Crown-ether enclosure generated by ionic liquid components—synthesis, crystal structure and Raman spectra of compounds of imidazolium based salts and 18-crown-6†
Mimoza
Gjikaj
*a,
Wolfgang
Brockner
a,
Jan
Namyslo
b and
Arnold
Adam
a
aInstitute of Inorganic and Analytical Chemistry, Clausthal University of Technology, Clausthal-Zellerfeld, Germany. E-mail: mimoza.gjikaj@tu-clausthal.de; Fax: +49 5323 722995; Tel: +49 5323 722887
bInstitute of Organic Chemistry, Clausthal University of Technology, Clausthal-Zellerfeld, Germany
First published on 17th September 2007
Abstract
Bis(1,3-dimethylimidazolium methylsulfate) [18-crown-6] (1), ([DMIm]2[CH3SO4]2[18-crown-6], bis(1-butyl-3-methylimidazolium methylsulfate) [18-crown-6] (2), ([BMIm]2[CH3SO4]2[18-crown-6]), bis(1-ethyl-3-methylimidazolium methanesulfonate) [18-crown-6] (3), ([EMIm]2 [CH3SO3]2[18-crown-6]) and bis(1-ethyl-3-methylimidazolium trifluoromethanesulfonate) [18-crown-6] (4), ([EMIm]2[CF3SO3]2[18-crown-6]) were prepared and characterized by single-crystal X-ray diffraction, NMR and Raman spectroscopy. Coulomb interactions between the ionic (liquid) components as well as hydrogen bonding are important. No significant close contacts are observed between the ionic components and the 18-crown-6 molecules. Crystal structures of all compounds consist of alternated layers of crown-ether molecules and respective ionic units compounds. Within the layers of the ionic components (compounds 1, 3 and 4), two cations are linked to each other by C–H⋯π interactions between one methyl carbon and the imidazolium ring of another cation.
Introduction
Room temperature ionic liquids (ILs) have been an area of interest for fundamental reasons associated with their negligible vapour pressure, good electrical conductivity, high ionic mobility, excellent thermal and chemical stabilities and solvent capability.1,2 These materials have been used as media for clean liquid–liquid extraction processes,3catalysts for organic4 and organometallic synthesis,5polymerisation and radical polymerisation,6 and as media for analytical and physical chemistry.7 Some of them possess liquid crystal8 and lubricant properties9 and can behave as sensors.10 One of the barriers to these applications is the relatively high viscosity compared to those of classical organic solvents.11 After the discovery of the first air- and water-stable ionic liquid compounds such as [EMIm]BF4 and [EMIm][CF3SO3], which are reported by Wilkes and Zaworotko, and Cooper and O'Sullivan,12 respectively, much effort has been devoted to the basic understanding of their structure properties; especially those composed of 1-alkyl-3-methylimidazolium cation.13–15In this work, we report the co-crystallisation of imidazolium based salts with 18-crown-6. Comparable (inclusion) compounds have been synthesized very recently by Bates, Gale and coworkers16–18 out of imidazolium salts and calixarene derivatives. These compounds show interesting structural architectures as well as the capacity to act as (selective) ion-pair receptors. Different kinds of interactions can also be studied thereby. Generally the complex formation of organic molecules via weak non-covalent interactions such as hydrogen bonds, π-stacking, charge-transfer interaction and electrostatic interaction is of great importance.19 These interactions and charge transfers have received increasing attention recently.20 The investigated compounds were characterised by X-ray analysis, Raman spectroscopy and NMR.
Results and discussions
All the studied imidazolium based salts formed compounds with 18-crown-6 (Scheme 1). | ||
| Scheme 1 Molecular structure of 1-alkyl-3-methylimidazolium cation (DMIm: R = methyl, BMIm: R = n-butyl and EMIm: R = ethyl). | ||
Four low melting alkylimidazolium salts, bis(1,3-dimethyl-imidazolium methylsulfate) [18-crown-6] (1), bis(1-butyl-3-methylimidazolium methylsulfate) [18-crown-6] (2), bis(1-ethyl-3-methylimidazolium methanesulfonate) [18-crown-6] (3), and bis(1-ethyl-3-methylimidazolium trifluoromethane-sulfonate)[18-crown-6] (4) have been prepared and their single-crystal structures determined.
All compounds consist of alternating layers of crown-ether molecules and the respective ionic components [DMIm]+, [CH3SO4]– for 1, [BMIm]+, [CH3SO4]– for 2, [EMIm]+, [CH3SO3]– for 3 and [EMIm]+, [CF3SO3]– for 4. The layers of crown ether are surrounded above and below from methyl groups of imidazolium with a distance of 1.976 Å for 1, 2.001 Å for 2, 2.011 Å for 3 and 2.090 Å for 4. The crystallographic data and details of the single crystal structure determinations are given in Table 1. Selected interatomic distances and angles for compounds 1–4 are collected in Table 2.
