Hydrogen transfer from guest molecule to radical in adjacent hydrate-cages
Kazunari
Ohgaki
*a,
Kentaro
Nakatsuji
a,
Kei
Takeya
a,
Atsushi
Tani
b and
Takeshi
Sugahara
a
aDivision of Chemical Engineering, Graduate School of Engineering Science, Osaka University, 1-3 Machikaneyama, Toyonaka, Osaka, 560-8531, Japan. E-mail: ohgaki@cheng.es.osaka-u.ac.jp; Fax: +81 (0)6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6850
6850![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6290; Tel: +81 (0)6
6290; Tel: +81 (0)6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6850
6850![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6290
6290
bDepartment of Earth and Space Science, Graduate School of Science, Osaka University, 1-1 Machikaneyama, Toyonaka, Osaka, 560-0043, Japan. E-mail: atani@ess.sci.osaka-u.ac.jp
First published on 20th November 2007
Abstract
Electron spin resonance measurement of gamma-ray-irradiated propane hydrates shows that the normal propyl radical withdraws hydrogen from the adjacent propane molecule through the hexagonal planes of the hydrate cage without water molecule bridging.
The transfer mechanisms and dynamics of protons and atomic hydrogen are of fundamental importance in physical chemistry. However, direct experimental observations of these transfer reactions at the atomic level are difficult. Recently, proton transfer has been observed in complicated radicals and in compounds such as polymers, enzymes, ion channels and membrane-spanning proteins using electron spin resonance (ESR) and fluorescence techniques.1,2 Nevertheless, hydrogen transfer has not yet been reported in light hydrocarbons like methane, ethane or propane. In the present study, we successfully observed hydrogen transfer between propyl radicals and propane molecules in the adjacent hexakaidecahedron hydrate-cages using ESR.
Clathrate hydrate is the solid crystal compound of water molecules (host cage) and so-called “guest molecules”. There are three types of unit cells of hydrates: structure I, structure II and structure H.3Propane generates structure II hydrates, which consist of 16 small cages and 8 large cages. Propane occupies only large cage at the rate of one propane molecule per large cage, which shares the hexagonal plane with adjacent large cages. The small cage is vacant.
Propane hydrate was synthesized at 277 K by mixing propane gas (0.6 MPa) and distilled water in a high-pressure cell. Small pieces of propane hydrate with a diameter of 1–2 mm were collected, and kept in plastic vials at 77 K with liquid nitrogen. The samples were irradiated by gamma rays at 77 K using a source of 60Co. Several pieces of propane hydrate (30–50 mg) were placed in an ESR sample tube at 77 K and were measured at 120 K with a commercial X-band ESR spectrometer (RE-1X; JEOL, Tokyo, Japan). The sample temperature was controlled by a nitrogen gas flow unit system. The power of the microwave was set at 1 mW. The spectra of both n-propyl and i-propyl radicals are unsaturated in the microwave power of 1 mW, because the saturation points of n-propyl and i-propyl radicals are located at 10 and 3 mW, respectively. The 100-kHz modulation field was 0.1 mT. In isothermal annealing experiments, the annealing and measurement temperatures were 240, 250 and 260 K, which is just below the equilibrium temperature of pure propane hydrate.
Fig. 1a shows the ESR spectrum of a mixture of normal propyl (n-propyl) and isopropyl (i-propyl) radicals in the irradiated propane hydrate before annealing at 250 K. The g-factors and hyperfine constants (Aα and Aβ) were determined: 2.0029 ± 0.0005, 2.2 ± 0.1 mT and 3.0 ± 0.1 mT for the n-propyl radical, and 2.0027 ± 0.0005, 2.4 ± 0.1 mT and 2.5 ± 0.1 mT for the i-propyl radical, respectively. Atomic hydrogen is unstable and it disappears promptly, even at 120 K.
 | ||
| Fig. 1 ESR spectra of propyl radicals in the propane hydrate cage. (a) ESR spectrum before annealing shows similar amounts of n-propyl (solid triangles) and i-propyl (open triangles) radicals. (b) During annealing at 250 K for 3 hours, the n-propyl radical decreased and the i-propyl radical increased. Each sample was quenched quickly to 120 K and ESR analysis was carried out at that temperature. | ||
The OH radical is not observed at 120 K, which is consistent with our previous report on gamma-ray-irradiated methane hydrate.4 The spectrum was resolved into two spectra derived from i-propyl (open triangles) and n-propyl (solid triangles) radicals. From the spectrum resolution, the amount of each radical was obtained using the intensity ratio. Fig. 1b shows a typical ESR spectrum after annealing for 3 hours at 250 K. We conclude that a lot of n-propyl radicals apparently change to i-propyl radicals.
