where he directs a research group at CHILL – modelled on QUILL), and is Adjunct Director of the Centre for Green Manufacturing at the University of Alabama. He has published over 300 papers and patents, co-authored four books, and co-edited eight books. |
“If you want to find something new, look for something new!” Yves Chauvin, Nobel Address, 2005.1
In a recent front page article in Chemical and Engineering News entitled ‘Out Of The Ivory Tower’, it was2 argued that “Ionic liquids are starting to leave academic labs and find their way into a wide variety of industrial applications”. By following the history of ionic liquids, the major developments in the area, and the development of industrial processes, we will demonstrate that this view is misleading. In fact, we map the parallel developments between academic research and industrial developments since the materials were first reported in 1914.
1 History doesn't repeat itself; historians merely repeat each other
1.1 In the beginning
The field of ionic liquids began in 1914 (see Fig. 1) with an observation by Paul Walden (see Fig. 2),3 who reported the physical properties of ethylammonium nitrate ([EtNH3][NO3]; mp 13–14 °C), which was formed by the neutralisation of ethylamine with concentrated nitric acid.4 It is extremely instructive to revisit this paper, as the thinking behind the work, and some of the issues of association are as relevant today as they were in 1914. He wrote: “In the following, I will disclose my investigations on the electric conductivity and, derived from the capillarity constant, the molecular size of some organic ammonium salts. Anhydrous salts were chosen, which melt at relatively low temperatures, approximately up to 100 °C. These low melting points limited the degree of thermolysis of both the solvent and the dissolved salts in the molten salt. Therefore, they allowed for the reproducibility of the observation of melts of anhydrous mineral salts at low temperatures previously only feasible at high temperatures. They offered the possibility to conduct all measurements by means of the methods and apparatus employed at usual temperatures. The conditions in these low-melting salts approximated thus those experimental conditions applied to investigate conventional aqueous and non-aqueous solvents in detail, which are anticipated by the osmotic theory of van't Hoff and the theory of electrolytic dissociation of Arrhenius.” And then later, “The general picture of these organic salts at low temperatures (below, or around 100 °C) thus corresponds to the experiences made with inorganic (single, non-complexing) molten salts at much higher temperatures (approximately between 300 and 600 °C). In either case, if the salt molecules are associated, and if this (approximate) degree of association increases from x = 6–9, a complete dissociation of the simple ions of the molten salt of course does not exist; although an electrolytic dissociation appears to be existent, but it affects certainly the presence of complex ions besides the non-complexing ions (as a result of a step-wise cleavage of the associated salt molecules). The degree of dissociation thus affects both types of ions and will reach different values for each particular salts (i.e. subject to the nature of the cation and anion), depending on the composition and type of binary salt.” Discussions and arguments about the state of association/dissociation are still current in 2007.5 | ||
| Fig. 1 Extracts from the first paper describing an ionic liquid.4 | ||
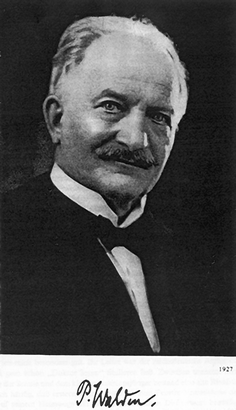 | ||
| Fig. 2 Paul Walden, the discoverer of ionic liquids (and the Walden inversion).3 | ||
However, despite Walden's clear exposition, and his discovery of a new class of liquids, this paper did not prompt any significant interest at the time. Today, however, this is widely acknowledged as the start of the field of ionic liquids, and it has left an important legacy. The current definition for ionic liquids is derived from here: ionic liquids are most commonly defined as materials that are composed of cations and anions which melt at or below 100 °C. This temperature does not have any chemical or physical significance, but has persisted until the present day; it is only now that it is being queried.
1.2 The industrial revolution
After two decades of silence, ionic liquids (in the guise of “liquefied quaternary ammonium salts”) appear in a patent from 1934 (see Fig. 3), which claimed that when halide salts of nitrogen-containing bases (such as 1-benzylpyridinium chloride, 1-ethylpyridinium chloride, etc.) were mixed with cellulose at the temperatures above 100 °C, the cellulose dissolved, and solutions of a different viscosity were formed.6 These new solutions, that contained the cellulose in a very reactive form, were suitable for various chemical reactions, i.e. etherification and esterification. The cellulose derivatives, if not precipitated directly, could be separated from the solutions using suitable precipitating agents and used for producing threads, films and artificial masses. This patent is a herald of what scientists are trying to achieve nowadays using cellulose, which will be described later (see Section 3.1.4). | ||
| Fig. 3 The first patent relating to an industrial application of ionic liquids.6 | ||
1.3 The shape of things to come
After another fallow phase in their history, ionic liquids re-emerged in the period just after World War II. In the patent literature in 1948,7 and later in the open literature,8 the application of mixtures of aluminium(III) chloride and 1-ethylpyridinium bromide to the electrodeposition of aluminium was described. The phase diagram of AlCl3 and 1-ethylpyridinium bromide system is shown in Fig. 4, and demonstrates that there is a narrow window of composition between 63 and 68 mole per cent of aluminium(III) chloride where the system is liquid at or below room temperature. This is an important discovery, but the selected system based on mixed bromide and chloride salts meant that the solvent was chemically complicated and hence difficult to investigate. This problem was eliminated after another 25 years, when the Osteryoung group in 1975,9 aided by Bernard Gilbert,10 studied in detail the chemical and physical properties of ionic liquids made from 1-butylpyridinium chloride and aluminium(III) chloride, the [C4py]Cl–AlCl3 system.† The phase diagram of AlCl3 and 1-butylpyridinium chloride, surprisingly given its importance, is poorly determined in the literature, and the published partial diagrams are shown in Fig. 5.11 The room temperature (20 °C) liquid range occurs between about 60 and 67% mole per cent of aluminium(III) chloride. But whilst being investigated for their fundamental properties, applications of these ionic liquids were also being studied12 and patented13 by the United States Air Force, who were (inter alia) interested in developing ionic liquids as battery electrolytes.14,15![Phase diagram for the [C2py]Br–AlCl3.8](/image/article/2008/CS/b006677j/b006677j-f4.gif) | ||
| Fig. 4 Phase diagram for the [C2py]Br–AlCl3.8 | ||
![Two partial phase diagrams for [C4py]Cl–AlCl3.11](/image/article/2008/CS/b006677j/b006677j-f5.gif) | ||
| Fig. 5 Two partial phase diagrams for [C4py]Cl–AlCl3.11 | ||
1.4 Let there be light
Whilst the work of Osteryoung's group (see Section 1.3) was a real breakthrough in the field, the system they had developed, [C4py]Cl–AlCl3, had serious limitations. It was liquid at room temperature, but only over a very narrow compositional range, and the cation was very easily reduced (a real constraint for work on battery applications and electrodeposition). In an elegant and inspired study,16 unfortunately never fully published in the open literature, Wilkes and Hussey used MNDO (MNDO = Modified Neglect of Differential Overlap) semi-empirical molecular orbital calculations to predict the LUMO energies for about sixty heterocyclic cations, and then correlated these with extant reduction potentials.14 The result was a prediction that 1,3-dialkylimidazolium cations, known since 1884,17 would be substantially more stable to reduction than the 1-alkylpyridinium cations. This led to the synthesis of the now archetypal ionic liquid system, [C2mim]Cl–AlCl3,18‡ and its subsequent characterisation.19–23 The phase diagram of this system21 is illustrated in Fig. 6.![The phase diagram for the [C2mim]Cl–AlCl3 system.21](/image/article/2008/CS/b006677j/b006677j-f6.gif) | ||
| Fig. 6 The phase diagram for the [C2mim]Cl–AlCl3 system.21 | ||
![The concentration of anions in the [C2mim]Cl–AlCl3 system as a function of composition, X(AlCl3).](/image/article/2008/CS/b006677j/b006677j-f7.gif) | ||
| Fig. 7 The concentration of anions in the [C2mim]Cl–AlCl3 system as a function of composition, X(AlCl3). | ||
From Fig. 6, it is immediately clear that the liquid range of this system is much wider that for the Hurley and Wier (Fig. 4) or Osteryoung (Fig. 5) systems: it is a low viscosity liquid at room temperature from X(AlCl3) = 0.33 to X(AlCl3) = 0.67 {where X(AlCl3) is the mole fraction of the notional aluminium(III) chloride content, although it should be clearly noted that there is no uncomplexed aluminium(III) chloride present}.
The most distinctive feature of the chloroaluminate(III) systems (which have been reviewed in detail elsewhere)24,25 is that, depending on the apparent mole fraction of aluminium(III) chloride, the ionic liquid is acidic {X(AlCl3) > 0.5}, basic {X(AlCl3) < 0.5}, or neutral {X(AlCl3) = 0.5}, referring to the Franklin acidity and basicity,26 although frequently assumed to refer to the Lewis acidity and basicity. The composition of the system is strongly dependent on X(AlCl3), as the primary equilibrium of eqn (1) (K ∼ 10–16.3 – and note how close this is to the auto-ionisation constant for water)23 dominates (see Fig. 7).
| 2[AlCl4]– ⇌ [Al2Cl7]– + Cl– | (1) |
For very acidic systems {X(AlCl3) = 0.67–0.75}, a further equilibrium, eqn (2), governs the anion concentration:
| 2[Al2Cl7]– ⇌ [Al3Cl10]– + [AlCl4]– | (2) |
A major drawback of all chloroaluminate(III) ionic liquids was their moisture sensitivity. Any trace of moisture decomposes these ionic liquids (although the hydrolysed aluminium species can be reconverted to the chloroaluminate(III) species by treatment with phosgene).29 This is a major disadvantage for industrial application, as moisture must be excluded from all the reagents and equipment involved in all operations. Similarly, all laboratory preparations must be carried out in an inert-atmosphere box, or by employing standard Schlenk techniques.
Thus, the predictions of Wilkes and Hussey16 were fully realised in the experiments, and the [C2mim]Cl–AlCl3 ionic liquid system also proved to be much less viscous than the [C4py]Cl–AlCl3 system, and had a much wider electrochemical window. This was the first genuine example of an ionic liquid system that was liquid at room-temperature: it opened up a field for electrochemistry specialists, and laid the foundations for the explosion of interest that was to follow.
1.5 Let there be air and water
In 1992, Wilkes and Zaworotko30 reported, under the title of “Air and water stable 1-ethyl-3-methylimidazolium based ionic liquids”, the preparation and characterisation of a new range of ionic liquids that still contained the 1-ethyl-3-methylimidazolium cation, but now also contained a range of alternative anions, [C2mim]X (X = [CH3CO2], [NO3] or [BF4]).30 These new salts could be prepared and used on the bench, rather than in an inert-atmosphere box. Since 1992, a wide range of ionic liquids has been developed incorporating many different anions: including hexafluorophosphate, ethanoate, trifluoroethanoate, sulfate, hydrogensulfate, alkylsulfate, nitrate, biscyanamide [N(CN)2]–, trifluoromethanesulfonate [CF3SO3]–, bis{(trifluoromethyl)sulfonyl}amide [N(CF3SO2)2]–, and tris{(trifluoromethyl)sulfonyl}methanide [C(CF3SO2)3]–. This is thus another landmark paper, and justifiably widely cited. However, on an independent line, we should not forget that the first ionic liquid, [EtNH3][NO3] (see Section 1.1), was a nitrate, and that in the same year as the Wilkes and Zaworotko paper appeared, and predating it, Cooper and O'Sullivan reported the syntheses of [C2mim][CF3SO3] and [C2mim][CH3SO3] under the title “New, stable, ambient-temperature molten salts”, but only in a conference proceedings volume.31At that time, it was not fully appreciated how much these ionic liquids absorbed water from the atmosphere. They still need to be handled in dry atmosphere, even though they do not react with water. In the presence of moisture, hexafluorophosphate ionic liquids are the least acceptable media for electrochemistry, as they release HF. Therefore, ionic liquids based on more hydrophobic anions such as trifluoromethanesulfonate [CF3SO3]– (triflate; [OTf]–), bis{(trifluoromethyl)sulfonyl}amide [N(CF3SO2)2]– (bistriflamide, [NTf2]–), and tris{(trifluoromethyl)sulfonyl}methanide [C(CF3SO2)3]– ([CTf3]–), have been developed by Grätzel and coworkers.32,33 These ionic liquids have received extensive attention not only because of their low reactivity with water, but also because of their large electrochemical windows: the electrochemical window for water is 1.23 V, and for ionic liquids could be 5–6 V (see Fig. 8).
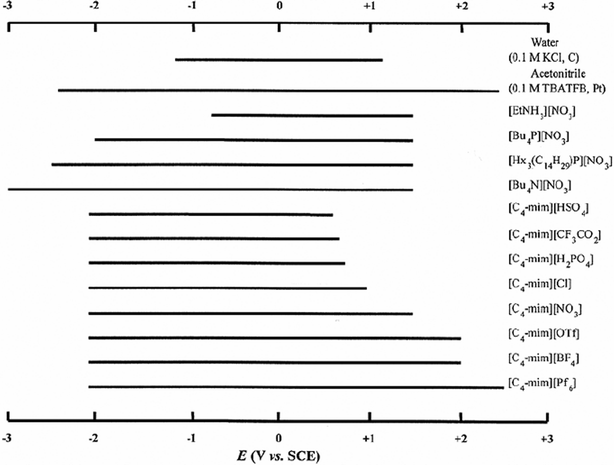 | ||
| Fig. 8 The electrochemical windows for some typical ionic liquids.34 | ||
Over the years that followed, new classes of cations, based on phosphonium and pyrrolidinium were developed. It soon became clear that over one million simple ionic liquids could be synthesised.35,36
1.6 Design and conquer
“A common mistake people make when trying to design something completely foolproof is to underestimate the ingenuity of complete fools.”Douglas Noel Adams, Mostly Harmless, 1992
The melting points of the Group 1 chlorides are given in Table 1. As can be seen, these are significantly above room temperature, and far too high to be used as a medium for organic chemistry. However, the eutectic compositions of mixed systems do melt at significantly lower temperatures than either component. Applying the understanding of lattice energies gained from the Kapustinskii equation,37 the effect of increasing the size of the anion can be seen in Table 2. The melting points of these simple tetrachloroaluminate(III) salts are in the range of the boiling points of high-boiling organic solvents. However, as this represents the lowest temperature at which these systems are liquids, and not the highest (as for organic solvents), it is necessary to bring the melting point down even further. This can be done by increasing the size of the cations: replacing the simple inorganic cations with organic cations depresses the melting point to temperatures at or below room temperature. Moreover, the asymmetry of the cation has been long recognised38 as an important factor in lowering the melting points: asymmetrical, “ugly” cations have a bigger effect than symmetrical “beautiful” cations. For example, salts of the “ugly” 1-butyl-3-methylimidazolium cation (which has only C1 symmetry, its only element of symmetry being the identity operator) melt at lower temperatures (by about 100 °C) than the analogous salts of the “prettier” 1-butylpyridinium cation (which has C2v symmetry). Moreover, conformational differences in the cations can frustrate crystallisation, leading to glass formation and/or polymorphism.39 The effect of symmetry in melting points is also reflected in the higher melting points reported for 1,3-dimethylimidazolium and 1,3-diethylimidazolium salts in comparison with the more unsymmetrical 1-ethyl-3-methylimidazolium or 1-butyl-3-methylimidazolium cation analogues.40 MacFarlane et al.41 also reported that salts derived from the ammonium or pyrrolidinium cations (see Fig. 9) were solid at room temperature when the cation substituents formed a highly symmetrical species, whereas lower symmetry forms produced liquids at room temperature (see Table 3), often displaying glass-forming behaviour. Finally, the phosphonium ionic liquids also display this phenomenon. In the series [P666n][PF6], the melting points (see Fig. 10) maximise at the salt with the symmetrical (Td) cation, [P6666][PF6], and drop away as one of the chains gets either longer or shorter, with concomitant symmetry lowering to C3v.