| 1 | 2 | 3 | 4 | |
|---|---|---|---|---|
| a R = Σ| |Fo| – |Fc| |/Σ||Fo|. wR2 = {Σ[w(|Fo|2 – |Fc|2)2]/Σ[w(|Fo|2)2]}1/2. | ||||
| Empirical formula | C24H48N4O14S2 | C30H60N4O14S2 | C26H52N4O12S2 | C26H46F6N4O12S2 |
| Formula weight/g mol–1 | 680.78 | 764.94 | 676.84 | 784.79 |
| Temperature/K | 223 | 223 | 223 | 223 |
| Wavelength/Å | 0.71073 | 0.71073 | 0.71073 | 0.71073 |
| Crystal system | Triclinic | Monoclinic | Triclinic | Triclinic |
| Space group |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (no. 2) (no. 2) |
P21/n (no. 14) |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (no. 2) (no. 2) |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (no. 2) (no. 2) |
| a/Å | 8.551(2) | 9.123(11) | 8.751(5) | 8.946(2) |
| b/Å | 9.298(2) | 16.188(1) | 9.126(4) | 9.165(2) |
| c/Å | 10.774(3) | 13.939(2) | 11.357(6) | 11.469(3) |
| α/° | 86.93(2) | 97.26(4) | 92.60(2) | |
| β /° | 80.09(2) | 98.03(1) | 97.99(4) | 98.72(2) |
| γ/° | 82.06(2) | 97.76(4) | 97.86(2) | |
| Volume/Å3 | 835.3(3) | 2038.2(4) | 880.1(7) | 918.6(4) |
| Z | 1 | 2 | 1 | 1 |
| Density (calc.)/g cm–3 | 1.353 | 1.246 | 1.277 | 1.419 |
| Abs. coefficient/mm–1 | 0.228 | 0.194 | 0.212 | 0.236 |
| F(000) | 364 | 824 | 364 | 412 |
| θ min, max/° | 2.21, 25.02 | 1.94, 25.02 | 2.25, 25.03 | 2.25, 25.02 |
| Index ranges | –9 ≤ h ≤ 10 | –10 ≤ h ≤ 10 | –10 ≤ h ≤ 9 | –10 ≤ h ≤ 9 |
| –11 ≤ k ≤ 11 | –19 ≤ k ≤ 18 | –10 ≤ k ≤ 10 | –10 ≤ k ≤ 10 | |
| –12 ≤ l ≤ 12 | –16 ≤ l ≤ 15 | –13 ≤ l ≤ 13 | –13 ≤ l ≤ 13 | |
| Unique reflections | 2903 | 3598 | 3085 | 3202 |
| Data/restrains/parameters | 2903/0/293 | 3598/0/336 | 3085/0/303 | 3202/0/319 |
| Goodness-of-fit on F2 | 1.030 | 1.090 | 1.038 | 1.080 |
| R indexes [I > 2σI] | R1 = 0.0621 | R1 = 0.0612 | R1 = 0.0725 | R1 = 0.0612 |
| wR2 = 0.1542 | wR2 = 0.1484 | wR2 = 0.1454 | wR2 = 0.1080 | |
| R indexes (all data) | R1 = 0.0845 | R1 = 0.0779 | R1 = 0.1172 | R1 = 0.1016 |
| wR2 = 0.1682 | wR2 = 0.1591 | wR2 = 0.1638 | wR2 = 0.1217 | |
| Largest diff. peak and hole/e Å3 | 0.576 and –0.295 | 0.418 and –0.289 | 0.365 and –0.261 | 0.261 and –0.232 |
| Compound 1 | |||
|---|---|---|---|
| S–O(1) | 1.397(4) | O(1)–S–O(2) | 116.2(3) |
| S–O(2) | 1.406(4) | O(1)–S–O(3) | 111.3(3) |
| S–O(3) | 1.436(4) | O(1)–S–O(4) | 107.3(3) |
| S–O(4) | 1.573(3) | O(2)–S–O(3) | 110.0(3) |
| O(4)–C(8) | 1.470(7) | C(8)–O(4)–S | 114.0(4) |
| Compound 2 | |||
| S–O(1) | 1.400(3) | O(1)–S–O(2) | 113.3(2) |
| S–O(2) | 1.400(3) | O(1)–S–O(3) | 113.4(2) |
| S–O(3) | 1.434(3) | O(1)–S–O(4) | 107.0(2) |
| S–O(4) | 1.565(3) | O(2)–S–O(3) | 112.7(2) |
| O(4)–C(11) | 1.466(9) | C(11)–O(4)–S | 114.2(5) |
| Compound 3 | |||
| S–O(1) | 1.429(4) | O(1)–S–O(2) | 112.6(3) |
| S–O(2) | 1.432(4) | O(1)–S–O(3) | 110.3(3) |
| S–O(3) | 1.440(4) | O(2)–S–O(3) | 113.9(3) |
| S–C(9) | 1.749(6) | O(1)–S–C(9) | 106.9(3) |
| O(2)–S–C(9) | 106.8(3) | ||
| O(3)–S–C(9) | 105.6(3) | ||
| Compound 4 | |||
| S–O(1) | 1.406(3) | O(1)–S–O(2) | 115.9(3) |
| S–O(2) | 1.428(3) | O(1)–S–O(3) | 115.0(3) |
| S–O(3) | 1.430(3) | O(2)–S–O(3) | 113.1(2) |
| S–C(9) | 1.807(4) | O(1)–S–C(9) | 104.0(2) |
| F(1)–C(9) | 1.314(5) | O(2)–S–C(9) | 103.4(2) |
| F(2)–C(9) | 1.308(5) | O(3)–S–C(9) | 103.3(2) |
| F(3)–C(9) | 1.297(5) | F(1)–C(9)–S | 112.0(3) |
| F(2)–C(9)–S | 111.3(3) | ||
| F(3)–C(9)–S | 116.6(3) | ||
Crystal structures
Bis(1, 3-dimethylimidazolium methylsulfate) [18-crown-6] (1) crystallizes in the triclinic P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group, consisting of alternating layers of crown-ether molecules and the respective ionic components [DMIm]+ and [CH3SO4]– (Fig. 1).
space group, consisting of alternating layers of crown-ether molecules and the respective ionic components [DMIm]+ and [CH3SO4]– (Fig. 1).
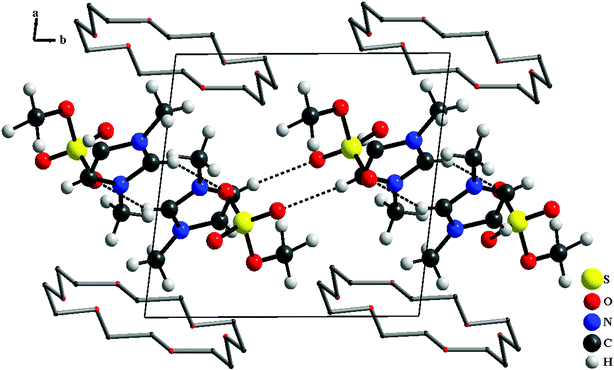 | ||
| Fig. 1 Perspective view of the unit cell of compound 1 along the c-axis and H atoms of crown ethers have been omitted for clarity. | ||
Within the layer of the ionic components, two cations are linked to each other by C–H⋯π interactions between one methyl carbon C(7) and the imidazolium ring of another cation (Fig. 2). The distance between the methyl carbon and the center of the imidazolium ring is estimated to be 3.682 Å, which is in excellent agreement with the values expected for the lowest benzene⋯methane interactions.21 The only significant hydrogen bonding are contacts between the two (acidic) ring-hydrogens C(2) and C(5), and the two terminal oxygen atoms of the sulfate anion. Each imidazolium cation participates in two hydrogen bonds, forming a chain with C–H⋯O distances 2.351 and 2.730 Å and C–H⋯O angles 150.0 and 175.8° (Table 3). The hydrogen bonding and the C–H⋯π stacking contacts help stabilize the extended crystal lattice and allow a close packing in the crystal structure.