Fig. 2 shows the results of the present isothermal annealing experiment at 260 K. Propane hydrate is thermodynamically stable at this temperature under 0.1 MPa.3 Although the total amount of radicals is almost constant, as shown in Fig. 2 (upper panel), the amount of the n-propyl radical decreases while that of the i-propyl radical increases. The amounts of change for the two radicals show good agreement with each other. The secondary radical (i-propyl) is more thermally stable than the primary radical (n-propyl) due to the effect of conjugation and the stereo structure of the radicals. Therefore, hydrogen transfer occurs, and changes from the n-propyl radical to the i-propyl radical.
 | ||
| Fig. 2 Representative results of isothermal annealing experiments at 260 K showing that the normalized total amount of both the n-propyl and i-propyl radicals, Nall(t)/Nall(0), is almost constant except for hydrate sublimation, because the decreasing amount of n-propyl radicals (ΔNn−pro/Nall(0): solid circles) is equal to the increasing amount of i-propyl radicals (ΔNi−pro/Nall(0): open circles). | ||
To investigate whether hydrogen transfer occurs in the isolated n-propyl radical (intramolecular reaction) or between the n-propyl radical and the propane molecule in the adjacent cage (intermolecular reaction), two additional experiments were performed. In the first experiment, we examined the gamma-ray-irradiated equimolar propane and SF6 mixed hydrate. The present isothermal annealing experiment at 250 K for the mixed hydrate showed that the transfer from n-propyl radical to i-propyl radical is extremely slow, that is, the hydrogen-picking reaction is interrupted by the addition of SF6 which competitively occupies only large hydrate cages. In the next experiment, the propane hydrate composed of D2O was also investigated in a similar manner. Neither of the propyl radicals takes in the heavy hydrogen atom from D2O.
These facts reveal that the hydrogen of the propane molecule transfers directly to the n-propyl radical in the adjacent large cage without involving the water molecules of the hydrate cages. If, during this hydrogen-picking process, the α-hydrogen of propane is removed by the n-propyl radical, then an n-propyl radical is produced again; thus a chain reaction should follow. When the β-hydrogen of propane is removed, an i-propyl radical is produced instead of an n-propyl radical; thus the reaction is stopped. The amount of i-propyl radical is increased absolutely in the latter process.
Assuming that hydrogen transfer from n-propyl to i-propyl is followed by an irreversible first-order reaction, the amount of the n-propyl radical, Nn−pro(t), is expressed as
 | (1) |
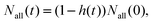 | (2) |
 | (3) |
 | ||
| Fig. 3 Decreasing rate for n-propyl radicals. The fraction of n-propyl radical decreases exponentially at each temperature (open triangles, 240 K; open circles, 250 K; solid circles, 260 K). | ||
 | ||
| Fig. 4 Arrhenius plot of the decreasing rate for n-propyl radicals. The activation energy of 34 ± 3 kJ mol−1 is estimated from the line. | ||
We have successfully observed hydrogen transfer in clathrate hydrates. In the hydrate cages, the concentrated radicals of light hydrocarbons are more stable than those in any other environment.5,6 The anomalous stability of radicals in hydrate cages facilitates the observation of intermolecular hydrogen transfer.
Acknowledgements
The authors would like to thank Dr H. Sato, Dr C. Yamanaka and Dr M. Katsura for helpful discussions. We are indebted to Dr T. Ikeda of the Institute of Scientific and Industrial Research of Osaka University for 60Co γ-ray irradiation. One of the authors (K.T.) would like to express special thanks to the 21st Century Center of Excellence (COE) program “Creation of Integrated EcoChemistry”. This study was supported in part by a grant from Research Fellowships of the Japan Society for the Promotion of Science for Young Scientists (No. 17-9677 to K.T.).References
- C. Tanner, C. Manca and S. Leutwyler, Science, 2003, 302, 1736 CrossRef CAS.
- A. Kajiwara, Kobunshi Ronbunshu, 2004, 61, 237 Search PubMed.
- E. D. Sloan, Jr, Clathrate Hydrate of Natural Gases, Mercel Dekker, New York, revised and expanded, 2nd edn, 1998 Search PubMed.
- K. Takeya, A. Tani, T. Yada, M. Ikeya and K. Ohgaki, Jpn. J. Appl. Phys., Part 1, 2004, 43, 353 CrossRef CAS.
- K. Gotoh, T. Miyazaki, K. Fueki and K. Lee, Radiat. Phys. Chem., 1987, 30, 89 CrossRef CAS.
- R. W. Fessenden and R. H. Schuler, J. Chem. Phys., 1963, 39, 2147 CrossRef CAS.
| This journal is © the Owner Societies 2008 |