![Some commonly used ionic liquid systems.36 The abbreviation [Cnmpyr]+ represents the 1-alkyl-1-methylpyrrolidinium cation, where the index n represents the number of carbon atoms in the linear alkyl chain. [Pwxyz]+, [Nwxyz]+ and [Sxyz]+ are normally used to represent tetraalkylphosphonium, tetraalkylammonium and trialkylsulfonium cations, respectively, where the indices w, x, y and z indicate the length of the corresponding linear alkyl chains.](/image/article/2008/CS/b006677j/b006677j-f9.gif) | ||
| Fig. 9 Some commonly used ionic liquid systems.36 The abbreviation [Cnmpyr]+ represents the 1-alkyl-1-methylpyrrolidinium cation, where the index n represents the number of carbon atoms in the linear alkyl chain. [Pwxyz]+, [Nwxyz]+ and [Sxyz]+ are normally used to represent tetraalkylphosphonium, tetraalkylammonium and trialkylsulfonium cations, respectively, where the indices w, x, y and z indicate the length of the corresponding linear alkyl chains. | ||
![The melting points of [P666n][PF6] as a function of n.42](/image/article/2008/CS/b006677j/b006677j-f10.gif) | ||
| Fig. 10 The melting points of [P666n][PF6] as a function of n.42 | ||
| System | mol% | mp/°C |
|---|---|---|
| LiCl | 100 | 610 |
| NaCl | 100 | 803 |
| KCl | 100 | 772 |
| CsCl | 100 | 646 |
| LiCl–CsCl | 60–40 | 355 |
| NaCl–KCl | 50–50 | 658 |
| CsCl–KCl | 35–65 | 610 |
| System | mol% | mp/°C |
|---|---|---|
| AlCl3 | 100 | 192 |
| LiCl–AlCl3 | 50–50 | 144 |
| NaCl–AlCl3 | 50–50 | 151 |
| KCl–AlCl3 | 50–50 | 256 |
| Structure | Tg/°C | Tm/°C |
|---|---|---|
| [N1111][N(SO2CF3)2] | 133 | |
| [N1124][N(SO2CF3)2] | –92 | –8 |
| [C1mpyr][N(SO2CF3)2] | 132 | |
| [C4mpyr][N(SO2CF3)2] | –87 | –18 |
Most common ionic liquids are formed through the combination of an organic heterocyclic cation, such as dialkylimidazolium, and an inorganic or organic anion, such as nitrate or methanesulfonate. Typical cations and anions of ionic liquids, and their common abbreviations, are shown in Fig. 9. However, it should be remembered that, in principle, any singly charged cation or anion could be used, and ionic liquids based on doubly charged species have been reported too.
So, a spate of qualitative and semi-qualitative observations led to the conclusion that, to design ionic liquids, the use of an ugly cation with a single positive charge was a good criterion. This was recently supported by a computational study of anion and cation effects on the melting points of imidazolium salts, using a QSPR approach.43 Not only was the experimental melting point curve for the series [Cnmim][PF6] remarkably well reproduced (see Fig. 11), but the predictions were rationalised in terms of the cationic structure (see Fig. 12). This quantified and rationalised the empirical observations, and confirmed that the empirical design criteria had a sound theoretical grounding. So, as scientists, we must recognise the wisdom and perspective of artist John Constable:
“I never saw an ugly thing in my life: for let the form of an object be what it may - light, shade, and perspective will always make it beautiful.”
![Predicted and observed melting points for a series of 1-alkyl-3-methylimidazolium hexafluorophosphate, [Cnmim][PF6], ionic liquids with increasing carbon chain length on the cation.43](/image/article/2008/CS/b006677j/b006677j-f11.gif) | ||
| Fig. 11 Predicted and observed melting points for a series of 1-alkyl-3-methylimidazolium hexafluorophosphate, [Cnmim][PF6], ionic liquids with increasing carbon chain length on the cation.43 | ||
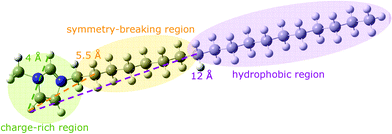 | ||
| Fig. 12 An optimised structure of a 1-methyl-3-octadecylimidazolium cation showing the structural regions that are important for determining the melting point. From left: at 4 Å the charge-rich region localised on the imidazolium ring; at 5.5 Å the symmetry-breaking region that decreases the melting point; from 12 Å onwards the hydrophobic region that increases the melting point due to van der Waals interactions.43 | ||
Another factor which influences melting point is the presence or absence of strong hydrogen bonds in the lattice. The presence of hydrogen bonding in the structures of 1-alkyl-3-methylimidazolium salt was first reported in 1986 (see Fig. 13),44 but despite the lack of recognition of the existence of CH⋯X– hydrogen bonds in those days,22 it soon became accepted as a real phenomenon.45 Hence, halide salts tend to have much higher melting points than, say, their tetrafluoroborate46 or hexafluorophosphate47 analogues.
1.7 Venimus, vidimus, vicimus (or, more aptly, ήρθαµε, εβλέίδαµε, κατακτήσαµε)
By the mid 1990s, the basic understanding of the ionic liquids concept was well known in a narrow scientific community, mostly electrochemists, but this area of esoteric curiosity was of little interest, or too specialised, for synthetic industrial applications. However there was a suggestion that ionic liquids could be used for green chemistry and industrial chemistry:38,40“The reactions we have observed represent the tip of an iceberg – all the indications are that room-temperature ionic liquids are the basis of a new industrial technology. They are truly designer solvents: either the cation or the anion can be changed, if not at will, then certainly with considerable ease, in order to optimise such phenomena as the relative solubilities of the reactants and products, the reaction kinetics, the liquid range of the solvent, the cost of the solvent, the intrinsic catalytic behaviour of the media, and air-stability of the system. For the first time, it is possible to design a solvent to optimise a reaction (with control over both yield and selectivity), rather than to let the solvent dictate the course of the reaction. […] This, quite literally, revolutionises the methodology of synthetic organic chemistry: it will never be the same again!”40
At that time, this suggestion must have seemed ludicrous. Only twenty papers per year were being published. There was no public hint of industrial applications. Nevertheless, behind the scenes, several industries (viz. BP, BNFL, Unilever) were filing patents related directly to potential uses of ionic liquids for large scale green industrial processes.48–50 By the late 1990s, with the publication of these patents on the use of ionic liquids as solvents for organic synthesis (both catalytic and non-catalytic) and the formation of an industrial academic consortium QUILL51 in Belfast involving sixteen companies, which aimed to develop generic ionic liquid technologies for industrial applications, there rose an increasing interest for green chemistry. In 2000, the dawn of the new millennium, at a crucial meeting in Crete, an Advanced Research Workshop sponsored by NATO and entitled “Green Industrial Applications of Ionic Liquids”, a round table was held. It outlined, in detail, a strategy for the development of ionic liquids for industrial applications and set criteria that would have to be met:52
(a) Ionic liquids are intrinsically interesting and worthy of study for advancing science (ionic vs. molecular solvents) with the expectation that something useful may be derived from their study;
(b) Combined with green chemistry, a new paradigm in thinking about synthesis in general, ionic liquids provide an opportunity for science/engineering/business to work together from the beginning of the field's development;
(c) Readily available, well characterised ionic liquids, free of intellectual property, are needed to encourage development of applications.
(d) Toxicity, biodegradation, bio-accumulation, safety, health, and environment (SHE) impact data are needed immediately;
(e) Ionic liquid research should include cost/benefit, economic, and life-cycle analyses;
(f) Regulatory road blocks to ionic liquids implementation should be tackled now;
(g) A public (free), verified, web-based database of physical, thermodynamic, and related data (i.e. not process specific) is needed, and work should start immediately on identifying the best methods to accomplish this;
(h) There is an urgent need to increase the number, but especially the areas of expertise, of ionic liquids researchers. A model of open collaboration needs to be encouraged;
(i) International collaboration, communication, and education regarding the results are needed; and
(j) A brochure should be developed to advance the understanding of ionic liquids and their applications.
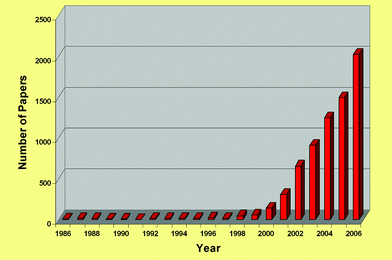
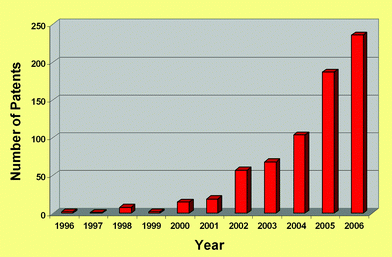
Now, in 2007, it can be seen that this was a very precise set of guidelines; all of the criteria have come to pass, especially (d),53 (g)54 (see Fig. 14) and (j),55,56 and we have recently expanded on the relationship between ionic liquids and green chemistry.57,58 The rate of publication in the open literature has been rising at a greater than exponential rate for years (see Fig. 15), and this is tracked by the rate of appearance of patents (see Fig. 16).59
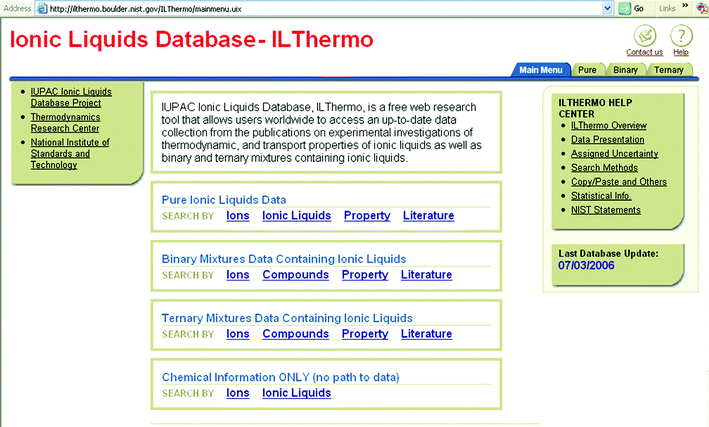 | ||
| Fig. 14 The home page of the IUPAC Ionic Liquids Database. | ||
Within the rest of this review, examples of applications of ionic liquids in industry will be shown. In retrospect, the Crete meeting can be seen as a crossroads§ in the development of ionic liquids, where the right path was chosen: it was attended by a healthy mix of academics and industrialists.
1.8 Out of the labyrinth
Daedalus designed it; Theseus emerged victorious from it, having slain the Minotaur, by following Ariadne's thread. To enter the world of ionic liquids, with over 8000 papers having been published in the last decade, can seem as daunting as the task which faced Theseus: what is needed is the aid of Ariadne. The required threads come in the form of the recent books published on the subject,52,60,61 and some useful reviews.25,36,62 But even here, a thread is needed: remarkably, a review of ionic liquids appears every two-to-three days, and papers are appearing faster than forty per week. The second edition of “Ionic Liquids in Synthesis” will be a welcome addition to the literature, and this is due to appear later this year.63Unlike the six hundred conventional solvents that are extensively used in industrial processes, ionic liquids represent a different paradigm. As there are at least a million simple ionic liquids (and a trillion ternary systems), they can be designed to optimise a specific reaction, for example to deliver maximum yield and purity of the isolated product. This is why ionic liquids¶ have been christened “designer solvents”.64 To give a simplistic example, ionic liquids can be composed of ions of low toxicity (even edible, in some cases),65 or conversely they can be made from such ions as cyanide or paraquat to produce exceptionally poisonous ionic liquids (gainsaying spurious claims that ionic liquids are intrinsically green).
It is essential to use solvents with negligible vapour pressure, and there are a few alternatives. One of the options is ionic liquids, which minimise the risk of atmospheric contamination and reduce associated health concerns. That is why ionic liquids are frequently called “green solvents”, as they can help to reduce the amount of solvent or catalyst in a chemical reaction, too. However it should be noted that one property of low vapour pressure does not make ionic liquids green. If ionic liquids are toxic and not biodegradable, they are not green.