![Interionic contacts between [DMIm]+ cation and [CH3SO4]– anion in compound 1; hydrogen bonding and C–H⋯π interactions are shown by dotted lines.](/image/article/2008/CE/b708724c/b708724c-f2.gif) | ||
| Fig. 2 Interionic contacts between [DMIm]+ cation and [CH3SO4]– anion in compound 1; hydrogen bonding and C–H⋯π interactions are shown by dotted lines. | ||
| Donor–H⋯Acceptor | D–H/Å | H⋯A/Å | D⋯A/Å | D–H⋯A/° |
|---|---|---|---|---|
| a x, y – 1, z. b x–1/2, –y + 1/2, z + 1/2. c x – 1, y, z. d –x + 1, –y + 1, –z + 2. e –x + 1, –y, –z + 2. f x, y + 1, z. | ||||
| Compound 1 | ||||
| C(2)–H(2)⋯O(2) | 0.824 | 2.738 | 3.476 | 150.0 |
| C(5)–H(5)⋯O(1)a | 0.808 | 2.351 | 3.157 | 175.8 |
| Compound 2 | ||||
| C(2)–H(2)⋯O(4) | 0.918 | 2.771 | 3.620 | 154.4 |
| C(4)–H(4)⋯O(1)b | 0.902 | 2.448 | 3.290 | 155.4 |
| C(5)–H(5)⋯O(2)c | 0.906 | 2.521 | 3.352 | 152.8 |
| Compound 3 | ||||
| C(2)–H(2)⋯O(3)e | 0.925 | 2.410 | 3.283 | 157.4 |
| C(5)–H(5)⋯O(2)d | 0.911 | 2.286 | 3.169 | 163.1 |
| Compound 4 | ||||
| C(2)–H(2)⋯O(2) | 0.918 | 2.533 | 3.292 | 140.4 |
| C(5)–H(5)⋯O(1)f | 0.830 | 2.373 | 3.180 | 164.2 |
S–O bond distances in [CH3SO4]– range from 1.396 to 1.572 Å, the O–S–O bond angles from 100.2 to 116.2° and C–O bond length in the [CH3SO4]– amounts to 1.473 Å (Table 2).
These data correspond well to those previously reported for the methylsulfate anion in [DMIm] [CH3SO4]22 and [NH4]6[calix][4] [arenesulfonate] [MeOSO3]·(H2O)2.23
Bis(1-butyl-3-methylimidazolium methylsulfate) [18-crown-6] (2) crystallizes in the monoclinic P21/n space group. Similar to compound 1 the structure consists of alternate layers of 18-crown-6 and the ionic units ([BMIm]+ and [CH3SO4]–). In the 1-butyl-3-methylimidazolium cation all C–C bonds of the alkyl chain are in a trans–trans conformation which projects the chain away from the imidazolium ring, with a torsion angle around C(7)–C(8) of 177.3° (Fig. 3).
![Trans–trans conformation of [BMIm]+ cation in compound 2.](/image/article/2008/CE/b708724c/b708724c-f3.gif) | ||
| Fig. 3 Trans–trans conformation of [BMIm]+ cation in compound 2. | ||
Distinguished by a trans–trans conformation of the butyl chain, each cation in the lattice has three neighbouring counter ions in contact at the van der Waals distance. The shortest hydrogen bonds are between C(4) and C(5) of the imidazolium ring and two oxygen atoms of the sulfate anion O(1) and O(2), with C–H⋯O distances of 2.448 and 2.521 Å, and C–H⋯O angles of 152.8 and 155.4° (Table 3). The next interaction is between C(2) and O(4) with C–H⋯O bond distance of 2.771 Å and C–H⋯O angle of 154.4° (Fig. 4). In compound 2, the orientation of the butyl chain away from the imidazolium ring allows no other significant van der Waals contacts or C–H⋯π interactions.24
![Interionic contacts between [BMIm]+ cation and [CH3SO4]– anion in compound 2; hydrogen bondings are shown by dotted lines.](/image/article/2008/CE/b708724c/b708724c-f4.gif) | ||
| Fig. 4 Interionic contacts between [BMIm]+ cation and [CH3SO4]– anion in compound 2; hydrogen bondings are shown by dotted lines. | ||
S–O bond distances in the [CH3SO4]– range from 1.400 to 1.564 Å, the O–S–O bond angles from 99.8 to 113.3° and C–O bond length is 1.469 Å. These data correspond well with those found in 1 (Table 2).
Both imidazolium salts 3 and 4 crystallized in the triclinic space groupP![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) . The crystal structures contain one crystallographically independent [EMIm]+ cation, [CH3SO3]– and crown ether for 3 and one crystallographically independent [EMIm]+ cation, [CF3SO3]– and crown ether for 4. The crystallographic data for 3 and 4 are given in Table 1. The ions are packed in the crystal lattice of compounds 3 and 4via weak interionic C–H⋯O hydrogen bonds as well as significant C–H⋯π interactions.
. The crystal structures contain one crystallographically independent [EMIm]+ cation, [CH3SO3]– and crown ether for 3 and one crystallographically independent [EMIm]+ cation, [CF3SO3]– and crown ether for 4. The crystallographic data for 3 and 4 are given in Table 1. The ions are packed in the crystal lattice of compounds 3 and 4via weak interionic C–H⋯O hydrogen bonds as well as significant C–H⋯π interactions.
Comparable to compound 1, two [EMIm]+ cations in both compounds 3 and 4 are linked to each other by C–H⋯π interactions between one methyl carbon (C(6) for 3 and C(6) for 4) and the imidazolium ring of another cation (Fig. 5 and 6). The distance between the methyl carbon and the center of the imidazolium ring is estimated to be 3.717 Å for 3 and 3.990 Å for 4, which agrees very well with the value expected for the lowest benzene⋯methane interactions.