It was almost universally believed that ionic liquids could not evaporate. However, recently, a paper by Earle et al. was published that demonstrated that, under the appropriate conditions of high temperature and low pressure, it was possible to distil ionic liquids, and that indeed mixtures of ionic liquids could be separated by fractional distillation.66 There was immediate doubt cast upon these findings, both in many e-mails addressed to the authors, and in a more open format, such as an article entitled “Are ionic liquids REALLY volatile?”.67 This was because the myth of involatility was so deeply embedded in the ionic liquid community that it was difficult to accept, especially as it seemed to cut across an almost theological creed that it was the lack of vapour pressure that made ionic liquids green.58 However, Wasserscheid, in commenting on this paper, states:68 “Do these developments bring into question the view that ionic liquids are ‘green solvents’? That reputation is built largely on their non-volatility, because they do not create the atmospheric pollution that can result from the volatility of classic organic solvents. The answer is no: Earle and colleagues clearly state that the vapour pressure of ionic liquids remains negligible at near ambient conditions, and many ionic liquids show no signs of distillation below the temperature of their thermal decomposition. What the proven volatility of some thermally stable ionic liquids under relatively mild (and close to technically realistic) conditions will most assuredly do, however, is make these substances more useful for us. New purification methods and reactions that use ionic liquids in the gas phase are territories that are now ripe to be explored. The possibility of separating two ionic liquids by distillation will, in particular, unlock the door to new routes towards the manufacture of ionic liquids, and towards the regeneration of spent ionic liquids from technical processes”. Now, a year on, the volatility of some ionic liquids is now widely accepted, and the nature of the gas-phase species has been established as tightly bound, discrete ion-pairs (and not clusters).69
Ionic liquids are intrinsically excellent candidates for industrial applications compared to volatile organic solvents. Organic solvents have been known for several centuries, and obviously occupy most of the solvent market in industry. However if the properties of ionic liquids and organic solvents are to be compared (see Table 4), it could be anticipated that industry may be a natural environment for ionic liquids. Since a lot of properties of ionic liquids have yet to be discovered, at the current level of development ionic liquids can nicely complement, and even sometimes work better than, organic solvents in a number of industrial processes. This statement should not however diminish the fact that ionic liquids have plenty of academic applications, and their unique properties due to their structure, which will be discussed later in this review, make them exciting compounds for theoretical and computational studies.71 A companion review for organic synthesis in an academic environment will appear in this journal later in 2008.72
| Property | Organic solvents | Ionic liquids |
|---|---|---|
| a The data summarised in this Table are not comprehensive, nor do they represent outliers; they are meant to give a brief visual comparison of typical values. Detailed values can be found elsewhere.70 | ||
| Number of solvents | >1000 | >1,000,000 |
| Applicability | Single function | Multifunction |
| Catalytic ability | Rare | Common and tuneable |
| Chirality | Rare | Common and tuneable |
| Vapour pressure | Obeys the Clausius–Clapeyron equation | Negligible vapour pressure under normal conditions |
| Flammability | Usually flammable | Usually nonflammable |
| Solvation | Weakly solvating | Strongly solvating |
| Polarity | Conventional polarity concepts apply | Polarity concept questionable |
| Tuneability | Limited range of solvents available | Virtually unlimited range means “designer solvents” |
| Cost | Normally cheap | Typically between 2 and 100 times the cost of organic solvents |
| Recyclability | Green imperative | Economic imperative |
| Viscosity/cP | 0.2–100 | 22–40,000 |
| Density/g cm–3 | 0.6–1.7 | 0.8–3.3 |
| Refractive index | 1.3–1.6 | 1.5–2.2 |
1.9 All things are ready, if our minds be so
As has been mentioned before, there are about 600 conventional solvents used in industry, compared to at least one million (106) simple ionic liquids that can be easily prepared in the laboratory.35 But that total is just for simple primary systems. At the moment only about 300 ionic liquids are commercialised, so one can imagine how many opportunities in this field are still undiscovered and why this field of chemistry is so tempting. If there are one million possible simple systems, then there are one billion (1012) binary combinations of these, and one trillion (1018) ternary systems possible!! And in case this seems like a cock-and-bull story, it is entirely plausible that to generate the required combination of reactivity, solubility and viscosity, industrial applications will need to work with ternary systems.49 At a zeroth order approximation, the cations in ionic liquids are responsible for their physical properties (such as melting point, viscosity, and density), whereas the anions control chemical properties and reactivity.36 Indeed, the choice of ionic liquid can control the outcome of a reaction; if the reaction between toluene and nitric acid is performed in three different ionic liquids, then three different products are formed (see Fig. 17).73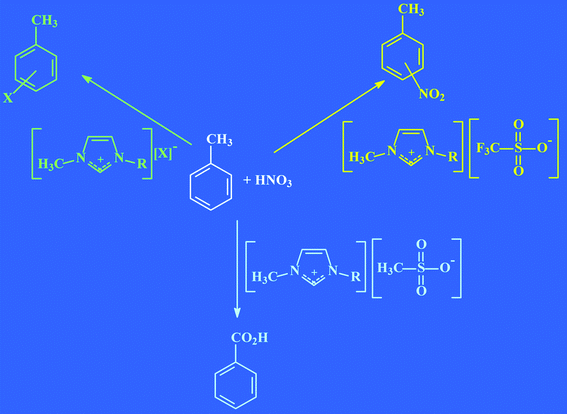 | ||
| Fig. 17 The reaction between toluene and nitric acid produces three different products in three different ionic liquids, each at better than 99% conversion.73 | ||
So, why are the ternary systems needed? The first choice, from a million, is to control and define the chemistry. Then, a second ionic liquid (now defining the solvent to one in a billion) may be added to fine tune the physical properties of the system (viz. viscosity, density, or thermal conductivity). Finally, a third cheap (but inert) ionic liquid (now defining the solvent to one in a trillion) can be added to lower the cost of the system. This means, as a new paradigm in chemistry, that the solvent can be fine-tuned to optimise the chemistry, the chemical engineering, and the cost of the system. Therefore ionic liquids can legitimately be called “designer” solvents, and offer a freedom and flexibility for process design previously unknown and undreamt.38,40,55,64
2 Suppliers of ionic liquids
2.1 To thine own self be true
It is self-evident that if one wants to use an ionic liquid, one has to have one. So, how do you get them? If working on a small scale, in academia or in industry, the best source of ionic liquids is to make them yourself. They are simple to prepare, and by controlling the synthesis, one controls the purity too. These routes have been discussed extensively elsewhere,36,60,74 and are outside the scope of this review, but it is crucial to monitor the purity of the ionic liquid, and to report it in all publications. As a bare minimum, all reports should include, in addition to NMR and MS analysis:(a) The method of synthesis of the ionic liquid
(b) The water content of the ionic liquid
(c) The halide content of the ionic liquid
Disgracefully few papers include this information, and this adds to the rather poor quality of many publications in this area.
The only viable alternatives to making them oneself are to either obtain them from a trusted synthetic collaborator, or to purchase them from a commercial supplier. In either case, it does not obviate the need for assessing the analytical purity of the ionic liquids as received and used. It is not always appreciated that even very hydrophobic ionic liquids absorb water rapidly from the atmosphere, and two examples of this are given in Fig. 18.
![Water absorption from the atmosphere as a function of time for [C4mim][BF4] (△) and [C4mim][PF6] (○).75](/image/article/2008/CS/b006677j/b006677j-f18.gif) | ||
| Fig. 18 Water absorption from the atmosphere as a function of time for [C4mim][BF4] (△) and [C4mim][PF6] (○).75 | ||
2.2 The price of everything, and the value of nothing
Obviously the important issues for industry are security of supply and cost.56 Considering firstly supply, this is no longer an issue as there are now many suppliers of ionic liquids, from the gram scale to the multi-ton scale {but it was a concern in 2000, see Section 1.7, point (b)}. Some of the key suppliers are listed in Table 5.| Supplier | Website |
|---|---|
| Merck KGaA/EMD Chemicals | http://ildb.merck.de/ionicliquids/en/startpage.htm |
| BASF | http://www.basionics.com |
| Cytec | http://www.cytec.com/ |
| SACHEM | http://www.sacheminc.com/products-and-services/ionic-liquids.html |
| DuPont | http://www.dupont.com/fluorointermediates/pdf/k15303.pdf |
| Scionix | http://www.scionix.co.uk |
| Solvent Innovation | http://www.solvent-innovation.com/index_overview.htm |
| IoLiTec | http://www.iolitec.de/ionic_e.htm |
| Accelergy | http://www.accelergy.com/ |
| ACROS | http://www.acros.com/_Rainbow/pdf/AO_IonicLiqui_Eur_def.pdf |
| Sigma-Aldrich | http://www.sigmaaldrich.com/catalog/search/TablePage/16255866 |
| Kanto Chemical Co. | http://www.kanto.co.jp/english/ |
| Nippon Gohsei | http://www.nichigo.co.jp/english/pro/chemical.html |
| Solchemar | http://www.solchemar.com/ |
| Chemada | http://www.chemada.com/cat0.htm |
Whilst it may be invidious to single out an individual supplier, it must be said that the Merck website is remarkably useful, containing an easily searchable database of ionic liquids, and summarising (and being searchable by) key physical properties: a typical search result is shown in Fig. 19. Moreover, Merck are unique in supplying ionic liquids at three different levels of specified purity, and have more ionic liquids in their catalogue than all the other suppliers combined. Merck further distinguishes itself with a focus on ionic liquids systems containing electrochemically stable anions, such as tris(pentafluoroethyl)trifluorophosphate and tetracyanoborate. Other sources worthy of highlighting are Cytec, who specialise in tetraalkylphosphonium ionic liquids, SACHEM, who specialise in tetraalkylammonium ionic liquids, BASF, who specialise in ionic liquids derived from 1-methylimidazole, and Scionix, who specialise in ionic liquids for electroplating and related processes, especially those derived from choline. All five of these suppliers can supply multi-ton quantities of ionic liquids.
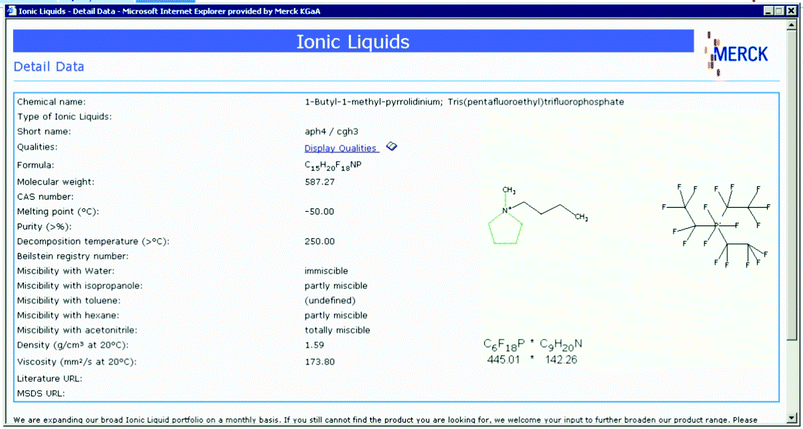 | ||
| Fig. 19 A typical screen capture from the Merck Ionic Liquids Database. | ||
Since ionic liquids are designer solvents, they can be based on ions of a very wide range regarding costs. For example, de facto, ionic liquids containing gold76 will be very expensive, and even more familiar ionic liquids based on the 1-alkyl-3-methylimidazolium cation, can also be quite costly, especially when coupled with the bis{(trifluoromethyl)sulfonyl}amide (or bistriflamide) ion. However not all ionic liquids are expensive, especially on an industrial scale: some ionic liquids, e.g. choline-based and tetraalkylphosphonium or tetraalkylammonium salts,77 are intrinsically less expensive than others.
Even getting ballpark figures for the cost is not straightforward: many of the websites only provide prices on application. However, some generalisations can be made; choline chloride sells at approximately the same price as toluene. On the Solchemar website, at the time of writing, 25 g of [C4mim]Cl was €25, but 2500 g was €599 (ca. £400, or £160 per kg) – four times cheaper per gram – which makes it only five times more expensive than dimethyl sulfoxide. So, to generalise, ionic liquids normally fall in the range of 5–20 times more expensive than molecular solvents, on a laboratory scale, but there are those which are cheaper, and those which are much more expensive. However, as it is at the heart of green chemistry to recycle ionic liquids, 10–20 recycles gives them the same cost per cycle as conventional organic solvents, and over 50 recycles makes them significantly cheaper.
3 I am come to survey the Tower this day
To return to the recent distorted view of the industrial development of ionic liquid technology,2 we will survey the Ivory Tower, but casting its walls wider than academia (where be these warders that they wait not here?)! The processes (either commercialised or pilot) will be considered according to the Company that has taken the work to scale, in no particular order.3.1 BASF
Of all the industrial giants, BASF has done the most publicly to implement ionic liquid technology. They possess the largest patent portfolio, have the broadest range of applications, and work openly with leading academics.The BASIL™ process is used for the production of the generic photoinitiator precursor alkoxyphenylphosphines (see Fig. 20). In the original process, triethylamine was used to scavenge the acid that was formed in the course of the reaction, but this made the reaction mixture difficult to handle as the waste by-product, triethylammonium chloride formed a dense insoluble paste. Replacing triethylamine with 1-methylimidazole results in the formation of 1-methylimidazolium chloride, an ionic liquid, which separates out of the reaction mixture as a discrete phase. This new process uses a much smaller reactor (see Fig. 21) than the initial process; the space-time yield is increased from 8 kg m–3 h–1 to 690,000 kg m–3 h–1, and the yield increased from 50% to 98%. 1-Methylimidazole is recycled, via base decomposition of 1-H-3-methylimidazolium chloride, in a proprietary process.79 The reaction is now carried out at a multi-ton scale, proving that handling large quantities of ionic liquids is practical. Its success was almost immediately recognised: the process won the ECN Innovation Award in 2004.80
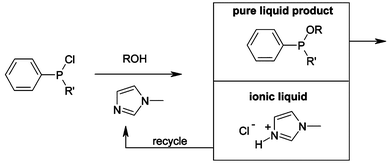 | ||
| Fig. 20 The BASIL™ process. | ||
 | ||
| Fig. 21 The BASIL jet stream reactor (© BASF 2007). | ||
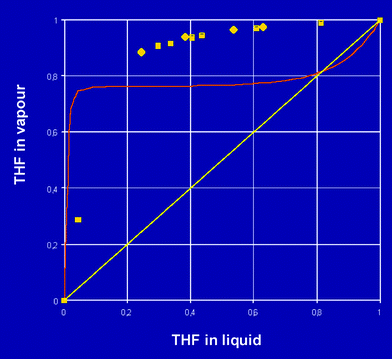 | ||
| Fig. 22 The phase diagram for the water–tetrahydrofuran system. The solid orange line represents the pure two component system; the yellow points represent the effect of adding the ionic liquid (© BASF 2007). | ||
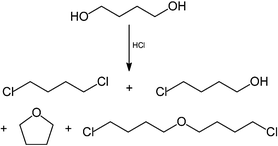
If the reaction is attempted with hydrogen chloride instead of phosgene, four products are formed, and the desired 1,4-dichlorobutane is only a minor by-product, the major products being tetrahydrofuran and 1-chlorobutan-4-ol:
However, if the hydrogen chloride is dissolved in an ionic liquid, then almost pure 1,4-dichlorobutane is obtained (98% selectivity).84
![SEM photo of aluminium deposited on sheet steel from [C2mim][AlCl4] at room temperature (© BASF 2007).](/image/article/2008/CS/b006677j/b006677j-f23.gif) | ||
| Fig. 23 SEM photo of aluminium deposited on sheet steel from [C2mim][AlCl4] at room temperature (© BASF 2007). | ||
3.2 Eastman Chemical Company
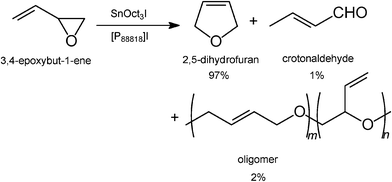
Although the BASIL™ process (see Section 3.1.1) was the first publicly announced room-temperature ionic liquid process, and was launched with a significant amount of publicity, Eastman Chemical Company had been running a process for the isomerisation of 3,4-epoxybut-1-ene to 2,5-dihydrofuran since December 1996.92,93This was operated by the Texas Eastman Division, in a plant with a 1400 metric tons per year capacity (see Fig. 24), at Longview until the end of 2004. The epoxidation of butadiene to 3,4-epoxybut-1-ene had been discovered in 1986 by Dr John R. Monnier of the Eastman Kodak Research Laboratories,94 and its isomerisation to 2,5-dihydrofuran was later discovered in 1988 by Dr Stephen N. Falling and coworkers of the Eastman Chemical Company.95 This isomerisation process critically requires a Lewis acid catalyst, and a Lewis basic ionic liquid, [P888![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18]I (ex Cytec). It operated with three continuous, stirred-tank reactors, a wiped-film evaporator, a distillation train, and a continuous, countercurrent, liquid–liquid extractor for recovery of the catalysts from December 1996 to December 2004.95 The phosphonium ionic liquid was selected over its ammonium analogue because of its greater thermal stability. The plant is now idle, because the market for the product has declined.
18]I (ex Cytec). It operated with three continuous, stirred-tank reactors, a wiped-film evaporator, a distillation train, and a continuous, countercurrent, liquid–liquid extractor for recovery of the catalysts from December 1996 to December 2004.95 The phosphonium ionic liquid was selected over its ammonium analogue because of its greater thermal stability. The plant is now idle, because the market for the product has declined.
 | ||
| Fig. 24 Texas Eastman Division EpB Chemical Semiworks Plant, Longview, Texas (© Eastman Chemical Company 2007). | ||
3.3 IFP (Institut Français du Pétrole) – Axens
Whereas Eastman Chemical Company and BASF commercialised the first ionic liquid processes, IFP was the first to operate an ionic liquid pilot plant.96 The Dimersol process, based on traditional technology, consists of the dimerisation of alkenes, typically propene (Dimersol-G) and butenes (Dimersol-X) to the more valuable branched hexenes and octenes.97 This is an important industrial process, with thirty-five plants in operation worldwide, each plant producing between 20,000 and 90,000 tonnes per year of dimer, with a total annual production of 3,500,000 tonnes.1,98 The longer-chain olefins produced in the dimerisation process are usually hydroformylated to alcohols (e.g. isononanols): isononanols are then converted into dialkyl phthalates, which are used as poly(vinyl chloride) plasticisers.The dimerisation reaction is catalysed by a cationic nickel complex of the general form [LNiCH2R′][AlCl4] (L = PR3) and is commonly operated without solvent. However, it has been found that the catalyst shows greater activity when it is dissolved in undesirable aromatic or halogenated hydrocarbons.1,97
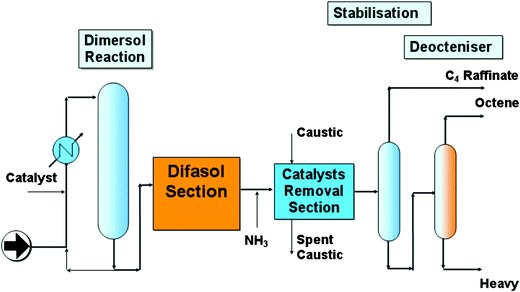
The use of chloroaluminate(III) ionic liquids as solvents for these nickel-catalysed dimerisation reactions has been developed and pioneered at IFP (France), especially by Nobel laureate Yves Chauvin and Hélène Olivier-Bourbigou.99,100 The reaction can be performed as a biphasic system between –15 °C and 5 °C, as the products form a second layer that can be easily separated and the catalysts remains selectively dissolved in the ionic liquid phase. The activity of the catalyst is much higher than in both solvent-free and conventional solvent systems, and the selectivity for desirable dimers is enhanced. This process has been patented as the Difasol process and can be retro-fitted (see Fig. 25 and Fig. 26) into existing Dimersol plants; it is operated by Axens, an IFP subsidiary, and is described in Chauvin's Nobel lecture.1
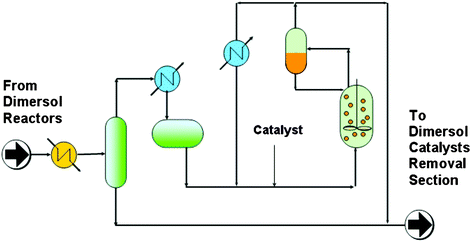 | ||
| Fig. 26 Difasol process scheme.99 | ||
The combined Dimersol–Difasol process was described for dimerisation of 2-methylbut-2-ene under laboratory conditions:99 the biphasic system is clearly superior (see Table 6).
In comparison with the homogeneous Dimersol process, the advantages of the biphasic Difasol system are:
• a much better use of the catalyst and therefore a reduced catalyst disposal and cost
• a better dimer selectivity
• a higher yield into dimers can be achieved in a single step even with a low concentration alkene feed
• a possible extension of the Dimersol process to higher less reactive olefins
• from an engineering point of view, the reactor size is much smaller than in the homogeneous system
When the Difasol reactor, which involves the use of an ionic liquid, was added to the existing Dimersol reactor, the process (Fig. 25) became much more efficient. The process uses a biphasic mixture (cf. the BASIL process, Section 3.1.1), which results in a much better use of the catalyst, and therefore a reduced catalyst disposal cost, and a better dimer selectivity. A higher yield into dimers can be achieved in a single step, even with a low-concentration alkene feed, and from an engineering point of view, the reactor size is much smaller than in the homogeneous system. Moreover, this new process can be possibly extended to higher, less reactive olefins.
3.4 Degussa
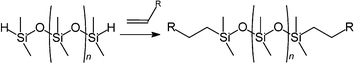
Like BASF (see Section 3.1), Degussa are developing ionic liquids on several distinguishable fronts. They also own a minority stake in Solvent Innovations.The hydrosilylation of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond with silane-functionalised polydimethylsiloxanes is a widely applied reaction in industrial synthesis for the production of organosilicon compounds on a technical scale. Degussa have used ionic liquids as a means of catalyst heterogenisation: the catalyst (ionic) is dissolved in the ionic liquid, from which there is no significant leaching, but in which the product is insoluble (see Fig. 27). Their aim was the synthesis of polyethersiloxanes, an important class of surface active compounds which find use in a broad range of industrial applications. The experimental procedure is rather simple, and can be described as a one-pot biphasic synthesis (Fig. 27).101,102
C double bond with silane-functionalised polydimethylsiloxanes is a widely applied reaction in industrial synthesis for the production of organosilicon compounds on a technical scale. Degussa have used ionic liquids as a means of catalyst heterogenisation: the catalyst (ionic) is dissolved in the ionic liquid, from which there is no significant leaching, but in which the product is insoluble (see Fig. 27). Their aim was the synthesis of polyethersiloxanes, an important class of surface active compounds which find use in a broad range of industrial applications. The experimental procedure is rather simple, and can be described as a one-pot biphasic synthesis (Fig. 27).101,102
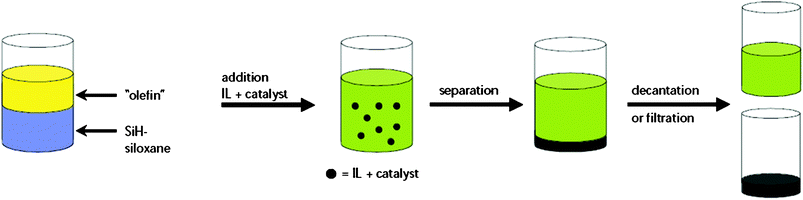 | ||
| Fig. 27 Schematic for the hydrosilylation process (yellow = alkene; blue = silane; green = product; black = catalyst dissolved in ionic liquid phase).102 | ||
Silane-functionalised polydimethylsiloxanes with different chain lengths and functionality patterns, and polyethers and ethers with different ethylene and propylene oxide content, were studied:101,103
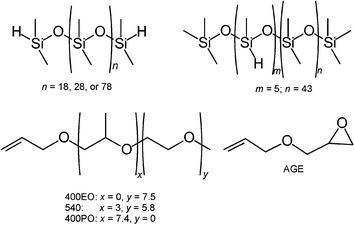
The catalysts used were H2[PtCl6] (ionic) and [(µ-Cl)2{PtCl(cyclohexene)}2] (molecular), and the ionic liquids studied were 1-butyl-4-methylpyridinium tetrafluoroborate, 1-butyl-3-methylpyridinium chloride, 1,2,3-trimethylimidazolium methylsulfate, and TEGO® IL K5MS (see Fig. 28). Hydrosilylation reactions in the presence of ionic liquids based on 1,3-dialkylimidazolium cations did not give the desired polyethersiloxanes, as the 2-H position of the cation was too reactive.
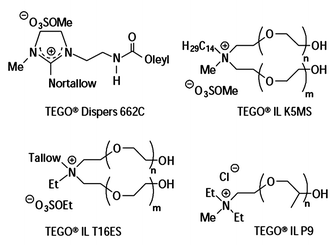 | ||
| Fig. 28 Ionic liquids used as paint additives for the Pliolite® range.102 | ||
Factors affecting the reaction included the influence of the hydrophilicity/hydrophobicity of the substrates and corresponding products on the catalytic performance of the various catalyst/ionic liquid solution combinations and, even more importantly, on the separation behaviour of the ionic liquid at the end of the reaction. A clean separation of the ionic liquid from the products is essential for the partitioning of the catalyst between the two phases. Naturally, without a clean separation and a much better solubility of the catalyst in the ionic liquid phase than in the product phase, a complete retention of the catalyst in the ionic liquid and the desired recovery of the catalyst cannot be achieved. The reaction was found to give high conversions (>99%) in short time periods, under most conditions. The principal conclusion was that the nature of the catalyst was the key variable. When the molecular catalyst, [(µ-Cl)2{PtCl(cyclohexene)}2], was used, it leached from the ionic liquid, and this leaching increased with increasing concentration of catalyst. However, using an ionic catalyst, H2[PtCl6], the leaching was below detectable levels (<1 ppm), and independent of catalyst concentration. It is now generally accepted that ionic catalysts in ionic liquids are anchored even more strongly than catalysts chemically attached to oxide or polymer surfaces.101,103
The hydrophobicity of the organosilicon products increases with increasing chain length and decreasing silane-functionalisation of the polydimethylsiloxane. The polyethers are more hydrophobic, the higher the content of propylene oxide. The ionic liquid phase separates more easily from the organosilicon products the more hydrophobic they are.
 | ||
| Fig. 29 Painted surfaces with paints with and without added ionic liquids (© Degussa 2007). | ||
3.5 Air products
Undoubtedly the talk which aroused the most interest at the first International Congress on Ionic Liquids (COIL-1) in Salzburg,93 was a presentation by Dan Tempel of Air Products.106 He revealed a new Air Products technology,107 which is based around entrainment by the complexing of reactive gases in ionic liquids. It produces at least twice the performance of the main rival process, which relies on the physical adsorption of gases on solids, and provides a method to deliver these reactive and hazardous gases in a safe, effective, more easily handled way, reducing both the risks and hazards in the work place – a true green chemistry success. The gases can be stored and transported at sub-atmospheric pressure (see Fig. 30), instead of the normal pressurised cylinders, adding dramatically to safety. It is marketed as Gasguard® Sub-Atmospheric System, Complexed Gas Technology (CGT); the gas is removed from the cylinder by the application of vacuum.108 | ||
| Fig. 30 CGT 11BF3 and CGT PH3 commercial offerings (2.2 litre cylinders) (© Air Products 2007). | ||
The system is based on the inherent Lewis acidity or basicity of the gases; Lewis acidic gases (such as boron(III) fluoride) are stored in Lewis basic ionic liquids (such as [Cnmim][BF4]), whereas Lewis basic gases (such phosphine, PH3, or arsine, AsH3) are stored in Lewis acid ionic liquids (such as [Cnmim][Cu2Cl3] or [Cnmim][Cu2Br3]). It can thus be used to deliver high purity gases to the electronics market.
3.6 Central Glass Co., Ltd., Japan
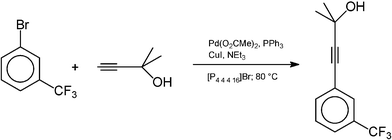
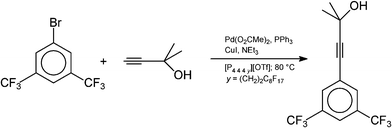
The Central Glass Company109 are, to the best of our knowledge, the first firm to produce pharmaceutical intermediates using ionic liquid technology, although a two-step synthesis of pravadoline had been described earlier in the open literature.110Sonogashira coupling is a palladium–copper catalysed reaction of aryl halides and terminal alkyl- or aryl-alkynes, allowing preparation of alkyl, aryl-, and diaryl-substituted alkynes, which can be used in optical, electronic and pharmaceutical applications. It is typically carried out in organic solvents (such as toluene, tetrahydrofuran, or N,N-dimethylmethanamide), and a stoicheiometric amount of base is required to trap the HX produced in the reaction. The Sonogashira coupling reaction in ionic liquids had been described several times in the open literature,111 but each time in the highly undesirable [C4mim][PF6] (the anion hydrolyses to release HF even in mild conditions).36
The following reaction was studied in detail by the Central Glass Co., Ltd.:
What they discovered was the use of 1,3-dialkylimidazolium cations led to reactions which were demonstrably less efficient than when conventional solvents were used (presumably due to carbene formation,36cf. Section 3.4.1). However, when tetraalkylphosphonium ionic liquids were used, the reaction was much more efficient than when carried out in organic solvents, or with neat reagents (see Fig. 31). From these results, the following reaction was developed and commercialised:
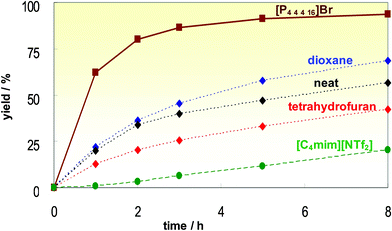 | ||
| Fig. 31 The Sonogashira reaction in various media. | ||
The product is separated from the catalyst-containing ionic liquid phase by extraction with hexane, and the by-product salt, [HNEt3]Br, removed with a counter-current flow of water. The remaining ionic liquid-catalyst solution can be recycled several times with little loss of catalytic activity, and a microflow reaction system can also be used for this reaction.
3.7 IoLiTec
IoLiTec (Ionic Liquids Technologies) are an interesting new company specialising in marketing ionic liquids (see Table 5), and developing applications.112 They have developed a practical and efficient technology to clean high value and sensitive surfaces, using ionic liquids as antistatic cleaning agents (see Fig. 32), the brush bristles being moistened by a fine spray of aqueous solution. Conventionally, dilute aqueous sodium chloride solutions have been used as the wetting agent, but the effect of replacing this with an ionic liquid is dramatically demonstrated in Fig. 33.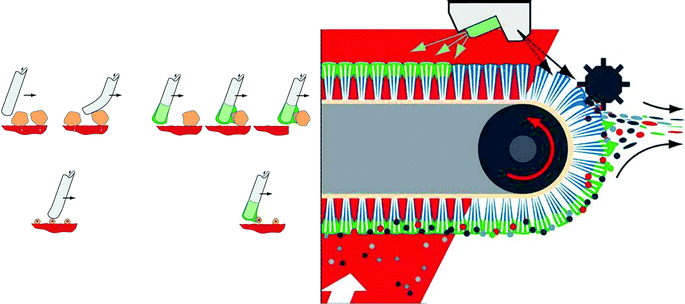 | ||
| Fig. 32 The removal of dust particles by brush bristles (left), which is much more efficient if they are coated with a conducting liquid film, applied by a spray from a fine nozzle (right) (© IoLiTec 2007). | ||
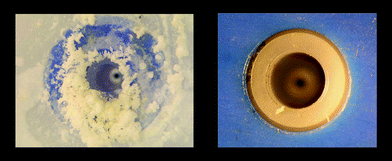 | ||
| Fig. 33 A spray nozzle for aqueous solutions of sodium chloride (left) and a hydrophilic ionic liquid (right), each after 10 h of operation (© IoLiTec 2007). | ||
IoLiTec has also interests in the application of ionic liquids in medicine (coated implants), food analytics, sensors, and heat pumps,112 and is currently involved in the development of various green applications based on ionic liquids, e.g. electrolytes for dye-sensitised solar cells (Grätzel cells or DSSCs),32,113 and phase change materials (PCMs)114 for the storage of solar energy and for sorption cooling media for “solar cooling”. Pilot installations for both DSSC and PCM applications are currently being constructed.