![Interionic contacts between [EMIm]+ cation and [CH3SO3]– anion in compound 3; hydrogen bonding and C–H⋯π interactions are shown by dotted lines.](/image/article/2008/CE/b708724c/b708724c-f5.gif) | ||
| Fig. 5 Interionic contacts between [EMIm]+ cation and [CH3SO3]– anion in compound 3; hydrogen bonding and C–H⋯π interactions are shown by dotted lines. | ||
![Interionic contacts between [EMIm]+ cation and [CF3SO3]– anion in compound 4. Hydrogen bonding and the C–H⋯π interactions are shown by dotted lines.](/image/article/2008/CE/b708724c/b708724c-f6.gif) | ||
| Fig. 6 Interionic contacts between [EMIm]+ cation and [CF3SO3]– anion in compound 4. Hydrogen bonding and the C–H⋯π interactions are shown by dotted lines. | ||
Two significant C–H⋯O hydrogen bonds involving H(2) and H(5) connect the cations and anions forming layered structures for compounds 3 and 4. C–H⋯O bond distances of 2.286 and 2.410 Å and C–H⋯O bond angles of 157.4 and 163.1° are found for compound 3, and two [CF3SO3]– anions with C–H⋯O bond distances of 2.373 and 2.533 Å, and C–H⋯O bond angles of 140.3 and 164.2° for compound 4 (Fig. 6 and Table 3).
In 3 the methanesulfonate ion is in a staggered conformation with C3v symmetry. The sulfonate group has an average S–O bond distance of 1.434 Å, an average O–S–O bond angle of 112.3°, and a C–S bond distance of 1.750 Å (Table 2).
The arrangement of the [CF3SO3]– anion in 4 agrees very well with this in several dehydrated salts, such as LiCF3SO3 and [EMIm] [SO3CF3].25 It also has a staggered conformation with C3v symmetry. The S–C (1.808 Å), average C–F (1.306 Å), and average S–O (1.421 Å) bond lengths for compound 4 (Table 2) are in best agreement with those measured by Dehnicke et al.26 and Jansen et al.27
Raman spectroscopic characterization
Solid title compounds are composed by the respective cationic and anionic units as well as by 18-crown-6 molecules. The dominant interactions are Coulombic. H-bridge bonding of the ionic units to 18-crown-6 molecules is not found. Consequently, the Raman spectra of the solid title compounds are superpositions of those from 18-crown-6 and the corresponding ionic components. Usually, asymmetrically alkyl substituted imidazolium cations are employed for prototype ILs. In this context the 1,3-dimethylimidazolium cation (in 1) is here exceptional, and unfortunately, no corresponding vibrational spectral data were available. Both the other cations, [BMIm]+ (in 2) and [EMIm]+ (in 3 and 4), have been investigated intensely by vibrational spectroscopy as relevant units in ILs.26–33 Especially, the rotational and vibrational freedom of the butyl group in [BMIm]+ can lead to at least two stable conformers. For [BMIm]+ Cl– diagnostic Raman modes allow to distinguish between these conformers being in equilibrium.28–30 For the trans–trans conformer (with a torsional angle tλ ≈ 180°) the Raman bands at 625, 730, and 790 cm–1 are characteristic.28,30Raman frequencies of the respective anions of the studied compounds are, more or less, known in detail. The Raman frequencies of the crystalline compounds are summarized along with their assignments in groups in Table S1 (ESI)† along with these of 18-crown-6. For the crystalline compound 2Raman spectra at –60 and –150 °C were recorded to extend the temperature range of the temperature dependence of the equilibrium between the trans–trans and trans–gauche[BMIm]+ conformers, demonstrated hitherto only between 50 and 110 °C.28,30 Unexpectedly, no significant change in the intensity relation of the 730 cm–1 Raman mode (trans–trans conformer) to the 602 cm–1 band (trans–gauche) could be determined for all temperatures (20, –60, and –150 °C). This can be understood by the assumption that the mentioned equilibrium is frozen and the rotational formation of the different conformers is hindered.
The crystal-structure determination of 2 was carried out at –50 °C (Table S1),† resulting in the monoclinic space groupP21/n (trans–trans conformer), without any indication of a structural disorder. So, the low temperature Raman frequencies of 2 are not in complete accordance with the crystal structure (determination). An explanation could be that the X-ray radiation during the measurement overcomes the rotational hindering from the trans–gauche to the trans–trans conformers of [BMIm]+. In Fig. 7 the Raman spectra of compound 2 at 20 and –60 °C are shown.
![FT-Raman
spectra (λexc. = 1064 nm) of crystalline [BMIm]2[CH3SO4]2[18-crown-6] at –60 and 20 °C. The Raman intensity is given in arbitrary units.](/image/article/2008/CE/b708724c/b708724c-f7.gif) | ||
| Fig. 7 FT-Raman spectra (λexc. = 1064 nm) of crystalline [BMIm]2[CH3SO4]2[18-crown-6] at –60 and 20 °C. The Raman intensity is given in arbitrary units. | ||
Considering the Raman spectra of the investigated compounds, it can be noticed that the vibrational modes of the ionic units and the 18-crown-6 molecules superimpose as mentioned; also, the “molecular groups” in the relevant species (e.g., CH3, CH2, CH, imidazole ring, –SO3) give rise to uncoupled vibrations and hence to Raman bands in typical frequency regions. In consequence, single component modes e.g. CH3 from CH3SO4–, CH3SO3– or CH3 (ring substituent) are superpositioned and not always at their original frequency value anymore. Therefore, the assignments are done to groups in Table S1 (ESI).†
The assignments of the Raman frequencies of 1–4 are first related to the ionic components (IL) and 18-crown-6 (cr). The noticeable modes of the respective anions, [CH3SO4]–, [CH3SO3]–, and [CF3SO3]–, are marked in italics in Table S1 (ESI).† Secondly, the frequency assignments are proposed to characteristic group frequencies in comparison to literature data partly based on sophisticated ab initio as well as on DFT computations. In the following, some related comments are given (c.f. Table S1, ESI).†
The most Raman bands of the title compounds belong to the imidazolium cations. The quasi-aromatic imidazolium contains “aromatic” C–H units with distinctive stretching modes in the area 3070–3200 cm–1, whereas the corresponding deformations are attributed to vibrations around 1300 cm–1.24,28–30 Furthermore, the imidazolium ring itself has characteristic bands in the region 1450–1570 cm–1 and at about 1150–1370 cm–1.29,31,33 The intensities of these modes can be affected by the kind and number of the ring (alkyl) substituents. The frequencies in the region 2800–3000 cm–1 are a result of the aliphatic CH3 and CH2 stretching vibrations (superpositioned by CH3groups of the respective anions and CH2 units of 18-crown-6). Corresponding CH3 and CH2 bending modes are found at 1350–1440 and around 1100–1200 cm–1, respectively. Butyl substituent in 2 further contributes a number of vibrations in the region from about 500 to 800 cm–1 caused by alkyl chain orientations. As mentioned, two different conformers, trans–trans and trans–gauche, could be identified by Raman spectroscopy at room temperature.28–30
Recently, [BMIm][CH3SO4] was studied in relation to the formation of solutions with some n-alkanes and aromatic hydrocarbons.34 Unfortunately, IR and Raman data of methylsulfate, [CH3SO4]–, are daunting,35 nevertheless the characteristic νs(SO3) can be attributed to the strong Raman band at 1061 cm–1 for 1 and 2, and νs(SO2) is assigned to the medium bands at 1144 cm–1 (1) and 1146 cm–1 (2), respectively. The methanesulfonate ion, [CH3SO3]–, in 3 exhibits C3v symmetry. The vibrational spectrum for this anion has been published for several compounds.36 In accordance with literature data, the characteristic Raman frequencies are assigned as νs(SO3): 1042 cm–1, νas(SO3): 1203 cm–1, δs(SO3): 551 cm–1 and δas(SO3): 526 cm–1.