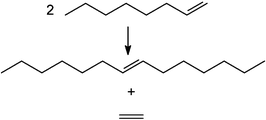
3.8 SASOL
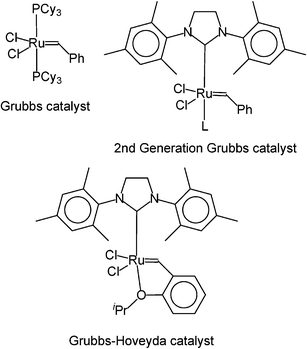
South African company SASOL has been investigating the potential of ionic liquids in metathesis115 and olefin trimerisation.116 This metal-catalysed reaction of two alkenes was the subject of the 2005 Nobel Prize in Chemistry,117 and an excellent set of books exist.118 The reaction focused upon is the self-metathesis of oct-1-ene to yield tetradec-7-ene and ethene:SASOL investigated this reaction in conventional solvents, with neat reagents, and in a range of ionic liquids.115,119 They used the conventional Grubbs catalyst, a second generation Grubbs catalyst, and a Grubbs-Hoveyda (third generation) catalyst:
Experiments combining the conventional Grubbs catalyst with ionic liquids were less than promising, but with the second generation catalyst dissolved in [C2dmim][NTf2] remarkable improvements were observed: the yield of tetradec-7-ene was over 20% higher than with either the neat reagents or with toluene as a solvent. Moreover, the selectivity towards tetradec-7-ene was stable with time, whereas it dropped with time in conventional solvents. However, the use of a Grubbs–Hoveyda catalyst (see above) gave an even more spectacular result: the results of the neat reaction and the reaction performed in [C2dmim][NTf2] are compared in Fig. 34. The ionic liquid performs better in every way. Moreover, SASOL investigated the effect of the chain length of the alkyl group on the imidazolium cation upon the formation of the product, with again a dramatic difference being noted: the shorter the chain length, the better the result (see Fig. 35). The reaction is homogeneous, and very selective, with little or no isomerisation. Operating conditions are mild, from ambient temperature up to about 80 °C, and high activity and long life can be obtained from the catalysts, in this case ruthenium, which is cheaper than either rhodium or palladium.
![The results of the self metathesis of oct-1-ene in the presence of a Grubbs–Hoveyda catalyst with neat reagent (left) and in [C2dmim][NTf2] (right), activity being the upper set of graphs, and selectivity being the lower.](/image/article/2008/CS/b006677j/b006677j-f34.gif) | ||
| Fig. 34 The results of the self metathesis of oct-1-ene in the presence of a Grubbs–Hoveyda catalyst with neat reagent (left) and in [C2dmim][NTf2] (right), activity being the upper set of graphs, and selectivity being the lower. | ||
![The results of the self metathesis of oct-1-ene in the presence of a Grubbs–Hoveyda catalyst in various ionic liquids, [Cndmim][NTf2].](/image/article/2008/CS/b006677j/b006677j-f35.gif) | ||
| Fig. 35 The results of the self metathesis of oct-1-ene in the presence of a Grubbs–Hoveyda catalyst in various ionic liquids, [Cndmim][NTf2]. | ||
3.9 BP
BP have heavily invested in ionic liquid technology, as is apparent in their patent portfolio,49,50,120 and in an article in their in-house magazine (see Fig. 36).121 But there have been few public announcements about processes. From the article in Frontiers,121 we learn from Steve Koonin (BP's chief scientist) “Ionic liquids represent a fascinating new area of fundamental science, and one that has the unusual potential for direct and immediate impacts on a number of our businesses. For processing applications that BP uses in areas such as refining, petrochemicals, upstream and reservoir activities, ionic liquids could replace most of the processing steps that we use today.” | ||
| Fig. 36 Ionic liquids featured on the cover of the BP in-house magazine (© BP 2006). | ||
However, they have revealed some details of their aromatic alkylation process in chloroaluminate ionic liquids.122 Most importantly, they gave a detailed description of an ethylbenzene manufacturing plant (see Fig. 37), in which benzene is treated with ethene in Franklin acidic compositions of the [Cnmim]Cl–AlCl3 (n = 2, 4 or 8) ionic liquid system at pressures up to 60 bar and 170 °C.122 In a conclusion which reflects the theme of this review, they stated that “calculations suggest that for any reaction catalysed by AlCl3, the increased cost of substituting ionic liquid, currently estimated as a factor of 5–6, could only be justified by a decrease in catalyst usage of a similar order, resulting from a combination of desirable features such as superior activity, selectivity and recyclability. The authors conclude that there is considerable scope for further ionic liquid formulation development for aromatics alkylation, requiring the combined skills of industry and academia working in collaboration.”122
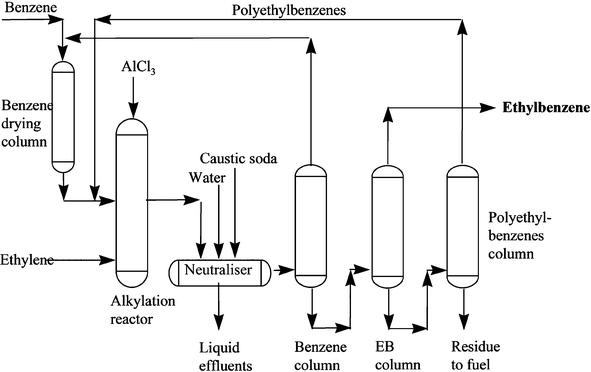 | ||
| Fig. 37 Schematic of an ethylbenzene manufacturing plant.122 | ||
3.10 ExxonMobil
Like BP, ExxonMobil have made no public pronouncements of their interests in developing ionic liquid processes, but they have a significant patent suite, and have been publishing in the open literature. Most of the work seems to be led by Dr Christian Mehnert. Areas of interests include biphasic carbonylation catalysis using conventional rhodium catalysts in Lewis-acidic ionic liquids,123 the electrochemical oxidation of sulfur compounds in naphtha,124 the use of supported ionic liquids,125,126 catalytic hydroformylation,127,128 hydrogenations,126,128 aldol condensations,129 and even ionic liquid synthesis.1303.11 Chevron and Chevron Phillips
Chevron, like BP and ExxonMobil, have used ionic liquids for a number of processes, but there are again no public statements about their state of commissioning. They have an extensive patent portfolio,131–136 and the main themes running through these are refinery alkylation,136 emulsification and high shear mixing,134 oligomerisation132,133 and hydrotreatment133 of alkenes (often to prepare lubricating oils), and removal of carbon dioxide from gas streams.1353.12 PetroChina
Unlike the other petrochemical companies, PetroChina have publicly announced a major process employing ionic liquids.137,138 The process, given the rather inharmonious name of Ionikylation,137 has been tested at pilot plant, and retrofitted into an existing 65,000 tonne per year sulfuric acid alkylation unit in China. The chemistry, alkylation of isobutene, parallels early BP and IFP work, in that it occurs in a strongly Lewis acidic ionic liquid based on aluminium(III) chloride. The retrofit not only increased the yield of the process (compared to sulfuric acid), but increased the process units capacity by 40% (to 248 tonnes per day), with attractive economics. This is by far the largest commercial usage of ionic liquids reported to date, and is certainly a forerunner of other commercial petrochemical processes.3.13 Eli Lilly
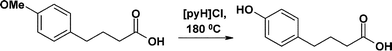
Eli Lilly have described a process for demethylating an aryl ether to quantitatively generate a phenol, on both a twenty-two litre and a 190 litre pilot plant scale.139 4-Methoxyphenylbutanoic acid is demethylated in three hours at 180 °C when melted with pyridinium hydrochloride, [pyH]Cl.139 4-Hydroxyphenylbutanoic acid, which is the product, is an important pharmaceutical intermediate.3.14 Pionics
Pionics is a Japanese firm specialising in lithium batteries.140 In association with Trekion,141 they have developed a very efficient and safe lithium battery. Pionics is now constructing a mass-production commercial plant in Shiga, Japan to produce self-extinguishing lithium ion rechargeable batteries, which should be in production by late 2007.1423.15 Scionix
Scionix,143 a University spin off from the University of Leicester and Whyte Chemicals, have developed a chromium electroplating process based on choline-chromium(III) derived ionic liquids.144 It is a high efficiency, low power, process, avoiding the conventional, toxic, chromium(VI) process. It is presently running at pilot plant scale, and is ripe for commercialisation. Scionix have also developed an interesting process for the electropolishing of stainless steel.145 Although not all the Scionix media are true ionic liquids, some being better described as deep eutectics (mixtures of ionic liquids and molecular components), many of the principles are similar. These choline-based systems have the huge advantage of being very cheap.3.16 Linde
Linde have developed a device that they call an “ionic compressor”.146 It is a machine for compressing gases at constant temperature (isothermal compression) and high pressure, using the ionic liquid as a type of liquid piston (ionic liquids have a very low compressibility). Linde state that “in contrast to a conventional piston compressor, with some 500 moving parts, we now need only eight.” The system maintains a constant gas pressure of 250 bar while delivering 500 cubic meters of natural gas per hour. The proposed early applications are dihydrogen and natural gas filling stations.4 Where does the future of ionic liquids lie
The field of ionic liquids is growing at a rate that was unpredictable even five years ago. The range of commercial applications is quite staggering; not just in the number, but in their wide diversity, arising from close cooperation between academia and industry. The concepts and paradigms of ionic liquids are new, and still not fully accepted in the wider community: it is hard for conservative scientists to throw away thousands of years of concepts grown from the fertile ground (ocean?) of molecular solvents, and if chemists are conservative, then chemical engineers are even more so. But, there is always a flipside, a mirror image, and there are now many laboratories all over the world (and the growth in China is spectacular) that work with ionic liquids. To restate the opening quote of this review: “If you want to find something new, look for something new!”. We predict that ionic liquids will be used commercially in all of the fields illustrated in Fig. 38. | ||
| Fig. 38 Causeway to the future||. | ||
A close examination of Fig. 38, however, reveals that these predictions are not haruspicy, but a natural extrapolation of where we are now. It is thus not really prediction but expectation. Pharmaceutical applications will burgeon;147 wider use will be made of the unique physical properties (they may even be sent to the moon);148 applications for their newly discovered volatility will be found;66,69 more petrochemical applications will be realised. But the most exciting applications will be the unpredictable ones. As ionic liquids can, in principle, replace conventional liquids wherever they are used, we have barely scratched the surface of the possible. The next few years will be truly fascinating.
Acknowledgements
Any review of the wide scope of this owes much to others than the authors. We are indebted to Drs Soghomon Boghasian, Markus Fanselow and Annegret Stark (for translation), Małgorzata Swadźba-Kwaśny (graphics), and Prof. Chuck Hussey, Prof. Christian Reichardt, Dr Will Pitner and Dr John Wilkes for comments and input to the manuscript. We are also extremely grateful to all the industrialists who commented on this review prior to its publication, and who granted us both copyright permission and access to previously unpublished information. In particular, we are continually grateful to the Industrial Advisory Board of QUILL and the EPSRC (Portfolio Partnership Scheme, grant number EP/D029538/1) for their continuing support.References
- Y. Chauvin, Angew. Chem., Int. Ed., 2006, 45, 3740–3747 CrossRef.
- P. L. Short, Out of the Ivory Tower, Chem. Eng. News, 2006, 84, 24th April, pp. 15–21 Search PubMed.
- W. Hückel, Chem. Ber., 1958, 91 Search PubMed , XIX–LXVI.
- P. Walden, Bull. Acad. Impér. Sci. St. Pétersbourg, 1914, 8, 405–422 Search PubMed.
- H. Tokuda, S. Tsuzuki, M. Susan, K. Hayamizu and M. Watanabe, J. Phys. Chem. B, 2006, 110, 19593–19600 CrossRef CAS.
- C. Graenacher, Cellulose solution, US Pat., 1943176 (1934) Search PubMed.
- F. H. Hurley, Electrodeposition of Aluminum, US Pat., 4,446,331 (1948) Search PubMed; T. P. Wier, Jr., US Pat., 4,446,350 (1948); T. P. Wier, Jr. and F. H. Hurley, US Pat., 4,446,349 (1948).
- F. H. Hurley and T. P. Wier, J. Electrochem. Soc., 1951, 98, 207–212 CrossRef CAS; F. H. Hurley and T. P. Wier, J. Electrochem. Soc., 1951, 98, 203–206 CrossRef CAS.
- H. L. Chum, V. R. Koch, L. L. Miller and R. A. Osteryoung, J. Am. Chem. Soc., 1975, 97, 3264–3265 CrossRef CAS.
- R. J. Gale, B. Gilbert and R. A. Osteryoung, Inorg. Chem., 1978, 17, 2728–2729 CrossRef CAS.
- S. Takahashi, N. Koura and R. Nakajima, Denki Kagaku, 1986, 54, 263–268 Search PubMed; S. Takahashi, I. Saeki, K. Tanaka and H. Kanezashi, Nisshin Steel Technical Report, 1989, 60, 36 Search PubMed; S. Takahashi, N. Koura, S. Kohara, M.-L. Saboungi and L. A. Curtiss, Plasma Ions, 1999, 2, 91–105 Search PubMed; R. T. Carlin and J. S. Wilkes, in ‘Chemistry of Non-Aqueous Solutions: Current Progress’, ed. G. Mamantov and A. I. Popov, Wiley-VCH, New York, 1994, pp. 277–306 Search PubMed.
- J. C. Nardi, C. L. Hussey, L. A. King and J. K. Erbacher, ‘The Electrolytic Removal of Aluminum From A Two-phase Aluminum-trialuminum Nickeldie Matrix’, FJSRL-TR-76-0018, Frank J. Seiler Research Laboratory Technical Report, 1976 Search PubMed.
- J. C. Nardi, C. L. Hussey and L. A. King, US Pat., 4,122,245 (1978).
- J. S. Wilkes, in ‘Ionic Liquids: Industrial Applications to Green Chemistry’, ed. R. D. Rogers and K. R. Seddon, ACS Symp. Ser, American Chemical Society, Washington D.C., 2002, vol. 818, pp. 214–229 Search PubMed; J. S. Wilkes, Green Chem., 2002, 4, 73–80 Search PubMed.
- J. S. Wilkes, in ‘Green Industrial Applications of Ionic Liquids’, ed. R. D. Rogers, K. R. Seddon and S. Volkov, NATO Science Series II: Mathematics, Physics and Chemistry, Kluwer, Dordrecht, 2002, vol. 92, pp. 295–320 Search PubMed.
- J. S. Wilkes and C. L. Hussey, ‘Selection of Cations for Ambient Temperature Chloroaluminate Molten Salts Using MNDO Molecular Orbital Calculations’, FJSRL-TR-82-0002, Frank J. Seiler Research Laboratory Technical Report, 1982 Search PubMed.
- O. Wallach, Ber. Chem. Dtsche. Ges., 1884, 16, 535 Search PubMed.
- J. S. Wilkes, J. A. Levisky, R. A. Wilson and C. L. Hussey, Inorg. Chem., 1982, 21, 1263–1264 CrossRef CAS.
- C. J. Dymek, C. L. Hussey, J. S. Wilkes and H. A. Øye, J. Electrochem. Soc., 1987, 134 Search PubMed , C510.