The triflate ion, [CF3SO3]– in 4 also exhibits C3v symmetry. All fundamental triflate vibrations are situated below 1400 cm–1 (meaning also below most of the CH3 and CH2 bending modes).37 Most of its vibrational modes can be ascribed to the SO3 and CF3 units, respectively. Very strong νs(SO3) at 1033 cm–1 dominates the Raman spectrum of 4 in the region below 1500 cm–1, and νas(SO3) comes out at 1246 cm–1 with weak–medium intensity. The deformation δs(SO3) is attributed to the weak–medium Raman band at 572 cm–1. The Raman mode at 1226 cm–1 is ascribed to the symmetric CF3 stretching, and δs(CF3) is the strong band at 756 cm–1. For further details, see Table S1 (ESI).†
Experimental results
All reactions and manipulations were carried out under a dry argon atmosphere using standard Schlenk, vacuum-line and glove box techniques. NMR spectra were recorded on a BRUKER Digital FT-NMR ‘AVANCE 400’ spectrometer at room temperature. Chemical shifts are reported in ppm relative to the 1H and 13C residue of the deuterated solvent (CDCl3). Raman spectra obtained from the bulk solids were recorded at 200 mV using Bruker IFS-FRA-106. To obtain measurement, respective samples were sealed under an argon atmosphere in glass capillaries, and data were recorded at room temperature, if not stated otherwise. The compounds 1, 3-dimethylimidazolium methylsulfate, 1-butyl-3-methylimidazolium methyl-sulfate, 1-ethyl-3-methylimidazolium, methanesulfonate, and 1-ethyl-3-methylimidazolium trifluoromethane-sulfonate from Merck were dried for 140 h in Schlenk tubes at 150 °C under reduced pressure and rigorous stirring before use. 18-Crown-6 (Merck) was dried over activated 4 Å molecular sieves and re-crystallized from ether.Syntheses
Bis(1,3-dimethylimidazolium methylsulfate) [18-crown-6] (1)
18-Crown-6 (1.135 mmol, 300 mg) was added to [DMIm] [CH3SO4] (1.135 mmol, 236 mg). The reaction mixture was heated at 150 °C for 2 h. After several days, colorless single crystals of compound 1 were formed. Mp 86 °C. 1H NMR (CDCl3), δ = 9.61 (s, 1H, H(2)), 7.25 (br s, 2H, H(4,5)), 4.02 (s, 3H, NCH3), 3.76 (s, 3 H, CH3OSO3–), 3.69 ([18-crown-6]).Bis(1-butyl-3-methylimidazolium methylsulfate) [18-crown-6] (2)
18-Crown-6 (1.135 mmol, 300 mg) was added to [BMIm] [CH3SO4] (1.13 mmol, 284 mg). The reaction mixture was heated at 250 °C for 4 h. After two weeks, colorless single crystals of compound 2 were formed. Mp 39 °C. 1H NMR (CDCl3), δ 9.65 (s, 1H, H(2)), 7.31 (dd, 3J = 1.9 Hz, 1H, H(4)), 7.25 (dd, 3J = 1.9 Hz, 1H, H(5)), 4.25 (t, 3J = 7.4 Hz, 2H, NCH2), 4.04 (s, 3H, NCH3), 3.76 (s, 3H, CH3OSO3–), 3.69 ([18-crown-6]), 1.88 (quint, 3J = 7.4 Hz, 2H, NCH2CH2), 1.39 (sext, 3J = 7.4 Hz, 2H, CH2CH3), 0.97 (t, 3J = 7.4 Hz, 3H, CH2CH3).Bis(1-ethyl-3-methylimidazolium methanesulfonate) [18-crown-6] (3)
18-Crown-6 (1.135 mmol, 300 mg) was added to [EMIm] [CH3SO3] (1.135 mmol, 234 mg). The reaction mixture was heated at 180 °C for 2 h. Colorless single crystals of 3 were formed within a week. Mp 51 °C. 1H NMR (CDCl3), δ 10.08 (s, 1H, H(2)), 7.25 (br s, 2H, H(4,5)), 4.35 (q, 3J = 7.4 Hz, 2H, CH2CH3), 4.06 (s, 3H, NCH3), 3.69 ([18-crown-6]), 2.82 (s, 3H, CH3SO3–), 1.59 (t, 3J = 7.4 Hz, 3H, CH2CH3).Bis(1-ethyl-3-methylimidazolium trifluoromethanesulfonate) [18-crown-6] (4)
18-Crown-6 (1.135 mmol, 300 mg) was added to [EMIm] [CF3SO3] (1.135 mmol, 295 mg). The reaction mixture was heated at 200 °C for 4 h. After several days colorless single crystals of 4 as an insoluble product were formed. Mp 69 °C. 1H NMR (CDCl3), δ 9.41 (s, 1H, H(2)), 7.23 (dd, 3J = 1.8 Hz, 1H, H(4)), 7.21 (dd, 3J = 1.8 Hz, 1H, H(5)), 4.31 (q, 3J = 7.5 Hz, 2H, CH2CH3), 4.01 (s, 3H, NCH3), 3.82 ([18-crown-6]), 1.61 (t, 3J = 7.5 Hz, 3H, CH2CH3).X-Ray diffraction analysis
All data were collected on a Stoe IPDS-II single-crystal X-ray diffractometer with graphite monochromated Mo Kα radiation (λ = 0.71073 Å) at 223 K. Crystal structure solution by direct methods using SHELXS-9738 yielded in all cases heavy atom positions. Subsequent difference Fourier analyses and least squares refinements with SHELXL-9738 allowed localization of the remaining atom positions. In the case of compounds 1, 3 and 4, the hydrogen positions could be obtained from the difference Fourier map while for compound 2 the hydrogen positions in C(10) were calculated using the riding model. The crystal data and structure refinement parameters of the four structures are summarized in Table 1. For preparation of the structure drawings, the programs Diamond39 and POV-Ray40 were applied.Further details on the crystal structure investigations may be obtained from the Cambridge Crystallographic Data Centre (CCDC, 12 Union Road, Cambridge CB2 1EZ, fax (+44)1223-336-033, e-mail deposit@ccdc.cam.ac.uk) on quoting the depository numbers CCDC-648112 for compound 1, CCDC-648113 for compound 2, CCDC-648115 for compound 3 and CCDC-648114 for compound 4. For crystallographic data in CIF or other electronic format see DOI: 10.1039/b708724c