- A. M. Elias and J. S. Wilkes, J. Chem. Eng. Data, 1994, 39, 79–82 CrossRef CAS; A. A. Fannin, L. A. King, D. J. Stech, R. L. Vaughn, J. S. Wilkes and J. L. Williams, J. Electrochem. Soc., 1982, 129, C122; H. A. Oye, M. Jagtoyen, T. Oksefjell and J. S. Wilkes, Mater. Sci. Forum, 1991, 73–75, 183–190 CrossRef CAS; J. S. Wilkes, J. A. Levisky, J. L. Pflug, C. L. Hussey and T. B. Scheffler, Anal. Chem., 1982, 54, 2378–2379 CrossRef CAS; J. S. Wilkes, J. S. Frye and G. F. Reynolds, Inorg. Chem., 1983, 22, 3870–3872 CrossRef CAS.
- A. A. Fannin, D. A. Floreani, L. A. King, J. S. Landers, B. J. Piersma, D. J. Stech, R. L. Vaughn, J. S. Wilkes and J. L. Williams, J. Phys. Chem., 1984, 88, 2614–2621 CrossRef.
- A. A. Fannin, L. A. King, J. A. Levisky and J. S. Wilkes, J. Phys. Chem., 1984, 88, 2609–2614 CrossRef.
- C. L. Hussey, T. B. Scheffler, J. S. Wilkes and A. A. Fannin, J. Electrochem. Soc., 1986, 133, 1389–1391 CrossRef CAS.
- C. L. Hussey, Pure Appl. Chem., 1988, 60, 1763–1772 CrossRef CAS.
- C. L. Hussey, Adv. Molten Salt Chem., 1983, 5, 185–230 Search PubMed.
- E. C. Franklin, J. Am. Chem. Soc., 1905, 27, 820 CrossRef CAS; E. C. Franklin, J. Am. Chem. Soc., 1924, 46, 2137–2151 CrossRef CAS.
- A. K. Abdul-Sada, A. M. Greenway, K. R. Seddon and T. Welton, Org. Mass Spectrom., 1993, 28, 759–765 CAS; A. K. Abdul-Sada, A. M. Greenway, K. R. Seddon and T. Welton, Org. Mass Spectrom., 1989, 24, 917–918 CAS.
- C. J. Dymek, Jr., C. L. Hussey, J. S. Wilkes and H. A. Øye, in ‘Joint (Sixth) International Symposium on Molten Salts’, ed. G. Mamantov, M. Blander, C. L. Hussey, C. Mamantov, M. L. Saboungi and J. S. Wilkes, The Electrochemical Society, Inc., Pennington, NJ, 1987, vol. 87-7, pp. 93–104 Search PubMed.
- A. K. Abdul-Sada, A. G. Avent, M. J. Parkington, T. A. Ryan, K. R. Seddon and T. Welton, J. Chem. Soc., Chem. Commun., 1987, 1643–1644 RSC; A. K. Abdul-Sada, A. G. Avent, M. J. Parkington, T. A. Ryan, K. R. Seddon and T. Welton, J. Chem. Soc., Dalton Trans., 1993, 3283–3286 RSC.
- J. S. Wilkes and M. J. Zaworotko, J. Chem. Soc., Chem. Commun., 1992, 965–967 RSC.
- E. I. Cooper and E. J. M. O'Sullivan, in ‘Eighth International Molten Salts Symposium’, ed. R. J. Gale, G. Blomgren and H. Kojima, The Electrochemical Society, Inc., Pennington, New Jersey, 1992, vol. 92-16, pp. 386–396 Search PubMed.
- M. Grätzel, J. Photochem. Photobiol., C, 2003, 4, 145–153 CrossRef CAS.
- P. Bônhote, A. P. Dias, N. Papageorgiou, K. Kalyanasundaram and M. Grätzel, Inorg. Chem., 1996, 35, 1168–1178 CrossRef CAS.
- W. R. Pitner, QUILL, The Queen's University of Belfast, unpublished results 2000.
- K. R. Seddon, in ‘The International George Papatheodorou Symposium: Proceedings’, ed. S. Boghosian, V. Dracopoulos, C. G. Kontoyannis and G. A. Voyiatzis, Institute of Chemical Engineering and High Temperature Chemical Processes, Patras, 1999, pp. 131–135 Search PubMed.
- A. Stark and K. R. Seddon, in ‘Kirk-Othmer Encyclopaedia of Chemical Technology’, ed. A. Seidel, John Wiley & Sons, Inc., Hoboken, New Jersey, 2007, vol. 26, pp. 836–920 Search PubMed.
- A. F. Kapustinskii, Q. Rev. Chem. Soc., 1956, 10, 283–294 RSC.
- K. R. Seddon, Kinet. Katal., 1996, 37, 743–748; K. R. Seddon, Kinet. Catal., 1996, 37, 693–697 CAS.
- J. D. Holbrey, W. M. Reichert, M. Nieuwenhuyzen, S. Johnston, K. R. Seddon and R. D. Rogers, Chem. Commun., 2003, 1636–1637 RSC.
- K. R. Seddon, J. Chem. Technol. Biotechnol., 1997, 68, 351–356 CrossRef CAS.
- D. R. MacFarlane, J. Sun, J. Golding, P. Meakin and M. Forsyth, Electrochim. Acta, 2000, 45, 1271–1278 CrossRef.
- M. J. Earle, A. Ramani, A. J. Robertson and K. R. Seddon, Chem. Commun., 2008 Search PubMed (to be submitted).
- I. López-Martin, E. Burello, P. N. Davey, K. R. Seddon and G. Rothenberg, ChemPhysChem, 2008, 8, 690–695.
- A. K. Abdul-Sada, A. M. Greenway, P. B. Hitchcock, T. J. Mohammed, K. R. Seddon and J. A. Zora, J. Chem. Soc., Chem. Commun., 1986, 1753–1754 RSC.
- A. G. Avent, P. A. Chaloner, M. P. Day, K. R. Seddon and T. Welton, J. Chem. Soc., Dalton Trans., 1994, 3405–3413 RSC; J. A. van den Berg and K. R. Seddon, Cryst. Growth Des., 2003, 3, 643–661 CrossRef CAS; C. B. Aakeröy, T. A. Evans, K. R. Seddon and I. Pálinkó, New J. Chem., 1999, 23, 145–152 RSC.
- J. D. Holbrey and K. R. Seddon, J. Chem. Soc., Dalton Trans., 1999, 2133–2139 RSC.
- C. M. Gordon, J. D. Holbrey, A. R. Kennedy and K. R. Seddon, J. Mater. Chem., 1998, 8, 2627–2636 RSC.
- R. C. Thied, K. R. Seddon, W. R. Pitner and D. W. Rooney, Nuclear fuel reprocessing, World Pat., WO 99 41752 (1999) Search PubMed; M. Fields, R. C. Thied, K. R. Seddon, W. R. Pitner and D. W. Rooney, Treatment of molten salt reprocessing wastes, World Pat., WO 99 14160 (1999) Search PubMed; P. N. Davey, C. P. Newman, K. R. Seddon and M. J. Earle, Improvements in Friedel–Crafts reactions, World Pat., WO 99 19288 (1999) Search PubMed; G. Roberts, C. M. Lok, C. J. Adams, K. R. Seddon, M. J. Earle and J. Hamill, Process for the preparation of branched fatty acids and their derivatives by the reaction of unsaturated linear fatty acids or their derivatives with ionic liquids, World Pat., WO 98 07679 (1998) Search PubMed; G. Roberts, C. M. Lok, C. J. Adams, K. R. Seddon, M. E. Earle and J. Hamill, Process and ionic catalysts for the preparation of branched fatty acids from linear saturated fatty acids, World Pat., WO 98 07680 (1998) Search PubMed; M. Fields, G. V. Hutson, K. R. Seddon and C. M. Gordon, Ionic liquids as solvents, World Pat., WO 98 06106 (1998) Search PubMed.
- A. K. Abdul-Sada, K. R. Seddon and N. J. Stewart, Ionic liquids of ternary melts, World Pat., WO 95 21872 (1995) Search PubMed.
- A. K. Abdul-Sada, M. P. Atkins, B. Ellis, P. K. G. Hodgson, M. L. M. Morgan and K. R. Seddon, Process and catalysts for the alkylation of aromatic hydrocarbons, World Pat., WO 95 21806 (1995) Search PubMed; A. K. Abdul-Sada, P. W. Ambler, P. K. G. Hodgson, K. R. Seddon and N. J. Stewart, Ionic liquids of imidazolium halide for oligomerization or polymerization of olefins, World Pat., WO 95 21871 (1995) Search PubMed.
- K. R. Seddon, Green Chem., 1999, 1 Search PubMed , G58–G59.
- ‘Green Industrial Applications of Ionic Liquids’, ed. R. D. Rogers, K. R. Seddon and S. Volkov, NATO Science Series II: Mathematics, Physics and Chemistry, vol. 92, Kluwer, Dordrecht, 2002 Search PubMed.
- DeChema, “BATIL (Biodegradability and Toxicity of Ionic Liquids), Berlin, 6th–9th May, 2007”, http://events.dechema.de/batil (2007) Search PubMed.
- National Institute of Standards and Technology, “IUPAC Ionic Liquids Database, ILThermo (NIST Standard Reference Database #147)”, http://ilthermo.boulder.nist.gov/ILThermo/mainmenu.uix (2006) Search PubMed.
- M. P. Atkins, P. Davey, G. Fitzwater, O. Rouher, K. R. Seddon and J. Swindall, “Ionic Liquids: A Map for Industrial Innovation”, Q001, QUILL, Belfast, 2004, http://quill.qub.ac.uk/map (2004) Search PubMed.
- G. Fitzwater, W. Geissler, R. Moulton, N. V. Plechkova, A. Robertson, K. R. Seddon, J. Swindall and K. Wan Joo,“Ionic Liquids: Sources of Innovation”, Q002, QUILL, Belfast, http://quill.qub.ac.uk/sources (2005) Search PubMed.
- N. V. Plechkova and K. R. Seddon, in ‘Methods and Reagents for Green Chemistry: An Introduction’, ed. P. Tundo, A. Perosa and F. Zecchini, John Wiley & Sons, Inc., New York, 2007, pp. 105–130 Search PubMed; D. R. MacFarlane and K. R. Seddon, Aust. J. Chem., 2007, 60, 3–5 Search PubMed.
- M. Deetlefs and K. R. Seddon, Chim. Oggi–Chem. Today, 2006, 24, 16–23 Search PubMed.
- M. Deetlefs, U. Hakala, K. R. Seddon and K. Wähälä, unpublished results, 2006.
- ‘Ionic Liquids in Synthesis’, ed. P. Wasserscheid and T. Welton, Wiley-VCH, Weinheim, 2003 Search PubMed.
- ‘Ionic Liquids IIIB: Fundamentals, Progress, Challenges, and Opportunities - Transformations and Processes’, ed. R. D. Rogers and K. R. Seddon, ACS Symp. Ser., vol. 902, American Chemical Society, Washington D.C., 2005 Search PubMed; ‘Ionic Liquids IIIA: Fundamentals, Progress, Challenges, and Opportunities - Properties and Structure’, ed. R. D. Rogers and K. R. Seddon, ACS Symp. Ser., vol. 901, American Chemical Society, Washington D. C., 2005 Search PubMed; ‘Ionic Liquids as Green Solvents: Progress and Prospects’, ed. R. D. Rogers and K. R. Seddon, ACS Symp. Ser., vol. 856, American Chemical Society, Washington D.C., 2003 Search PubMed; ‘Ionic Liquids: Industrial Applications for Green Chemistry’, ed. R. D. Rogers and K. R. Seddon, ACS Symp. Ser., vol. 818, American Chemical Society, Washington D.C., 2002 Search PubMed; ‘Electrochemical Aspects of Ionic Liquids’, ed. H. Ohno, Wiley-Interscience, Hoboken, 2005 Search PubMed; ‘Ionic Liquids: The Front and Future of Material Development’, ed. H. Ohno, CMC Press, Tokyo, 2003 Search PubMed.
- F. Endres and S. Z. El Abedin, Phys. Chem. Chem. Phys., 2006, 8, 2101–2116 RSC; F. van Rantwijk, R. M. Lau and R. A. Sheldon, Trends Biotechnol., 2003, 21, 131–138 CrossRef CAS; R. A. Sheldon, F. van Rantwijk and R. Madeira Lau, in ‘Ionic Liquids as Green Solvents: Progress and Prospects’, ed. R. D. Rogers and K. R. Seddon, ACS Symp. Ser., vol. 856, American Chemical Society, Washington D.C., 2003, pp. 192–205 Search PubMed; R. A. Sheldon, R. M. Lau, M. J. Sorgedrager, F. van Rantwijk and K. R. Seddon, Green Chem., 2002, 4, 147–151 Search PubMed; M. J. Earle and K. R. Seddon, in ‘Clean Solvents: Alternative Media for Chemical Reactions and Processing’, ed. M. Abraham and L. Moens, ACS Symp. Ser., vol. 819, American Chemical Society, Washington, 2002, pp. 10–25 RSC; M. J. Earle, in ‘Ionic Liquids: Industrial Applications for Green Chemistry’, ed. R. D. Rogers and K. R. Seddon, ACS Symp. Ser., vol. 818, American Chemical Society, Washington D.C., 2002, pp. 90–105 RSC; D. W. Rooney and K. R. Seddon, in ‘Handbook of Solvents’, ed. G. Wypych, ChemTech Publishing, Toronto, 2001, pp. 1459–1484 RSC; M. J. Earle and K. R. Seddon, Pure Appl. Chem., 2000, 72, 1391–1398 RSC; T. Welton, Chem. Rev., 1999, 99, 2071–2084 Search PubMed; J. L. Anderson, D. W. Armstrong and G.-T. Wei, Anal. Chem., 2006, 78, 2893–2902 Search PubMed; J. D. Holbrey and K. R. Seddon, Clean Prod. Process., 1999, 1, 223–236 Search PubMed; P. J. Scammells, J. L. Scott and R. D. Singer, Aust. J. Chem., 2005, 58, 155–169 CrossRef CAS.
- ‘Ionic Liquids in Synthesis’, ed. P. Wasserscheid and T. Welton, Wiley-VCH, Weinheim, 2nd edn., 2007 Search PubMed.
- M. Freemantle, Designer solvents – Ionic liquids may boost clean technology development, Chem. Eng. News, 1998, 76, 30th March, pp. 32–37 Search PubMed.
- M. Freemantle, Sweeteners for ion liquids: novel designer solvents are derived from anions of sugar substitutes, Chem. Eng. News, 2004, 82, March 15th, p. 10 Search PubMed.
- M. J. Earle, J. M. S. S. Esperanca, M. A. Gilea, J. N. C. Lopes, L. P. N. Rebelo, J. W. Magee, K. R. Seddon and J. A. Widegren, Nature, 2006, 439, 831–834 CrossRef CAS.
- Anonymous, Are ionic liquids REALLY volatile?, Solvent Innovation Newsletter, 2006, March, p. 1 Search PubMed.
- P. Wasserscheid, Nature, 2006, 439, 797–797 CrossRef CAS.
- J. P. Leal, J. M. S. S. Esperança, M. E. M. da Piedade, J. N. C. Lopes, L. P. N. Rebelo and K. R. Seddon, J. Phys. Chem. A, 2007, 111, 6176–6182 CrossRef CAS.
- ‘CRC Handbook of Chemistry and Physics’, ed. D. R. Lide, CRC Press, Boca Raton, 73rd edn., 1992 Search PubMed.
- R. W. Berg, Monatsh. Chem., 2007, 138, 1045–1075 CrossRef CAS.
- È. Boros and K. R. Seddon, Chem. Soc. Rev., 2008 Search PubMed , to be submitted.
- M. J. Earle, S. P. Katdare and K. R. Seddon, Org. Lett., 2004, 6, 707–710 CrossRef CAS.
- M. Deetlefs and K. R. Seddon, Green Chem., 2003, 5, 181–186 RSC; A. J. Carmichael, M. Deetlefs, M. J. Earle, U. Fröhlich and K. R. Seddon, in ‘Ionic Liquids as Green Solvents: Progress and Prospects’, ed. R. D. Rogers and K. R. Seddon, ACS Symp. Ser., American Chemical Society, Washington D.C., 2003, vol. 856, pp. 14–31 Search PubMed.
- K. R. Seddon, A. Stark and M.-J. Torres, Pure Appl. Chem., 2000, 72, 2275–2287 CrossRef CAS.
- M. Deetlefs, H. G. Raubenheimer and M. W. Esterhuysen, Catal. Today, 2002, 72, 29–41 CrossRef CAS; M. Hasan, I. V. Kozhevnikov, M. R. H. Siddiqui, A. Steiner and N. Winterton, Inorg. Chem., 1999, 38, 5637–5641 CrossRef CAS.
- J. Pernak, A. Syguda, I. Mirska, A. Pernak, J. Nawrot, A. Pradzynska, S. T. Griffin and R. D. Rogers, Chem.–Eur. J., 2007, 13, 6817–6827 CrossRef CAS; K. J. Fraser, E. I. Izgorodina, M. Forsyth, J. L. Scott and D. R. MacFarlane, Chem. Commun., 2007, 3817–3819 RSC.
- M. Freemantle, Chem. Eng. News, 2003, 81, 31st March, 9 Search PubMed; K. R. Seddon, Nat. Mater., 2003, 2, 363–364 Search PubMed; R. D. Rogers and K. R. Seddon, Science, 2003, 302, 792–793 CrossRef CAS; M. Maase and K. Massonne, in ‘Ionic Liquids IIIB: Fundamentals, Progress, Challenges, and Opportunities – Transformations and Processes’, ed. R. D. Rogers and K. R. Seddon, ACS Symp. Ser., American Chemical Society, Washington D.C., 2005, vol. 902, pp. 126–132 CrossRef; BASF, “Acid scavenging: The BASIL process”, http://www2.basf.de/en/intermed/nbd/products/ionic_liquids/processes/acid.htm?id=mGwEvAf1Ebw23hM (2005) CrossRef; M. Maase, in ‘Multiphase Homogeneous Catalysis’, ed. B. Cornils, W. A. Herrmann, I. T. Horvath, W. Leitner, S. Mecking, H. Olivier-Bourbigou and D. Vogt, Wiley-VCH, Weinheim, 2005, vol. 2, pp. 560–566 CrossRef.
- M. Maase, K. Massonne, K. Halbritter, R. Noe, M. Bartsch, W. Siegel, V. Stegmann, M. Flores, O. Huttenloch and M. Becker, Method for the separation of acids from chemical reaction mixtures by means of ionic fluids, World Pat., WO 2003 062171 (2003) Search PubMed.
- J. Baker, ECN Innovation Awards 2004 – the winners!, Eur. Chem. News, 2004, pp. 18–19 Search PubMed.
- Y. Beste, M. Eggersmann and H. Schoenmakers, Chem.-Ing.-Tech., 2005, 77, 1800–1808 CrossRef CAS; Y. A. Beste, M. Eggersmann and H. Schoenmakers, Chem.-Ing.-Tech., 2004, 76, 1407 CrossRef CAS.
- BASF, “Extractive distillation”, http://www2.basf.de/en/intermed/nbd/products/ionic_liquids/processes/distillation.htm?id=mGwEvAf1Ebw23hM (2005) Search PubMed; W. Arlt, M. Seiler, C. Jork and T. Schneider, Ionic liquids as selective additives for the separation of close-boiling or azeotropic mixtures, World Pat., WO 2002 074718 (2002) Search PubMed.
- M. J. Earle and S. P. Katdare, Oxidative halogenation of aromatic compounds, World Pat., WO 2002 30852 (2002) Search PubMed.
- BASF, “Chlorination with “nucleophilic HCl””, http://www2.basf.de/en/intermed/nbd/products/ionic_liquids/processes/chlorination.htm?id=mGwEvAf1Ebw23hM (2005) Search PubMed; V. Stegmann and K. Massonne, Method for producing haloalkanes from alcohols, WO Pat., 2005 026089 (2005) Search PubMed.
- T. A. Ryan, C. Ryan, E. A. Seddon and K. R. Seddon, ‘Phosgene and Related Carbonyl Halides’, ed. R. J. H. Clark, Elsevier, Amsterdam, 1996 Search PubMed.
- R. D. Rogers, Green Chem., 2004, 6, G17–G19 RSC.
- BASF, “Cellulose processing”, http://www2.basf.de/en/intermed/nbd/products/ionic_liquids/applications/cellulose/?id=mGwEvAf1Ebw23hM (2005) Search PubMed; M. Maase and V. Stegmann, Solubility of cellulose in ionic liquids with addition of amino bases, World Pat., WO 2006 108861 (2006) Search PubMed; M. Maase and V. Stegmann, Solutions of cellulose in ionic liquids, DE Pat., 102005017715 (2006) Search PubMed.
- R. P. Swatloski, J. D. Holbrey, S. K. Spear and R. D. Rogers, Proc.-Electrochem. Soc., 2002, 2002–19, 155–164 CAS; R. P. Swatloski, R. D. Rogers and J. D. Holbrey, Dissolution and processing of cellulose using ionic liquids, cellulose solution, and regenerating cellulose, World Pat., WO 2003 029329 (2003) Search PubMed; M. B. Turner, S. K. Spear, J. G. Huddleston, J. D. Holbrey and R. D. Rogers, Green Chem., 2003, 5, 443–447 Search PubMed; R. P. Swatloski, S. K. Spear, J. D. Holbrey and R. D. Rogers, J. Am. Chem. Soc., 2002, 124, 4974–4975 RSC; M. B. Turner, S. K. Spear, J. D. Holbrey and R. D. Rogers, Biomacromolecules, 2004, 5, 1379–1384 CrossRef CAS; J. D. Holbrey, R. P. Swatloski, J. Chen, D. Daly and R. D. Rogers, Polymer dissolution and blend formation in ionic liquids, World Pat., WO 2005 098546 (2005) CrossRef CAS; J. S. Moulthrop, R. P. Swatloski, G. Moyna and R. D. Rogers, Chem. Commun., 2005, 1557–1559 CrossRef CAS; M. B. Turner, S. K. Spear, J. D. Holbrey, D. T. Daly and R. D. Rogers, Biomacromolecules, 2005, 6, 2497–2502 Search PubMed; R. C. Remsing, R. P. Swatloski, R. D. Rogers and G. Moyna, Chem. Commun., 2006, 1271–1273 RSC; R. P. Swatloski, J. D. Holbrey, J. L. Weston and R. D. Rogers, Chim. Oggi, 2006, 24, 31–32 CrossRef CAS; R. P. Swatloski, J. D. Holbrey, J. L. Weston and R. D. Rogers, Chim. Oggi, 2006, 24, 34–35 RSC.
- A. D. French, N. R. Bertoniere, R. M. Brown, H. Chanzy, D. Gray, K. Hattori and W. Glasser, in ‘Kirk-Othmer Encyclopaedia of Chemical Technology’, ed. A. Seidel, John Wiley & Sons, Inc., Hoboken, New Jersey, 2007, vol. 5, pp. 360–394 Search PubMed.
- BASF, “Aluminium plating”, http://www2.basf.de/en/intermed/nbd/products/ionic_liquids/processes/liquid.htm?id=mGwEvAf1Ebw23hM (2005) Search PubMed.
- M. Bukowski, F. Endres, R. Hempelmann and H. Natter, Procedure for the electrochemical deposition of metals, alloys and semiconductors from ionic liquids and low melting salt mixtures, DE Pat., 10108893 (2002) Search PubMed; F. Endres, ChemPhysChem, 2002, 3, 144 Search PubMed; F. Endres, M. Bukowski, R. Hempelmann and H. Natter, Angew. Chem., Int. Ed., 2003, 42, 3428–3430 CrossRef CAS; W. Freyland, C. A. Zell, S. Z. El Abedin and F. Endres, Electrochim. Acta, 2003, 48, 3053–3061 CrossRef CAS; M. Bukowski, H. Natter, F. Endres and R. Hempelmann, GDCh-Monographie, 2004, 29, 92–99 CrossRef CAS; S. Z. El Abedin, E. M. Moustafa, R. Hempelmann, H. Natter and F. Endres, Electrochem. Commun., 2005, 7, 1111–1116 Search PubMed; S. Z. El Abedin and F. Endres, ChemPhysChem, 2006, 7, 58–61 CrossRef; S. Z. El Abedin, E. M. Moustafa, R. Hempelmann, H. Natter and F. Endres, ChemPhysChem, 2006, 7, 1535–1543 CrossRef; F. Endres, S. Z. El Abedin and N. Borissenko, Z. Phys. Chem. (Munich), 2006, 220, 1377–1394 CrossRef; Q. X. Liu, S. Z. El Abedin and F. Endres, Surf. Coat. Technol., 2006, 201, 1352–1356 CrossRef CAS; H. Natter, M. Bukowski, R. Hempelmann, S. Z. El Abedin, E. M. Moustafa and F. Endres, Z.Phys. Chem. (Munich), 2006, 220, 1275–1291 CrossRef CAS.
- S. N. Falling, J. R. Monnier, G. W. Phillips, J. S. Kanel and S. A. Godleski, First International Congress on Ionic Liquids (COIL-1), 19th–22nd June, Salzburg, Austria, 2005.
- J. D. Holbrey, N. V. Plechkova and K. R. Seddon, Green Chem., 2006, 8, 411–414 RSC.
- J. R. Monnier and P. J. Muehlbauer, Selective monoepoxidation of olefins, US Pat., 4,897,498 (1990) Search PubMed.
- G. W. Phillips, S. N. Falling, S. A. Godleski and J. R. Monnier, Continuous process for the manufacture of 2,5-dihydrofurans from γ,δ-epoxybutenes, US Pat., 5,315,019 (1994) Search PubMed; S. N. Falling, S. A. Godleski and L. McGarry, Process for the separation of oligomeric materials from a catalyst mixture, US Pat., 5,238,889 (1993) Search PubMed.
- IFP, ‘Annual Report’, 2004.
- Y. Chauvin, J. F. Gaillard, D. V. Quang and J. W. Andrews, Chem. Ind., 1974, 375–378 Search PubMed.
- Axens, “Dimersol-X”, http://www.axens.net/html-gb/offer/offer_processes_70.html.php (2007) Search PubMed.
- H. Olivier-Bourbigou, F. Hugues, in ‘Green Industrial Applications of Ionic Liquids’, ed. R. D. Rogers, K. R. Seddon and S. Volkov, NATO Science Series II: Mathematics, Physics and Chemistry, Kluwer, Dordrecht, 2002, vol. 92, pp. 67–84 Search PubMed.
- Y. Chauvin, A. Hirschauer and H. Olivier, J. Mol. Catal., 1994, 92, 155–165 CrossRef CAS; Y. Chauvin, S. Einloft and H. Olivier, Ind. Eng. Chem. Res., 1995, 34, 1149–1155 CrossRef CAS; H. Olivier-Bourbigou and V. Lecocq, in ‘Science and Technology in Catalysis 2002’, Studies in Surface Science and Catalysis, Kodansha Ltd, Tokyo, 2003, vol. 145, pp. 55–60 Search PubMed; F. Favre, A. Forestière, F. Hugues, H. Olivier-Bourbigou and J. A. Chodorge, Oil Gas-Eur. Mag., 2005, 31, 83–87 Search PubMed; Y. Chauvin, B. Gilbert and I. Guibard, J. Chem. Soc., Chem. Commun., 1990, 1715–1716 Search PubMed.
- B. Weyershausen, K. Hell and U. Hesse, in ‘Ionic Liquids IIIB: Fundamentals, Progress, Challenges, and Opportunities - Transformations and Processes’, ed. R. D. Rogers and K. R. Seddon, ACS Symp. Ser., American Chemical Society, Washington D.C., 2005, vol. 902, pp. 133–143 Search PubMed.
- A. Hoff, C. Jost, A. Prodi-Schwab, F. G. Schmidt and B. Weyershausen, Ionic Liquids: New designer compounds for more efficient chemistry, Elements: Degussa Science Newsletter, 2004, 9, pp. 10–15 Search PubMed.
- B. Weyershausen, K. Hell and U. Hesse, Green Chem., 2005, 7, 283–287 RSC.
- B. Weyershausen and K. Lehmann, Green Chem., 2005, 7, 15–19 RSC.
- M. Holzapfel, C. Jost, A. Prodi-Schwab, F. Krumeich, A. Wuersig, H. Buqa and P. Novak, Carbon, 2005, 43, 1488–1498 CrossRef CAS; A. Prodi-Schwab, C. Jost and M. Pascaly, First International Congress on Ionic Liquids (COIL-1), 19th–22nd June, Salzburg, Austria, 2005.
- D. J. Tempel, P. H. Henderson, J. R. Brzozowski, R. M. Pearlstein, J. J. Hart and D. Tavianini, First International Congress on Ionic Liquids (COIL-1), 19th–22nd June, Salzburg, Austria, 2005.
- D. J. Tempel, P. B. Henderson, J. R. Brzozowski, R. M. Pearlstein and D. Garg, Ionic liquid based mixtures for gas storage and delivery, US Pat., 2006060818 (2006) Search PubMed; D. J. Tempel, P. B. Henderson and J. R. Brzozowski, Ionic liquid based mixtures for gas storage and delivery, US Pat., 2006060817 (2006) Search PubMed.
- Air Products, “A New System for Delivering Ion Implantation Gases”, http://www.airproducts.com/markets/Electronics/Newsletter/SolutionsUpdate2.htm (2007) Search PubMed.
- Central Glass Co. (Japan), http://www.cgco.co.jp/english/index.html (2007).
- M. J. Earle, K. R. Seddon and P. B. McCormac, Green Chem., 2000, 2, 261–262 RSC.
- T. Fukuyama, M. Shinmen, S. Nishitani, M. Sato and I. Ryu, Org. Lett., 2002, 4, 1691–1694 CrossRef CAS; I. Kmentova, B. Gotov, V. Gajda and S. Toma, Monatsh. Chem., 2003, 134, 545–549 CrossRef CAS; S. B. Park and H. Alper, Chem. Commun., 2004, 1306–1307 RSC.