Summary and concluding remarks
Four 1-alkyl-3-methylimidazolium based compounds with anions [CH3SO4]–, [CH3SO3]– and [CF3SO3]– co-crystallize with 18-crown-6 in 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry. Single-crystal structure for the title compounds could be obtained along with their NMR and Raman spectra. For the 1-butyl-3-methylimidazolium cation (compound 2), the distinctive Raman bands trans–trans and trans–gauche conformers29 could be confirmed. The Raman spectra at –60 and –150 °C differ only marginally from those one at room temperature. This may be a hindering for the generation of different rotational conformers of [BMIm]+ at low temperatures, whereas the single-crystal structure determination (–50 °C) results in the monoclinic space groupP21/n (trans–trans conformer) without any indication of structural disorder.
1 stoichiometry. Single-crystal structure for the title compounds could be obtained along with their NMR and Raman spectra. For the 1-butyl-3-methylimidazolium cation (compound 2), the distinctive Raman bands trans–trans and trans–gauche conformers29 could be confirmed. The Raman spectra at –60 and –150 °C differ only marginally from those one at room temperature. This may be a hindering for the generation of different rotational conformers of [BMIm]+ at low temperatures, whereas the single-crystal structure determination (–50 °C) results in the monoclinic space groupP21/n (trans–trans conformer) without any indication of structural disorder.
One reason for the enclosure of 18-crown-6 in imidazolium based salts is obviously the formation of dense packing, or alternatively a space filling. The enclosure of 18-crown-6 stabilizes the new structures according to their melting temperatures in relation to these of the corresponding pure imidazolium based salts.
From the crystal structure conclusive information can be drawn for the relevant species in the liquid state and also for possible reaction mechanisms. The use of crown ethers in molten salt systems (ILs) can result in the synthesis of new compounds by exchange reactions or equilibrium shifting as, e.g.
| AB + CD + crown → [A(crown)D]↓ + C |
| + |
| + D |
| – |
| . |
Acknowledgements
The authors thank Karin Bode for the careful recording of the Raman spectra and Dr Viktor Zapolskii for the determination of the melting points.References
- (a) T. Welton, Chem. Rev., 1999, 99, 2071 CrossRef CAS; (b) K. R. Seddon, J. Chem. Technol. Biotechnol., 1997, 68, 351 CrossRef CAS; (c) P. Wasserscheid and W. Keim, Angew. Chem., Int. Ed., 2000, 39, 3773 CrossRef; (d) R. Hagiwara, Electrochemistry, 2002, 70, 130 Search PubMed; (e) P. Wasserscheid and T. Welton, Ionic Liquids in Synthesis, Wiley-VCH Verlag, Weinheim, 2003 Search PubMed.
- (a) C. L. Hussey, Pure Appl. Chem., 1988, 60, 1763 CrossRef CAS; (b) J. S. Wilkes, J. A. Levisky and C. L. Hussey, Inorg. Chem., 1982, 21, 1263 CrossRef CAS; (c) C. L. Hussey, Advances in Molten Salts Chemistry, ed. G. Mamantov and C. Mamantov, Elsevier, New York, 1983, vol. 5, p. 185 Search PubMed; (d) M. K. Dieter, C. J. Dymek, N. E. Heimer, J. W. Rovang and J. S. Wilkes, J. Am. Chem. Soc., 1988, 110, 2722 CrossRef CAS; (e) H. L. Ngo, K. LeCompte, L. Hargens and A. B. McEwen, Thermochim. Acta, 2000, 357–358, 97 CrossRef CAS.
- (a) J. G. Huddleston, H. D. Willauer, R. P. Swatloski, A. E. Visser and R. D. Rogers, Chem. Commun., 1998, 1765 RSC; (b) A. Bosmann, L. Datsevich, A. Jess, A. Lauter, C. Schmitz and P. Wasserscheid, Chem. Commun., 2001, 2494 RSC.
- (a) R. Sheldon, Chem. Commun., 2001, 2399 RSC; (b) J. D. Holbrey and K. R. Seddon, Clean Prod. Process., 1999, 1, 223 CrossRef.
- (a) M. Hasan, I. V. Kozhevnikov, M. R. H. Siddiqui, C. Femoni, A. Steiner and N. Winterton, Inorg. Chem., 2001, 40, 795 CrossRef CAS; (b) P. J. Dyson, M. C. Grossel, N. Srinivasan, T. Vine, T. Welton, D. J. Williams, A. J. P. White and T. J. Zigras, J. Chem. Soc., Dalton Trans., 1997, 3465 RSC; (c) M. Hasan, I. V. Kozhevnikov, M. R. H. Siddiqui, A. Steiner and N. Winterton, J. Chem. Res., 2000, 8, 392 Search PubMed; (d) D. Crofts, P. J. Dyson, K. M. Sanderson, N. Srinivasan and T. Welton, J. Organomet. Chem., 1999, 573, 292 CrossRef CAS.
- (a) R. T. Carlin and J. S. Wilkes, J. Mol. Catal., 1990, 63, 125 CrossRef CAS; (b) T. Biedroń and P. Kubisa, Macromol. Rapid Commun., 2001, 22, 1237 CrossRef CAS; (c) H. Zhang, K. Hong and J. W. Mays, Polym. Prepr. (Am. Chem. Soc., Div. Polym. Chem.), 2001, 42, 583 CAS; (d) A. Noda and M. Watanabe, Electrochim. Acta, 2000, 45, 1265 CrossRef CAS; (e) Y. Pang, X. Li, H. Ding, G. Shi and L. Jin, Electrochim. Acta, 2007, 52, 6172 CrossRef CAS.
- (a) A. Berthod and S. Carda-Broch, J. Liq. Chromatogr. Relat. Technol., 2003, 26, 1493 CrossRef CAS; (b) G. A. Baker, Sh. N. Baker, S. Pandey and F. V. Bright, Analyst, 2005, 130, 800 RSC.
- (a) C. J. Bowlas, D. W. Bruce and K. R. Seddon, Chem. Commun., 1996, 1625 RSC; (b) C. M. Gordon, J. D. Holbrey, A. R. Kennedy and K. R. Seddon, J. Mater. Chem., 1998, 8, 2627 RSC; (c) J. H. Davis, Jr., K. J. Forrester and T. Merrigan, Tetrahedron Lett., 1998, 39, 8955 CrossRef.
- C. Ye, W. Liu, Y. Chen and L. Yu, Chem. Commun., 2001, 2244 RSC.
- L. Yu, D. Gracia, R. Ren and X. Zeng, Chem. Commun., 2005, 2277 RSC.
- C. Hardacre, J. D. Holbrey, P. B. McCormac, S. E. J. McMath, M. Nieuwenhuyzen and K. R. Seddon, J. Mater. Chem., 2001, 11, 346 RSC.
- (a) J. S. Wilkes and M. J. Zaworotko, J. Chem. Soc., Chem. Commun., 1992, 965 RSC; (b) E. I. Cooper and E. J. M. O'Sullivan, Proc. Electrochem. Soc., 1992, 92–16, 386.
- (a) A. A. Fannin, D. A. Floreani, L. A. King, J. S. Landers, B. J. Piersma, D. J. Stech, R. L. Vaughn, J. S. Wilkes and J. L. Williams, J. Phys. Chem., 1984, 88, 2614 CrossRef; (b) P. Bonhôte, A.-P. Dias, M. Armand, N. Papageorgiou, K. Kalyanasundaram and M. Grätzel, Inorg. Chem., 1996, 35, 1168 CrossRef CAS; (c) J. D. Holbrey and K. R. Seddon, J. Chem. Soc., Dalton Trans., 1999, 2133 RSC; (d) J. G. Huddleston, A. E. Visser, W. M. Reichert, H. D. Willauer, G. A. Broker and R. D. Rogers, Green Chem., 2001, 3, 156 RSC.
- (a) S. V. Dzyuba and R. A. Bartsch, ChemPhysChem, 2002, 3, 161 CrossRef CAS; (b) W. Xu, E. I. Cooper and C. A. Angell, J. Phys. Chem. B, 2003, 107, 6170 CrossRef CAS; (c) W. Xu, L.-M. Wang, R. A. Nieman and C. A. Angell, J. Phys. Chem. B, 2003, 107, 11749 CrossRef CAS; (d) W. Xu and C. A. Angell, Science, 2003, 302, 422 CrossRef CAS; (e) R. Hagiwara, K. Matsumoto, Y. Nakamori, T. Tsuda, Y. Ito, H. Matsumoto and K. Momota, J. Electrochem. Soc., 2003, 150, D195 CrossRef CAS.
- (a) A. Noda, K. Hayamizu and M. Watanabe, J. Phys. Chem. B, 2001, 105, 4603 CrossRef CAS; (b) H. Tokuda, K. Hayamizu, K. Ishii, M. A. B. H. Susan and M. Watanabe, J. Phys. Chem. B, 2004, 108, 16593 CrossRef CAS; (c) H. Tokuda, K. Hayamizu, K. Ishii, M. A. B. H. Susan and M. Watanabe, J. Phys. Chem. B, 2005, 109, 6103 CrossRef CAS.
- R. Custelcean, L. H. Delmau, B. A. Moyer, J. L. Sessler, W.-S. Cho, D. Gross, G. W. Bates, S. J. Brooks, M. E. Light and P. A. Gale, Angew. Chem., Int. Ed., 2005, 44, 2537 CrossRef CAS.
- G. W. Bates, P. A. Gale and M. E. Light, CrystEngComm, 2006, 8, 300 RSC.
- G. W. Bates, M. Kostermans, W. Dehaen, P. A. Gale and M. E. Light, CrystEngComm, 2006, 8, 444 Search PubMed.
- (a) E. Weber and F. Vögtle, in Comprehensive Supramolecular Chemistry, ed. J. Atwood, J. Davies, D. MacNicol and F. Vögtle, Elsevier, Oxford, 1996, vol. 2, p. 1 Search PubMed; (b) D. B. Amabilino, F. M. Raymo and J. F. Stoddart, in Comprehensive Supramolecular Chemistry, ed. J. Atwood, J. Davies, D. MacNicol and F. Vögtle, Elsevier, Oxford, 1996, vol. 9, p. 86 Search PubMed; (c) J. C. Ma and D. A. Dougherty, Chem. Rev., 1997, 97, 1303 CrossRef CAS; (d) M. Nishio, Y. Umezawa, M. Hirota and Y. Takeuchi, Tetrahedron, 1995, 51, 8665 CrossRef CAS; (e) S. Kiviniemi, M. Nissinen, M. T. Lämsä, J. Jalonen, K. Rissanen and J. Pursiainen, New J. Chem., 2000, 24, 47 RSC; (f) D. Braga, F. Grepioni and E. Tedesco, Organometallics, 1998, 17, 2669 CrossRef CAS; (g) J. Dupont, P. A. Z. Suarez, R. F. de Souza, R. A. Burrow and J.-P. Kintzinger, Chem.–Eur. J., 2000, 6, 2377 CrossRef CAS.