- The Biotech/Life Sciences Portal, “A New Class of Materials: Ionic Liquids”, http://www.bio-pro.de/en/region/freiburg/magazin/01053/index.html (2007) Search PubMed.
- M. Grätzel, M. K. Nazeeruddin and B. O'Regan, Photovoltaic cells, World Pat., WO 91 16719 (1991) Search PubMed.
- M. Kenisarin and K. Mahkamov, Renew. Sust. Energy Rev., 2007, 11, 1939–1965 Search PubMed.
- M. J. Green, Ionic Liquids: A Road-Map to Commercialisation, 22nd April 2004, Royal Society of Chemistry, London, 2004.
- A. Ranwell and M. A. Tshamano, in ‘Ionic Liquids: Industrial Applications to Green Chemistry’, ed. R. D. Rogers and K. R. Seddon, ACS Symp. Ser, American Chemical Society, Washington D.C., 2002, vol. 818, pp. 147–161 Search PubMed.
- Nobel Foundation, “The Nobel Prize in Chemistry 2005”, http://nobelprize.org/nobel_prizes/chemistry/laureates/2005/index.html (2007) Search PubMed.
- R. H. Grubbs, ‘Handbook of metathesis’, Wiley-VCH John Wiley distributors, Weinheim Chichester, 2003 Search PubMed.
- A. Ranwell, C. L. Dwyer and M. Ajam, IP.com Journal, 2004, 4, 4 Search PubMed; D. B. G. Williams, M. Ajam and A. Ranwell, Organometallics, 2006, 25, 3088–3090 CrossRef CAS; A. Stark, M. Ajam, M. Green, H. G. Raubenheimer, A. Ranwell and B. Ondruschka, Adv. Synth. Catal., 2006, 348, 1934–1941 CrossRef CAS.
- P. W. Ambler, P. K. G. Hodgson and N. J. Stewart, Butene mixted feed polymerization in presence of ionic liquid, EP Pat., EP 558187 (1993) Search PubMed; P. W. Ambler, P. K. G. Hodgson and N. J. Stewart, Preparation of butene polymers using an ionic liquid, US Pat., 5304615 (1994) Search PubMed; I. R. Collins, M. J. Earle, S. P. Exton, N. V. Plechkova and K. R. Seddon, Ionic liquids, making emulsion containing ionic salt, and surfactant uses, World Pat., 2006111712 (2006) Search PubMed; B. Ellis, Ionic liquids, World Pat., WO 96 18459 (1996) Search PubMed; W. Keim, W. Korth and P. Wasserscheid, Preparation of ionic liquids for catalysts, World Pat., 00 16902 (2000) Search PubMed; K. Anderson, M. Fanselow and J. D. Holbrey, Method for separating emulsions, World Pat., WO 2006 131699 (2006) Search PubMed.
- N. Morgan, Standby for Supersolvents, BP Frontiers, 2005, August, 6–10 Search PubMed.
- M. P. Atkins, C. Bowlas, B. Ellis, F. Hubert, A. Rubatto and P. Wasserscheid, in ‘Green Industrial Applications of Ionic Liquids’, ed. R. D. Rogers, K. R. Seddon and S. Volkov, NATO Science Series II: Mathematics, Physics and Chemistry, Kluwer, Dordrecht, 2002, vol. 92, pp. 49–66 Search PubMed.
- R. Y. Saleh, Preparation of aromatic aldehydes from alkylaromatics and carbon monoxide in the presence of acidic ionic liquids, World Pat., WO 00 15594 (2000) Search PubMed.
- R. C. Schucker and W. C. Baird, Jr., Electrochemical oxidation of sulfur compounds in naphtha using ionic liquids, US Pat., 6274026 (2001) Search PubMed; R. C. Schucker, Electrochemical oxidation of sulfur compounds in naphtha, US Pat., 6338788 (2002) Search PubMed.
- C. P. Mehnert and R. A. Cook, Supported ionic liquid compositions, World Pat., WO 2002 098560 (2002) Search PubMed; C. P. Mehnert, R. A. Cook, N. C. Dispenziere and M. Afeworki, J. Am. Chem. Soc., 2002, 124, 12932–12933 Search PubMed; C. P. Mehnert, Chem.–Eur. J., 2004, 11, 50–56 CrossRef CAS.
- C. P. Mehnert, E. J. Mozeleski and R. A. Cook, Chem. Commun., 2002, 3010–3011 RSC.
- C. P. Mehnert, R. A. Cook, N. C. Dispenziere and E. J. Mozeleski, Polyhedron, 2004, 23, 2679–2688 CrossRef CAS.
- E. van Driessche, P. L. Buess, R. F. Caers, A. van Vliet, R. Y. Saleh, J. M. Vargas, K. L. Riley and M. Agosto, Process for the manufacture of alcohols by the catalytic hydroformylation and hydrogenation of alkenes, World Pat., WO 2005 058782 (2005) Search PubMed.
- C. P. Mehnert, N. C. Dispenziere and R. A. Cook, Chem. Commun., 2002, 1610–1611 RSC; C. P. Mehnert and N. C. Dispenziere, Process for conducting aldol condensation reactions in ionic liquid media, particularly imidazolium and pyridinium salts, World Pat., WO 2003 002502 (2003) Search PubMed.
- C. P. Mehnert, N. C. Dispenziere and R. A. Cook, Method for preparing high-purity ionic liquids, US Pat., 2004074842 (2004) Search PubMed.
- A. Wood, Chem. Week, 2001, 163, 22–22 Search PubMed; C. L. Munson, L. C. Boudreau, M. S. Driver and W. L. Schinski, Separation of olefins from paraffins using ionic liquid solutions, US Pat., 6339182 (2002) Search PubMed; D. J. O'Rear, L. C. Boudreau, M. S. Driver and C. L. Munson, Removal of mercaptans from hydrocarbon streams using ionic liquids, World Pat., WO 2002 034863 (2002) Search PubMed; L. C. Boudreau, M. S. Driver, C. L. Munson and W. L. Schinski, Separation of dienes from olefins using ionic liquids and metal salt complexing agents, US Pat., 2003125599 (2003) Search PubMed; K. D. Hope, D. W. Twomey and D. A. Stern, Method for manufacturing ionic-liquid catalysts, World Pat., WO 2003 089389 (2003) Search PubMed; C. B. Campbell and G. P. Sinquin, 4-Branched-alkyl-o-xylenesulfonate surfactants for enhanced petroleum recovery, World Pat., WO 2005 018300 (2005) Search PubMed.
- K. D. Hope, M. S. Driver and T. V. Harris, High-viscosity poly(alpha-olefins) prepared with ionic liquid catalysts, US Pat., 6395948 (2002) Search PubMed; D. J. O'Rear, T. V. Harris, S. J. Miller, R. R. Krug and B. K. Lok, Manufacture of lubricating base oils from highly paraffinic feedstocks by alkane dehydrogenation and alkene oligomerization, US Pat., 2002003102 (2002) Search PubMed; K. D. Hope, D. W. Twomey, D. A. Stern and J. B. Collins, Method for manufacturing high viscosity poly(a-olefins) using ionic liquid catalysts, World Pat., WO 2003 089390 (2003) Search PubMed; D. R. Johnson, C. A. Simmons, D. H. Mohr, S. J. Miller, S. K. Lee, W. L. Schinski and M. S. Driver, Integrated manufacture of lubricating oil basestocks from primary Fischer-Tropsch synthesis products, US Pat., 6605206 (2003) Search PubMed; T. V. Harris, M. Cheng and G.-D. Lei, Combined dehydration-adsorption-oligomerization of Fischer-Tropsch feedstocks for production of lubricating base oils, US Pat., 2004267071 (2004) Search PubMed; B. L. Small and R. Schmidt, Chem.–Eur. J., 2004, 10, 1014–1020 Search PubMed; K. D. Hope, D. A. Stern and E. A. Benham, Process and system to contact an ionic liquid catalyst with oxygen to improve a chemical reaction such as oligomerization or polymerization, World Pat., WO 2005 042447 (2005) Search PubMed; K. D. Hope, D. W. Twomey, M. S. Driver, D. A. Stern, J. B. Collins and T. V. Harris, Method for manufacturing high viscosity polyalphaolefins using ionic liquid catalysts, US Pat., 2005 0113621 (2005) Search PubMed; K. D. Hope and D. A. Stern, Method and system to recycle non-isomerized a-olefins in an ionic liquid-catalyzed oligomerization process, US Pat., 2006247482 (2006) Search PubMed.
- S. J. Miller and R. R. Krug, Two-stage dimerization of C5-11-alkene fractions for production of basestocks for lubricating oils, World Pat., WO 2002 055633 (2002) Search PubMed; D. R. Johnson, T. V. Harris and M. S. Driver, Combined hydrotreating-pyrolysis-ionic liquid-based oligomerization for manufacture of lubricating base oils from Fischer Tropsch fractions, US Pat., 2004267070 (2004) Search PubMed.
- L. H. Bergman, K. D. Hope, E. A. Benham and D. A. Stern, Method and system to add high shear to improve an ionic liquid catalyzed chemical reaction, US Pat., 2005 0119423 (2005) Search PubMed; L. H. Bergman, K. D. Hope, E. A. Benham and D. A. Stern, Method and system to add high shear to improve an ionic liquid catalyzed chemical reaction, World Pat., WO 2005 042151 (2005) Search PubMed.
- D. Chinn, D. Vu, M. S. Driver and L. C. Boudreau, Carbon dioxide removal from gas using ionic liquid absorbents, US Pat., 2005129598 (2005) Search PubMed; D. Chinn, D. Q. Vu, M. S. Driver and L. C. Boudreau, CO2 removal from gas using ionic liquid absorbents, US Pat., 2006251558 (2006) Search PubMed.
- S. Elomari, S. Trumbull, H. K. C. Timken and R. Cleverdon, Alkylation process using chloroaluminate ionic liquid catalysts, US Pat., 2006 0135839 (2006) Search PubMed; H. K. C. Timken, S. Elomari, S. Trumbull and R. Cleverdon, Integrated alkylation process using ionic liquid catalysts, World Pat., WO 2006 073749 (2006) Search PubMed; H. K. C. Timken, S. Elomari, S. Trumbull and R. Cleverdon, Chloroaluminate-based ionic liquid catalysts for ethylene-isoalkane alkylation for manufacture of high-octane gasoline alkylate, US Pat., 2006131209 (2006) Search PubMed.
- Z. C. Liu, R. Zhang, C. M. Xu and R. G. Xia, Oil Gas J., 2006, 104, 52–56 Search PubMed.
- Z. Liu, C. Xu and C. Huang, Method for manufacturing alkylate oil with composite ionic liquid used as catalyst, US Pat., 0133056 (2004) Search PubMed.
- C. R. Schmid, C. A. Beck, J. S. Cronin and M. A. Staszak, Org. Process Res. Dev., 2004, 8, 670–673 Search PubMed.
- Pionics, “Clean Energy and Ecology on Earth”, http://www.pionics-inet.co.jp/e-index.html (2007) Search PubMed.
- Trekion, “Ionic liquid”, http://www.trekion.co.jp/English/e_home.html (2007) Search PubMed.
- D. Teramoto, R. Yokoyama, H. Kagawa, T. Sada and N. Ogata, EUCHEM Conference on Molten Salts and Ionic Liquids, 16th–22nd September, 2006, Hammamet, Tunisia, 2006.
- Scionix, “Ionic Liquid Technology From Scionix”, http://www.scionix.co.uk/ (2007) Search PubMed.
- A. P. Abbott, G. Capper, D. L. Davies, R. K. Rasheed, J. Archer and C. John, Trans. Inst. Met. Finish., 2004, 82, 14–17 Search PubMed; A. P. Abbott, G. Capper, D. L. Davies and R. K. Rasheed, Chem.–Eur. J., 2004, 10, 3769–3774 CrossRef CAS; A. P. Abbott, G. Capper, D. L. Davies, H. Munro, R. K. Rasheed and V. Tambyrajah, ACS Symp. Ser., 2003, 856, 439–452 CAS.
- A. P. Abbott, D. L. Davies, G. Capper, R. K. Rasheed and V. Tambyrajah, Ionic liquids and their use as solvents, World Pat., WO 2002 026701 (2002) Search PubMed; A. P. Abbott, D. L. Davies, G. Capper, R. K. Rasheed and V. Tambyrajah, Ionic liquids and their use, World Pat., WO 2002 026381 (2002) Search PubMed; A. Abbott, D. Davies and P. Jenkins, Speciality Chemicals Magazine, 2004, 24, 36–37 Search PubMed; A. P. Abbott, G. Capper, K. J. McKenzie and K. S. Ryder, Electrochim. Acta, 2006, 51, 4420–4425 Search PubMed; A. P. Abbott, G. Capper, K. J. McKenzie, A. Glidle and K. S. Ryder, Phys. Chem. Chem. Phys., 2006, 8, 4214–4221 Search PubMed; A. P. Abbott, G. Capper, B. G. Swain and D. A. Wheeler, Trans. Inst. Met. Finish., 2005, 83, 51–53 CrossRef CAS.
- R. Adler and H. Mayer, Procedure for lubricating and/or cooling a compressor by using ionic liquids, DE Pat., 102005026916 (2006) Search PubMed; M. Kömpf, Mobility under high pressure Linde Technol., 2006, pp. 24–26 Search PubMed; R. Adler, “The demand is astounding”, Linde Technol., 2006, pp. 27–29 Search PubMed.
- W. L. Hough, M. Smiglak, H. Rodriguez, R. P. Swatloski, S. K. Spear, D. T. Daly, J. Pernak, J. E. Grisel, R. D. Carliss, M. D. Soutullo, J. H. Davis and R. D. Rogers, New J. Chem., 2007, 31, 1429–1436 RSC.
- E. F. Borra, O. Seddiki, R. Angel, D. Eisenstein, P. Hickson, K. R. Seddon and S. P. Worden, Nature, 2007, 447, 979–981 CrossRef CAS.
Footnotes |
| † [Cnpy]+ stands for the 1-alkylpyridinium cation, where the index n represents the number of carbon atoms in the linear alkyl chain. |
| ‡ [Cnmim]+ stands for the 1-alkyl-3-methylimidazolium cation, where the index n represents the number of carbon atoms in the linear alkyl chain. |
| § But, thankfully, not Tyburn! |
| ¶ Ionic liquids are also called room temperature molten salts, fused salts, NAILs, OILs, liquid organic salts, or ionic fluids; these are currently being phased out. They are sometimes grouped with supercritical fluids under the broader term of neoteric solvents. |
| || The diagram of ionic liquid applications is represented as a hexagon, the most perfect crystalline structure in nature. The authors of this review live in Northern Ireland, where the Giant's Causeway is a World Heritage site. Composed of over 40,000 hexagonal basalt columns, it was formed from cooling molten salts, and is evocative of the ubiquity of ionic liquids, here a medium for crystal growth. |
| This journal is © The Royal Society of Chemistry 2008 |

![Hydrogen bonding in the structure of [C2mim]I (distances in Å).44](/image/article/2008/CS/b006677j/b006677j-f13.gif)