- Y. Yoshida, K. Muroi, A. Otsuka, G. Saito, M. Takahashi and T. Yoko, Inorg. Chem., 2004, 43, 1458 CrossRef CAS.
- S. Tsuzuki, K. Honda, T. Uchimaru, M. Mikami and K. Tanabe, J. Am. Chem. Soc., 2000, 122, 3746 CrossRef CAS.
- J. D. Holbrey, W. M. Reichert, R. P. Swatloski, G. A. Broker, W. R. Pitner, K. R. Seddon and R. D. Rogers, Green Chem., 2002, 4, 407 RSC.
- S. G. Bott, A. W. Coleman and J. L. Atwood, J. Am. Chem. Soc., 1988, 110, 610 CrossRef CAS.
- J. D. Holbrey, W. M. Reichert, M. Nieuwenhuyzen, S. Johnston, K. R. Seddon and R. D. Rogers, Chem. Commun., 2003, 1636 RSC.
- (a) M. Tremayne, P. Lichtfoot, M. A. Mehta, P. G. Bruce, K. D. M. Harris, K. Shankland, C. J. Gilmore and G. Bricogne, J. Solid State Chem., 1992, 100, 191 CrossRef CAS; (b) A. R. Choudhury, N. Winterton, A. Steiner, A. I. Cooper and K. A. Johnson, CrystEngComm, 2006, 8, 742 RSC.
- (a) S. Chitsaz, H. Folkerts, J. Grebe, T. Gröb, K. Harms, W. Hiller, M. Krieger, W. Massa, J. Merle, M. Möhlen, B. Neumüller and K. Dehnicke, Z. Anorg. Allg. Chem., 2000, 626, 775 CrossRef CAS; (b) T. Gröb, G. Seybert, W. Massa, A. Greiner and K. Dehnicke, Z. Anorg. Allg. Chem., 2001, 627, 1 CrossRef CAS.
- (a) G. Korus and M. Jansen, Z. Anorg. Allg. Chem., 1997, 623, 1625 CrossRef; (b) G. Korus and M. Jansen, Z. Anorg. Allg. Chem., 2001, 627, 1559; (c) L. Hildebrandt, R. Dinnebier and M. Jansen, Z. Anorg. Allg. Chem., 2005, 631, 1660 CrossRef CAS; (d) R. Dinnebier, N. Sofina and M. Jansen, Z. Anorg. Allg. Chem., 2004, 630, 1613 CrossRef CAS.
- (a) S. Hayashi, R. Ozawa and H. Hamaguchi, Chem. Lett., 2003, 32, 498 CrossRef; (b) S. Saha, S. Hayashi, A. Kobayashi and H. Hamaguchi, Chem. Lett., 2003, 32, 740 CrossRef CAS; (c) R. Ozawa, S. Hayashi, S. Saha, A. Kobayashi and H. Hamaguchi, Chem. Lett., 2003, 32, 948 CrossRef CAS.
- R. W. Berg, M. Deetlefs, K. R. Seddon, I. Shim and J. M. Thompson, J. Phys. Chem. B, 2005, 109, 19018 CrossRef CAS.
- (a) P. A. Hunt, J. Phys. Chem. B, 2007 DOI:10.1021/jp067182p; (b) P. A. Hunt and I. R. Gould, J. Phys. Chem. A, 2006, 110, 2269 CrossRef CAS; (c) P. A. Hunt, I. R. Gould and B. Kirchner, Aust. J. Chem., 2007, 60, 9 CrossRef CAS.
- K. Matsumoto, R. Hagiwara, R. Yoshida, Y. Ito, Z. Mazej, P. Benkic, B. Zemva, O. Tamada, H. Yoshino and S. Matsubara, Dalton Trans., 2004, 144 RSC.
- S. A. Katsyuba, P. J. Dyson, E. E. Vandyukova, A. V. Chernova and A. Vidis, Helv. Chim. Acta, 2004, 87, 2556 CrossRef CAS.
- S. Hayashi, S. Saha, R. Ozawa, A. Kobayashi and H. Hamaguchi, Chem. Lett., 2003, 32, 332 CrossRef.
- U. Domanska, A. Pobudkowska and F. Eckert, Green Chem., 2006, 8, 268 RSC.
- L. L. Van Loon and H. C. Allen, J. Phys. Chem. B, 2004, 108, 17666 CrossRef.
- (a) J. Golding, S. Forsyth, D. R. MacFarlane, M. Forsyth and G. B. Deacon, Green Chem., 2002, 4, 223 RSC; (b) J. H. R. Clarke and L. A. Woodward, Trans. Faraday Soc., 1968, 64, 1041 RSC; (c) M. S. Wickleder, Z. Anorg. Allg. Chem., 2002, 628, 1848 CrossRef; (d) W. K. Thompson, Spectrochim. Acta, 1972, 28A, 1479 CrossRef.
- (a) B. Sandner, J. Tübke, S. Wartewig and S. Shashkov, Solid State Ionics, 1996, 83, 87 CrossRef CAS; (b) R. A. Silva, G. G. Silva and M. A. Pimenta, J. Raman Spectrosc., 2001, 32, 369 CrossRef CAS; (c) D. H. Johnson and D. F. Shriver, Inorg. Chem., 1993, 32, 1045 CrossRef CAS.
- G.M. Sheldrick, SHELX–97, SHELXL–97, Universität Göttingen, Germany, 1997.
- DIAMOND–Visual Crystal Structure Information System, CRYSTAL IMPACT, Bonn, Germany, 2004.
- POV-Ray TM, Version 3, Copyright by the POV-Ray-Team, 1997.
Footnote |
† Electronic supplementary information (ESI) available![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Crystal structures of compounds 1–4 (Fig. S1–S6); spectroscopy data (Fig. S7–S14; Raman frequencies of the crystalline title compounds along with their estimated intensities and proposed assignments (Table S1). See DOI: 10.1039/b708724c Crystal structures of compounds 1–4 (Fig. S1–S6); spectroscopy data (Fig. S7–S14; Raman frequencies of the crystalline title compounds along with their estimated intensities and proposed assignments (Table S1). See DOI: 10.1039/b708724c |
| This journal is © The Royal Society of Chemistry 2008 |
